Abstract
Background
Small-scale studies correlated the presence of thyroid autoimmunity with both improved or worsened breast cancer outcome.
Objectives
We aimed to clarify this association in a large cohort using the phase III, randomized, controlled Taxotere as Adjuvant Chemotherapy Trial (TACT, CRUK01/001).
Methods
TACT women >18 years old with node-positive or high-risk node-negative early breast cancer (pT1–3a, pN0–1, M0), with stored plasma (n = 1,974), taken 15.5 (median; IQR 7.0–24.0) months after breast surgery were studied. Patients had also received chemotherapy (100%), radiotherapy (1,745/1,974; 88.4%), hormonal therapy (1,378/ 1,974; 69.8%), or trastuzumab (48/1,974; 2.4%). History of thyroid diseases and/or related treatments was not available. The prognostic significance of autoantibodies to thyroid peroxidase (TPOAb; positive ≥6 kIU/L), free-thyroxine and thyrotropin (combined: euthyroid, hypothyroid, hyperthyroid) was evaluated for disease-free survival (DFS), overall-survival (OS), and time-to-recurrence (TTR), with Cox regression models in univariate and multivariable analyses. The extended median follow-up was 97.5 months.
Results
No difference in DFS was found by TPOAb status (unadjusted hazard ratio [HR]: 0.97, 95%CI: 0.78–1.19; p = 0.75) and/or thyroid function (unadjusted HR [hypothyroid vs. euthyroid]: 1.15, 95% CI: 0.79–1.68; p = 0.46; unadjusted HR [hyperthyroid vs. euthyroid]: 1.14, 95% CI: 0.82–1.61; p = 0.44). Similar results were obtained for OS, TTR, multivariable analyses, when TPOAb titre by tertiles was considered, and in a subgroup of 123 patients with plasma collected before adjuvant treatments.
Conclusions
No evidence for a prognostic role of TPOAb and/or thyroid function in moderate-to-high-risk early breast cancer was found in the largest and longest observational study to date.
Keywords: Autoimmune thyroid disease, Autoimmunity, Hyperthyroidism, Hypothyroidism, Thyroid function, Thyroid peroxidase antibodies, Breast cancer
Introduction
An association between breast cancer (BC) and benign thyroid disorders has been debated for decades, reported in several [1, 2] but not all [3] studies; the most recent meta-analyses and reviews reached contrasting conclusions [1, 4, 5, 6]. Hypothyroidism was found to correlate with both an increased [7, 8] or reduced [9, 10, 11] risk of developing BC, whilst other authors did not report a significant correlation [12, 13]. BC has been particularly associated with thyroid autoimmunity (TA); a higher prevalence of anti-thyroid peroxidase (TPO) autoantibodies (TPOAb) was found among BC patients compared with healthy controls [8, 14]. Furthermore, a better BC outcome has been report ed in TPOAb-positive (TPOAb+) versus TPOAb-negative (TPOAb–) patients in some [15, 16, 17, 18] but not all [19] studies.
Currently, no validated major blood prognostic markers for BC are available; carcinoembryonic antigen and cancer antigen 15.3 are the most used, but have low specificity and sensitivity [20]. Circulating tumour DNA and tumour cells seem very promising markers; however, further studies are needed to validate them in routine clinical practice [21]. It would therefore be valuable if TPOAb could be confirmed as a blood BC prognostic marker.
Two studies evaluated 5-year outcomes in 142 [15] and 47 [16] BC women: Smyth et al. [15] reporting TPOAb– as a poor prognostic factor for disease-free survival (DFS) and overall survival (OS), and Fiore et al. [16] reporting 6.7% mortality in patients positive for anti-thyroid autoantibodies (TAb), mainly TPOAb+, compared with 46.9% in TAb-negative patients. Farahati et al. [17] evaluated 314 newly diagnosed BC patients and found no distant metastases among TPOAb+ patients compared with 6.6% among TPOAb– patients. In contrast, Jiskra et al. [19] followed 84 BC patients for 136 months (median), finding no impact of TPOAb on DFS or OS.
The aim of the present study was to clarify the impact of TPOAb on BC prognosis in a large well-powered patient cohort with long-term follow-up, according to the “REporting recommendations for tumour MARKer prognostic studies (REMARK)” guidelines [22]. The Taxotere as Adjuvant Chemotherapy Trial (TACT) recruited 4,162 women diagnosed with moderate-to-high-risk early BC, evaluating whether sequential docetaxel (Taxotere) after anthracycline therapy would improve patient outcome compared with standard anthracycline chemotherapy: analyses were conducted at 62 months’ [23] and 97.5 months’ [24] follow-up, both showing no evidence of a difference between the two chemotherapy regimens. Of relevance, stored plasma was available in a significant number of these patients.
Furthermore, TPO is expressed in BC tissue [25], providing a possible mechanistic link: a thyroid/breast, shared, autoimmune response might target tumour cells and improve BC outcome. If TPOAb+ was confirmed as associated with a better BC outcome, new BC therapeutic approaches based on antigen-specific immunotherapies targeting TPO could be explored.
Materials and Methods
Patients
The TACT study [23] was a multi-centre, open-label, phase III, randomized, controlled trial of women aged >18 years, diagnosed with operable early BC (pT1–3a, pN0–1, M0), with indication for adjuvant chemotherapy, including both lymph-node positive (node+) and lymph-node negative (node–) patients, but at high risk (e.g., tumour grade 3, hormonal-receptor expression negative, or lymphovascular invasion).
Between February 2001 and June 2003, 4,162 women were enrolled across 103 UK and one Belgian centres. All subjects underwent surgery, mastectomy or wide local excision, and were randomized (1: 1 ratio) to the experimental regimen FEC-D (n = 2,073; fluorouracil, epirubicin, cyclophosphamide [FEC] followed by docetaxel) or the centre's choice of control chemotherapy, either FEC (n = 1,265) or E-CMF (n = 824; epirubicin followed by cyclophosphamide, methotrexate, and fluorouracil [CMF]). Adjuvant radiotherapy was mandatory after wide local excision or used after mastectomy according to local guidelines. Endocrine treatments (tamoxifen or aromatase inhibitor monotherapy, tamoxifen followed by aromatase inhibitor) were administered to patients with oestrogen receptor (ER)-positive expression (ER+). Patients with human epidermal growth factor receptor-2 (HER2)-positive expression (HER2+) were allowed to enter clinical trials assessing trastuzumab. All subjects have given their informed consent, and the study protocol has been approved by the institute's Committee on Human Research.
Laboratory Measurements
Following a protocol amendment (in November 2002), blood was taken for future translational research at the time of randomization, or at the women's next follow-up visit. Plasma samples were stored at −20°C for 6.5–13 years (range) at The Institute of Cancer Research (London, UK), and transferred to the Thyroid Research Group (Cardiff, UK) for TPOAb, thyrotropin (TSH), and free-thyroxine (FT4) analyses (in October 2014) using an ADVIA Centaur automated immunoassay analyzer (Bayer plc, UK) and Chemiluminescent Microparticle Immunoassay methods by the ARCHITECT® System (ABBOTT Laboratories, USA). According to the assay cut-off, TPOAb values were dichotomized as ≥6 kIU/L (positive: TPOAb+) versus <6 kIU/L (negative: TPOAb–); TPOAb+ were also categorized into tertiles. FT4 and TSH normal ranges were 9.0–19.1 pmol/L and 0.30–4.40 mIU/L, respectively. They were also combined in a thyroid function status variable: euthyroid (FT4 and TSH within the normal ranges), hypothyroid (FT4 <9.0 pmol/L and/or TSH >4.40 mIU/L), and hyperthyroid (FT4 >19.1 pmol/L and/or TSH <0.3 mIU/L).
Statistical Analysis
According to TPOAb prevalence in age-matched females of the general population [26, 27], 20% of BC individuals were expected to be TPOAb+. Power calculations indicated that 1,158 and 1,430 samples are required to provide 80 and 90% power, respectively, to detect a 81% 5-year DFS in TPOAb+ versus 73% in TPOAb– subjects (hazard ratio [HR], 0.64; two-sided log-rank test with a 0.05 probability of a type I error), consistent with a 74.9% 5-year DFS rate in the whole TACT cohort [23].
Baseline characteristics, BC treatments, and DFS-related characteristics were compared between TACT patients included or not in this study, and presented by dichotomized TPOAb and thyroid function status. Correlations between thyroid biomarkers were assessed using the Spearman rank method.
The primary outcome was to assess TPOAb prognostic significance in relation to DFS; secondary outcomes were TPOAb prognostic significance in relation to OS and time-to-recurrence (TTR), and thyroid function in relation to DFS, OS, and TTR.
For DFS, OS, and TTR, Kaplan-Meier curves were plotted and biomarkers compared with the log-rank test, assessed firstly in a univariate Cox proportional hazards regression model stratified by the centre's choice of control chemotherapy regimen and ER status, and subsequently included in a multivariable Cox model along with known BC prognostic factors: age, HER2 status, nodal involvement, tumour size, and tumour grade. Additional variables, i.e., trial treatment (experimental vs. control), type of surgery, trastuzumab use, radiotherapy, and menopausal status, were included if, by step-wise selection (p < 0.05), shown to add value. TPOAb, TSH, and FT4 were subsequently considered for inclusion if providing independent prognostic information. Interaction tests were used to explore differential effects within subgroups. HR with 95% CI was obtained, with HR < 1 indicating a better BC prognosis.
All patients with a biomarker value available were included in the analysis, as per an intention-to-treat analysis. All analyses were conducted using Stata version 13.1 (STATACORP, TX, USA) [23, 24].
Results
All available TACT plasma samples (n = 2,000) were analyzed for thyroid biomarkers, and 1,974 samples were considered for the statistical analyses (“analysis population”; online suppl. Fig. 1 for all online suppl. material, see www.karger.com/doi/10.1159/460246). The median (IQR; range) blood collection time was 15.5 (7.0–24.0; 0.5–57.2) months after surgery.
Online supplementary Table 1 reports the analysis population's characteristics; the median (IQR; range) follow-up was 96.7 (87.4–106.3; 3.4–126.4) months. Overall 5-year estimates for DFS, OS, and TTR were 79.5% (95% CI, 77.6–81.2), 87.4% (95% CI, 85.9–88.8), and 81.1% (95% CI, 79.3–82.8), respectively.
Distribution of TPOAb and Thyroid Function
TPOAb+ was detected in 406/1,974 (20.6%) patients, distributed in the following tertiles: 137 (6.9%) 6–40 kIU/L (T1), 134 (6.7%) 41–238 kIU/L (T2), 135 (6.8%) 240–2,000 kIU/L (T3). Baseline characteristics were largely comparable between TPOAb+ and TPOAb– patients (Table 1), apart from age, with TPOAb+ patients being slightly older than TPOAb– patients (mean age ±SD, 50.2 ± 7.7 vs. 48.8 ±8.5 years, respectively; p = 0.005).
Table 1.
Baseline characteristics and treatments for breast cancer by autoantibodies to thyroid peroxidase (TPOAb) and thyroid function status
| Autoantibodies to TPO |
Thyroid function status |
||||||
|---|---|---|---|---|---|---|---|
| TPOAb- (n = 1,568) | TPOAb+ (n = 406) | p value | hypothyroid(n = 96) | euthyroid (n = 1,760) | hyperthyroid (n = 118) | p value | |
| Mean age ± SD, years | 48.8±8.5 | 50.2±7.7 | 0.005a | 50.5±6.6 | 48.9±8.5 | 50.7±7.6 | 0.03d |
| Age group, n (%) | |||||||
| <40 years | 257 (16.4) | 49 (12.1) | 8 (8.3) | 287 (16.3) | 11 (9.3) | ||
| 40–49 years | 575 (36.7) | 151 (37.2) | 0.08b | 36 (37.5) | 647 (36.8) | 43 (36.4) | 0.62b |
| 50–59 years | 590 (37.6) | 167 (41.1) | 45 (46.9) | 657 (37.3) | 55 (46.6) | ||
| ≥60 years | 146 (9.3) | 39 (9.6) | 7 (7.3) | 169 (9.6) | 9 (7.6) | ||
| Nodal status, n (%) | |||||||
| Node negative | 314 (20.0) | 93 (22.9) | 18 (18.8) | 367 (20.9) | 22 (18.6) | ||
| 1–3 positive nodes | 719 (45.9) | 171 (42.1) | 0.62b | 33 (34.4) | 808 (45.9) | 49 (41.5) | 0.61b |
| ≥4 positive nodes | 535 (34.1) | 142 (35.0) | 45 (46.9) | 585 (33.2) | 47 (39.8) | ||
| Tumour grade, n (%) | |||||||
| Grade 1 | 77 (4.9) | 23 (5.7) | 4 (4.2) | 88 (5.0) | 8 (6.8) | ||
| Grade 2 | 603 (38.5) | 155 (38.2) | 0.74b | 35 (36.5) | 681 (38.7) | 42 (35.6) | 0.72b |
| Grade 3 | 883 (56.3) | 228 (56.2) | 57 (59.4) | 986 (56.0) | 68 (57.6) | ||
| Unknown | 5 (0.3) | 0 (0.0) | 0 (0.0) | 5 (0.3) | 0 (0.0) | ||
| Tumour size, n (%) | |||||||
| ≤2 cm | 578 (36.9) | 147 (36.2) | 25 (26.0) | 659 (37.4) | 41 (34.8) | ||
| >2 and ≤5 cm | 857 (54.7) | 220 (54.2) | 0.59b | 61 (63.5) | 952 (54.1) | 64 (54.2) | 0.38b |
| <p>5 cm | 132 (8.4) | 39 (9.6) | 10 (10.4) | 148 (8.4) | 13 (11.0) | ||
| Unknown | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 0 (0.0) | ||
| ER and HER2 status, n (%) | |||||||
| ER+ | 1,107 (70.6) | 289 (71.2) | 69 (71.9) | 1,248 (70.9) | 79 (67.0) | ||
| And HER2+ | 198 (12.6) | 49 (12.1) | 13 (13.5) | 220 (12.5) | 14 (11.9) | ||
| And HER2− | 772 (49.2) | 201 (49.5) | 46 (47.9) | 873 (49.6) | 54 (45.8) | 0.62c (ER) | |
| And HER2 unknown | 137 (8.7) | 39 (9.6) | 0.85c (ER) | 10 (10.4) | 155 (8.8) | 11 (9.3) | 0.84c (HER2) |
| ER− | 461 (29.4) | 117 (28.8) | 0.45c (HER2) | 27 (28.1) | 512 (29.1) | 39 (33.1) | |
| And HER2+ | 118 (7.5) | 43 (10.6) | 8 (8.3) | 141 (8.0) | 12 (10.2) | ||
| And HER2− | 289 (18.4) | 61 (15.0) | 15 (15.6) | 313 (17.8) | 22 (18.6) | ||
| And HER2 unknown | 54 (3.4) | 13 (3.2) | 4 (4.2) | 58 (3.3) | 5 (4.2) | ||
| Molecular subgroup, n (%) | |||||||
| ER+/HER2−1 | 784 (50.0) | 203 (50.0) | 47 (49.0) | 885 (50.3) | 55 (46.6) | ||
| HER2+ | 316 (20.2) | 92 (22.7) | 0.40c | 21 (21.9) | 361 (20.5) | 26 (22.0) | 0.94c |
| Triple negative | 277 (17.7) | 59 (14.5) | 14 (14.6) | 301 (17.1) | 21 (17.8) | ||
| Type of surgery and radiotherapy use, n (%) | 0.74c (surgery) | 0.99c (surgery) | |||||
| Mastectomy | 854 (54.5) | 225 (55.4) | 53 (55.2) | 962 (54.7) | 64 (54.2) | ||
| With radiotherapy2 | 688 (80.6) | 177 (78.7) | 0.61c (radiotherapy) | 47 (88.7) | 772 (80.2) | 46 (71.9) | 0.33c (radio-therapy) |
| Wide local excision | 714 (45.5) | 181 (44.6) | 43 (44.8) | 798 (45.3) | 54 (45.8) | ||
| With radiotherapy3 | 704 (98.6) | 176 (97.2) | 41 (95.3) | 787 (98.6) | 52 (96.3) | ||
| Endocrine treatment in ER+ | |||||||
| patients, n (%)4 | |||||||
| Tamoxifen monotherapy | 696 (62.9) | 167 (57.8) | 43 (62.3) | 772 (61.9) | 48 (60.8) | ||
| Tamoxifen followed by AI | 354 (32.0) | 100 (34.6) | 0.13c | 20 (29.0) | 409 (32.8) | 25 (31.7) | 0.09c |
| AI monotherapy | 46 (4.2) | 15 (5.2) | 6 (8.7) | 53 (4.3) | 2 (2.5) | ||
| No endocrine treatment/unknown | 11 (1.0) | 7 (2.4) | 0 (0.0) | 14 (1.1) | 4 (5.1) | ||
| Trastuzumab in HER2+ | |||||||
| patients, n (%)5 | |||||||
| Yes | 40 (12.7) | 8 (8.7) | 0.36c | 1 (4.8) | 44 (12.2) | 3 (11.5) | 0.71c |
| No/Not known | 276 (87.3) | 84 (91.3) | 20 (95.2) | 317 (87.8) | 23 (88.5) | ||
| Chemotherapy, n (%) | |||||||
| Control (FEC) | 498 (31.8) | 128 (31.5) | 27 (28.1) | 568 (32.3) | 31 (26.3) | ||
| Control (E-CMF) | 271 (17.3) | 61 (15.0) | 0.52c | 16 (16.7) | 301 (17.1) | 15 (12.7) | 0.90c |
| FEC-D | 799 (51.0) | 217 (53.4) | 53 (55.2) | 891 (50.6) | 52 (44.1) | ||
AI, aromatase inhibitors; ER+, positive oestrogen receptor (ER); ER−, negative ER; E-CMF, epirubicin 100 mg/m2 for 4 cycles followed by CMF (cyclophosphamide 600 mg/m2, methotrexate 40 mg/m2, and fluorouracil 600 mg/m2) for 4 cycles; FEC, fluorouracil 600 mg/m2, epirubicin 60 mg/m2, and cyclophosphamide 600 mg/m2 for 8 cycles; FEC-D, FEC for 4 cycles followed by docetaxel 100 mg/m2 for 4 cycles; HER2+, positive human epidermal growth factor receptor-2 (HER2); HER2−, negative HER2; SD, standard deviation; TPOAb+, positive TPOAb; TPOAb−, negative TPOAb; Triple negative, negative HER2, ER, and progesterone receptor.
Includes ER−, progesterone receptor positive, HER2−
denominators calculated using patients treated with mastectomy
denominators calculated using patients treated with wide local excision
denominators calculated using ER+ patients
denominators calculated using HER2+ patients.
t test
trend test, note “unknowns” excluded from the test
Fisher exact test
ANOVA.
Plasma material was sufficient to determine FT4 and TSH values in 1,974/1,974 (100%) and 1,971/1,974 (99.8%) samples, respectively. Among the 1,974 patients, 1,760 (89.2%) were euthyroid, 96 (4.9%) hypothyroid, and 118 (6.0%) hyperthyroid; all 3 subgroups had similar baseline characteristics (Table 1), apart from age, with hypothyroid and hyperthyroid patients being slightly older than euthyroid patients (mean age ±SD 50.5 ± 6.6 and 50.7 ± 7.6 vs. 48.9 ± 8.5 years, respectively; p = 0.03).
As shown in online supplementary Figure 2, FT4 and TSH were inversely correlated (Spearman rank, −0.23; p < 0.001) and TPOAb was positively associated with TSH (Spearman rank, 0.24; p < 0.001). The inverse correlation between TPOAb and FT4 was weak (Spearman rank, −0.04; p = 0.09). TPOAb+ cases were more prevalent among hypothyroid and hyperthyroid patients compared with the euthyroid group (73/96 [76.0%] hypothyroid; 45/118 [38.1%] hyperthyroid; 288/1,760 [16.4%] euthyroid; p < 0.001).
TPOAb and BC Prognosis
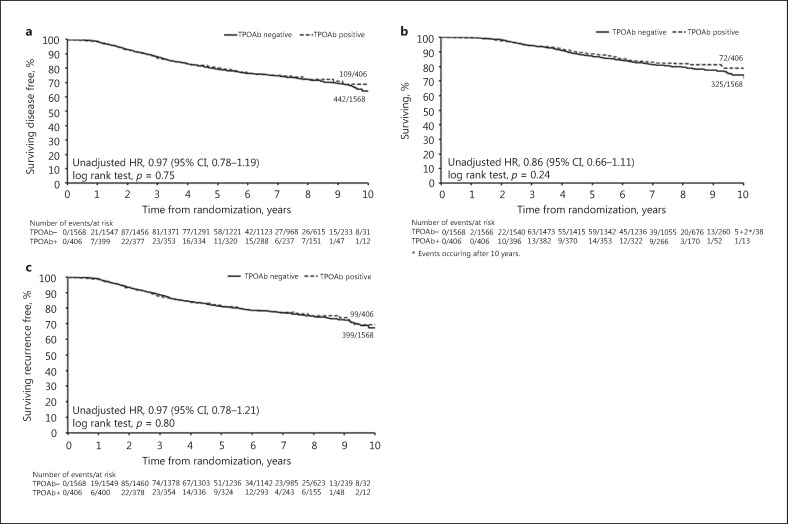
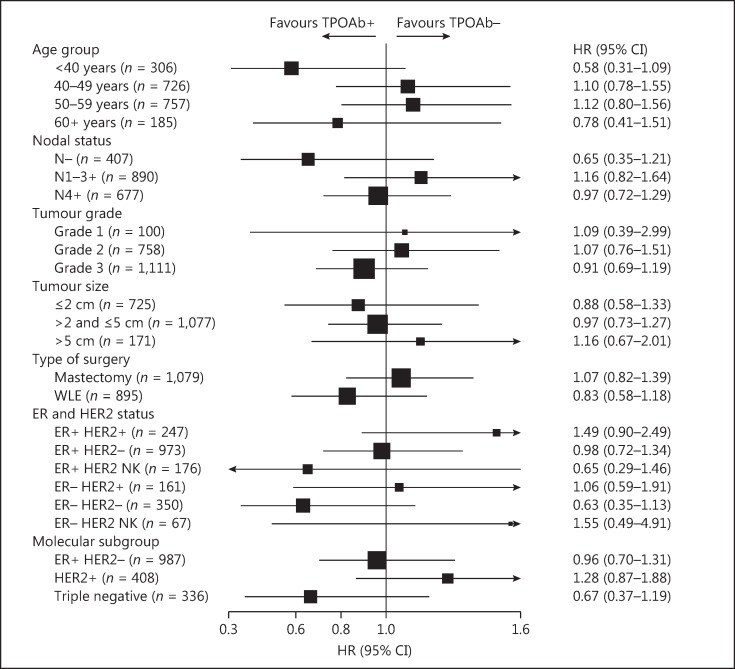
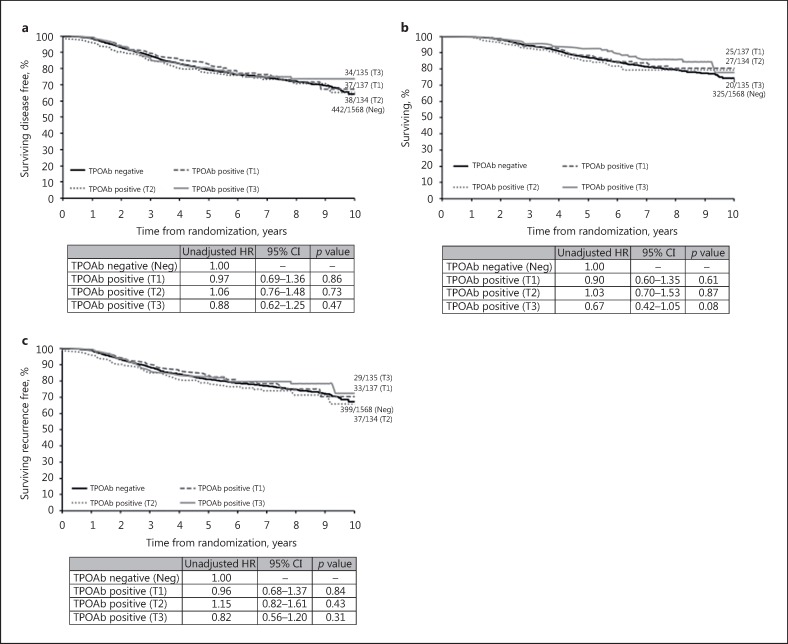
The majority of DFS events were related to distant recurrence in both the TPOAb+ and TPOAb– groups (online suppl. Table 2). There was no evidence of a difference in DFS between TPOAb+ and TPOAb– patients (unadjusted HR: 0.97, 95% CI: 0.78–1.19; p = 0.75, Fig. 1a; adjusted HR: 1.00, 95% CI: 0.81–1.24; p = 0.98, Table 2). Subgroup analyses showed no evidence of any significant interaction effects (Fig. 2). Similarly, there was no evidence of a difference by TPOAb status on OS (unadjusted HR: 0.86, 95% CI: 0.66–1.11; p = 0.24, Fig. 1b; adjusted HR: 0.89, 95% CI: 0.69–1.14; p = 0.35, not shown) and TTR (unadjusted HR: 0.97, 95% CI: 0.78–1.21; p = 0.80, Fig. 1c; adjusted HR: 1.02, 95% CI: 0.81–1.27; p = 0.89, not shown). TPOAb+ tertiles showed no evidence of a prognostic effect in both univariate (Fig. 3) and multivariable (data not shown) analyses for DFS, OS, and TTR.
Fig. 1.
Univariate analyses by dichotomized autoantibodies to thyroid peroxidase (TPOAb). Kaplan-Meier curves relative to breast cancer (BC) outcome (median follow-up 96.7 months) in patients positive (≥6 kIU/L) and negative (<6 kIU/L) for TPOAb. HR, hazard ratio (HR <1 indicates a favourable BC outcome); 95% CI, 95% confidence interval. a Disease-free survival. b Overall survival. c Time to recurrence.
Table 2.
Multivariable analysis for disease-free survival by di chotomized autoantibodies to thyroid peroxidase (TPOAb)
| HR | 95% CI | p value | |
|---|---|---|---|
| TPOAb status | |||
| Negative (n = 1,568) | 1.00 | – | – |
| Positive (n = 406) | 1.00 | 0.81–1.24 | 0.98 |
| Nodal status | |||
| Positive (n = 1,567) | 1.00 | – | – |
| Negative (n = 407) | 0.49 | 0.37–0.64 | <0.001 |
| HER2 status | |||
| Negative (n = 1,323) | 1.00 | – | – |
| Positive (n = 408) | 1.19 | 0.97–1.46 | 0.09 |
| Unknown (n = 243) | 0.93 | 0.71–1.23 | 0.63 |
| Age group | |||
| <40 years (n = 306) | 1.00 | – | – |
| 40–49 years (n = 726) | 0.78 | 0.61–1.00 | 0.05 |
| 50–59 years (n = 757) | 0.75 | 0.59–0.96 | 0.02 |
| ≤60 years (n = 185) | 0.95 | 0.69–1.31 | 0.76 |
| Tumour grade | |||
| Grade 1 (n = 100) | 1.00 | – | – |
| Grade 2 (n = 758) | 1.15 | 0.74–1.78 | 0.55 |
| Grade 3 (n = 1,111) | 1.39 | 0.89–2.17 | 0.14 |
| Unknown (n = 5) | 0.77 | 0.10–5.75 | 0.80 |
| Tumour size1 | |||
| ≤2 cm (n = 725) | 1.00 | – | – |
| >2 and ≤5 cm (n = 1,077) | 1.37 | 1.12–1.66 | 0.002 |
| >5 cm (n = 171) | 1.88 | 1.41–2.52 | <0.001 |
| Type of surgery | |||
| Mastectomy (n = 1,079) | 1.00 | – | – |
| WLE (n = 895) | 0.79 | 0.66–0.95 | 0.01 |
HER2, human epidermal growth factor receptor-2; HR, hazard ratio (HR <1 indicates a favourable breast cancer outcome); WLE, wide local excision; 95% CI, 95% confidence interval.
The patient with unknown tumour size (n = 1) has not been considered for this analysis.
Fig. 2.
Exploratory subgroup analyses for disease-free survival by dichotomized autoantibodies to thyroid peroxidase (TPOAb). ER+, positive oestrogen receptor (ER); ER–, negative ER; HER2+, positive human epidermal growth factor receptor-2 (HER2); HER2–, negative HER2; NK, not known; N–, lymph-node negative; N1–3+, 1–3 lymph-nodes positive; N4+, 4 or more lymph-nodes positive; TPOAb+, positive TPOAb; TPOAb–, negative TPOAb; Triple negative, negative HER2, ER, and progesterone receptor; WLE, wide local excision; 95% CI, 95% confidence interval.
Fig. 3.
Univariate analyses by autoantibodies to thyroid peroxidase (TPOAb) categorized into tertiles. Kaplan-Meier curves relative to breast cancer (BC) outcome (median follow-up 96.7 months) in patients negative (<6 kIU/L) and positive for TPOAb categorized into tertiles: 6–40 kIU/L (T1), 41–238 kIU/L (T2), 240–2,000 kIU/L (T3). HR, hazard ratio (HR <1 indicates a favourable BC outcome); 95% CI, 95% confidence interval. a Disease-free survival. b Overall survival. c Time to recurrence.
Two sensitivity analyses included 126 node+ patients not treated with radiotherapy, similar to Fiore et al.'s cohort [16], and 123 patients with blood taken before any adjuvant therapy. The median (IQR; range) time of blood collection after surgery was 12.4 (4.9–21.6; 0.7–47.2) months and 1.1 (0.9–1.4; 0.5–5.9) months, respectively. There was no evidence of a significant impact on DFS by TPOAb status in either of the two analyses, with unadjusted HRs of 1.48 (95% CI, 0.68–3.25; p = 0.32) and 0.83 (95% CI, 0.35–2.03; p = 0.69), respectively.
Thyroid Function and BC Prognosis
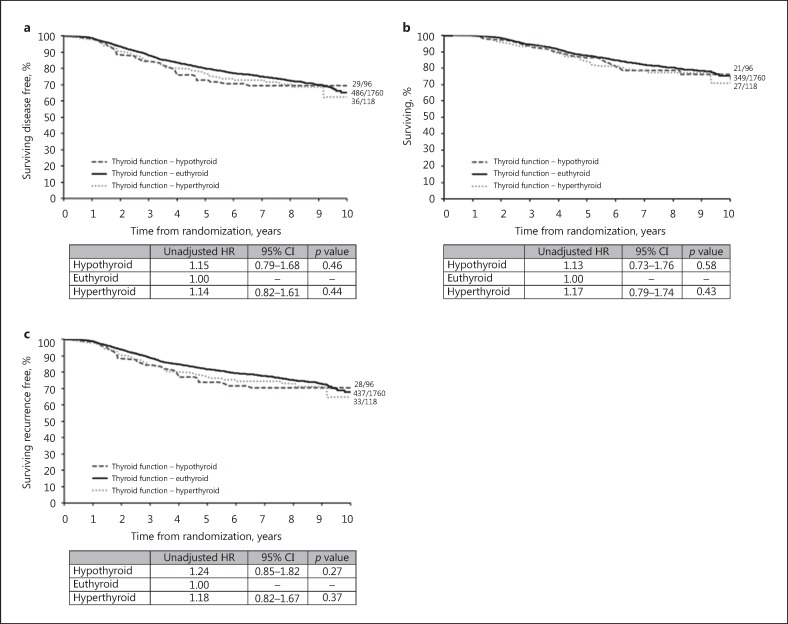
There was no evidence of a significant difference for DFS, OS, and TTR by thyroid function status in either univariate (Fig. 4) or multivariable (data not shown) analyses, and when considering FT4 and TSH separately (DFS, online suppl. Table 3 OS and TTR, not shown).
Fig. 4.
Univariate analyses by thyroid function status. Kaplan-Meier curves relative to breast cancer (BC) outcome (median follow-up 96.7 months) according to thyroid function status. Euthyroid, free-thyroxine (FT4) 9.0–19.1 pmol/L and/or thyrotropin (TSH) 0.30–4.40 mIU/L; hyperthyroid, FT4 >19.1 pmol/L and/or TSH <0.3 mIU/L; hypothyroid, FT4 <9.0 pmol/L and/or TSH >4.40 mIU/L. HR, hazard ratio (HR <1 indicates a favourable BC outcome); 95% CI, 95% confidence interval. a Disease-free survival. b Overall survival. c Time to recurrence.
Discussion
In this large cohort of moderate-to-high-risk early BC patients receiving adjuvant systemic treatments, we found that neither the presence nor the titre of plasma TPOAb, assessed after BC diagnosis and measured with standard assays, had a substantial impact on long-term recurrence or mortality; similar findings were observed for thyroid status. These results confirm one previous finding [19], but contrast with two other studies [15, 16]. We believe that our study is reliable, considering that our patient cohort is the largest to date, with one of the longest follow-ups, and focused on a well-defined BC population. Previous studies used smaller patient cohorts with shorter follow-ups [15, 16, 19], mixed different BC stages [19], or provided no information about BC stage [15], histological [15, 19] and molecular subtypes [15, 16, 19], and adjuvant treatments received [15, 19]; they may be susceptible to bias and random findings. In addition, the BC population analyzed in this study is very similar to that of Fiore et al. [16], who recruited non-metastatic aggressive BC cases all treated with chemotherapy.
The long survival of our patient cohort could obscure a minor prognostic effect of TPOAb and/or thyroid function on BC, hypothetically detectable only among patients not suitable for standard treatments (e.g., medical contraindications) and targeted therapies (e.g., triple negative BC). This is possible but unlikely, since our exploratory analysis conducted among different BC subtypes confirmed our negative results. Furthermore, the multivariable analyses confirmed nodal status and tumour size as the two most important BC prognostic factors [28], proving that the cohort used was appropriate for the research question, and the model reasonably sensitive. Similarly, the better BC prognosis characterizing the intermediate age group (50–59 years) is consistent with the results of a recent large cohort study [29].
Our study cannot exclude a role of different TA parameters on BC prognosis, i.e., the presence of goitre [15] or incidental TA-related 18F-FDG PET/CT uptake [18]. Furthermore, differences in the alternative splicing of TPO in the breast as compared to the thyroid have been described [25]; therefore, this might also result in different TPO epitopes being targeted.
TPOAb prevalence in our cohort, similar to our a priori predicted value, reflects TPOAb prevalence among women of the general population [26, 30], increasing with age [26, 31]. It remains possible that TPOAb+ rates are higher in the BC population, as our study was not designed to compare TPOAb prevalence among BC patients and the general population.
The principal limitations of the present study are the lack of clinical history for thyroid diseases or medications and that, similarly to previous studies [15, 19], blood was mainly collected during/after adjuvant BC therapy. The first limitation might influence the prognostic role of thyroid function, but marginally that of TPOAb, since they should exert an effect when either pre-existing or appearing at a later time [32]; however, the evidence that thyroid function influences BC outcome is weak [6]. The finding of more cases of hyper- (6.0%) than hypothyroidism (4.9%) may reflect overtreatment with levothyroxine in some individuals.
Regarding BC adjuvant treatments, an increased risk of hypothyroidism after chemotherapy [33, 34] or radiotherapy [35, 36] for BC has been suggested in a few small studies, but this has not been confirmed by others [37]. Tamoxifen can exert a modulation of thyroid function, mainly via an anti-thyroid effect [38, 39], and the stress related to the surgical procedure itself has been suggested to cause immunomodulation [40]. However, no clear large-scale effects of adjuvant treatments for BC, including trastuzumab, on thyroid function and immunity have been described, and our sensitivity analysis in a subgroup of 123 patients in whom blood was collected before BC adjuvant therapy showed no evidence of TPOAb prognostic ability, even if the wide 95% CI suggests a lack of statistical power.
To draw definitive conclusions, a prospective study collecting blood before cancer treatments would be ideal, but this is difficult to realize because of the large patient numbers required, as shown by our a priori power calculation. Furthermore, this study analyzed moderate-to-high-risk early BC only. BC is a heterogeneous disease, with many subtypes characterized by different clinical behaviour and prognosis; it could be possible that TPOAb and/or thyroid function affect the prognosis of certain specific BC subtypes and stages only, therefore, they should all be investigated separately, with much higher total patient numbers required to reach significant and definitive results.
In conclusion, the present study is to our knowledge the largest currently available, investigating the impact of blood TPOAb and thyroid function on BC prognosis, providing a detailed description of the BC population analyzed, and therefore representing a key-work to clarify this debate over decades. We found that TPOAb and thyroid function, both measured with standard assays and after BC diagnosis, appear not to influence substantially the long-term recurrence and mortality of moderate-to-high-risk early BC in the modern era. Major confounding in this conclusion due to BC treatments seems unlikely. Future studies might explore different BC stages and/or specific subtypes, also searching for non-conventional or breast-specific immune responses to particular TPO epitopes, to determine whether aspects of TA other than standard TPOAb and thyroid function may be relevant to BC outcome.
Disclosure Statement
The authors have nothing to disclose.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgements
This study was supported by the Tenovus Cancer Care, Cardiff, UK (2013 Tenovus Innovation Grant number TIG2014-28).
The authors thank all the patients who participated in this study and all the investigators involved in the TACT trial (funded by Cancer Research UK; ICR-CTSU also receives core funding from Cancer Research UK).
Additionally, the authors thank Dr. C. Evans and her team (Department of Medical Biochemistry, School of Medicine, Cardiff University, UK) for running the laboratory assays.
References
- 1.Hardefeldt PJ, Eslick GD, Edirimanne S. Benign thyroid disease is associated with breast cancer: a meta-analysis. Breast Cancer Res Treat. 2012;133:1169–1177. doi: 10.1007/s10549-012-2019-3. [DOI] [PubMed] [Google Scholar]
- 2.Prinzi N, Baldini E, Sorrenti S, De Vito C, Tuccilli C, Catania A, Carbotta S, Mocini R, Coccaro C, Nesca A, Bianchini M, De Antoni E, D'Armiento M, Ulisse S. Prevalence of breast cancer in thyroid diseases: results of a cross-sectional study of 3,921 patients. Breast Cancer Res Treat. 2014;144:683–688. doi: 10.1007/s10549-014-2893-y. [DOI] [PubMed] [Google Scholar]
- 3.Sarlis NJ, Gourgiotis L, Pucino F, Tolis GJ. Lack of association between Hashimoto thyroiditis and breast cancer: a quantitative research synthesis. Hormones (Athens) 2002;1:35–41. doi: 10.14310/horm.2002.1152. [DOI] [PubMed] [Google Scholar]
- 4.Angelousi AG, Anagnostou VK, Stamatakos MK, Georgiopoulos GA, Kontzoglou KC. Mechanisms in endocrinology: primary HT and risk for breast cancer: a systematic review and meta-analysis. Eur J Endocrinol. 2012;166:373–381. doi: 10.1530/EJE-11-0838. [DOI] [PubMed] [Google Scholar]
- 5.Moeller LC, Führer D. Thyroid hormone, thyroid hormone receptors, and cancer: a clinical perspective. Endocr Relat Cancer. 2013;20:R19–R29. doi: 10.1530/ERC-12-0219. [DOI] [PubMed] [Google Scholar]
- 6.Smyth PP. The thyroid and breast cancer. Curr Opin Endocrinol Diabetes Obes. 2016;23:389–393. doi: 10.1097/MED.0000000000000273. [DOI] [PubMed] [Google Scholar]
- 7.Mittra I, Hayward JL. Hypothalamic-pituitary-thyroid axis in breast cancer. Lancet. 1974;1:885–889. doi: 10.1016/s0140-6736(74)90344-4. [DOI] [PubMed] [Google Scholar]
- 8.Kuijpens JL, Nyklictek I, Louwman MW, Weetman TA, Pop VJ, Coebergh JW. Hypothyroidism might be related to breast cancer in post-menopausal women. Thyroid. 2005;15:1253–1259. doi: 10.1089/thy.2005.15.1253. [DOI] [PubMed] [Google Scholar]
- 9.Cristofanilli M, Yamamura Y, Kau SW, Bevers T, Strom S, Patangan M, Hsu L, Krishnamurthy S, Theriault RL, Hortobagyi GN. Thyroid hormone and breast carcinoma. Primary hypothyroidism is associated with a reduced incidence of primary breast carcinoma. Cancer. 2005;103:1122–1128. doi: 10.1002/cncr.20881. [DOI] [PubMed] [Google Scholar]
- 10.Tosovic A, Becker C, Bondeson AG, Bondeson L, Ericsson UB, Malm J, Manjer J. Prospectively measured thyroid hormones and thyroid peroxidase antibodies in relation to breast cancer risk. Int J Cancer. 2012;131:2126–2133. doi: 10.1002/ijc.27470. [DOI] [PubMed] [Google Scholar]
- 11.Sogaard M, Farkas DK, Ehrenstein V, Jorgensen JO, Dekkers OM, Sorensen HT. Hypothyroidism and hyperthyroidism and breast cancer risk: a nationwide cohort study. Eur J Endocrinol. 2016;174:409–414. doi: 10.1530/EJE-15-0989. [DOI] [PubMed] [Google Scholar]
- 12.Hedley AJ, Jones SJ, Spiegelhalter DJ, Clements P, Bewsher PD, Simpson JG, Weir RD. Breast cancer in thyroid disease: fact or fallacy? Lancet. 1981;1:131–133. doi: 10.1016/s0140-6736(81)90712-1. [DOI] [PubMed] [Google Scholar]
- 13.Hellevik AI, Asvold BO, Bjoro T, Romundstad PR, Nilsen TI, Vatten LJ. Thyroid function and cancer risk: a prospective population study. Cancer Epidemiol Biomarkers Prev. 2009;18:570–574. doi: 10.1158/1055-9965.EPI-08-0911. [DOI] [PubMed] [Google Scholar]
- 14.Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F, Cecchetti D, Martino E, Pinchera A. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metab. 1996;81:990–994. doi: 10.1210/jcem.81.3.8772562. [DOI] [PubMed] [Google Scholar]
- 15.Smyth PP, Shering SG, Kilbane MT, Murray MJ, McDermott EW, Smith DF, O'Higgins NJ. Serum thyroid peroxidase autoantibodies, thyroid volume, and outcome in breast carcinoma. J Clin Endocrinol Metab. 1998;83:2711–2716. doi: 10.1210/jcem.83.8.5049. [DOI] [PubMed] [Google Scholar]
- 16.Fiore E, Giustarini E, Mammoli C, Fragomeni F, Campani D, Muller I, Pinchera A, Giani C. Favorable predictive value of thyroid autoimmunity in high aggressive breast cancer. J Endocrinol Invest. 2007;30:734–738. doi: 10.1007/BF03350810. [DOI] [PubMed] [Google Scholar]
- 17.Farahati J, Roggenbuck D, Gilman E, Schutte M, Jagminaite E, Seyed Zakavi R, Loning T, Heissen E. Anti-thyroid peroxidase antibodies are associated with the absence of distant metastases in patients with newly diagnosed breast cancer. Clin Chem Lab Med. 2012;50:709–714. doi: 10.1515/CCLM.2011.819. [DOI] [PubMed] [Google Scholar]
- 18.Kim SS, Kim IJ, Kim SJ, Lee JY, Bae YT, Jeon YK, Kim BH, Kim YK. Incidental diffuse thyroid 18F-FDG uptake related to autoimmune thyroiditis may be a favorable prognostic factor in advanced breast cancer. J Nucl Med. 2012;53:1855–1862. doi: 10.2967/jnumed.112.108811. [DOI] [PubMed] [Google Scholar]
- 19.Jiskra J, Barkmanova J, Limanova Z, Lanska V, Smutek D, Potlukova E, Antosova M. Thyroid autoimmunity occurs more frequently in women with breast cancer compared to women with colorectal cancer and controls but it has no impact on relapse-free and overall survival. Oncol Rep. 2007;18:1603–1611. [PubMed] [Google Scholar]
- 20.Cheung KL, Graves CR, Robertson JF. Tumour marker measurements in the diagnosis and monitoring of breast cancer. Cancer Treat Rev. 2000;26:91–102. doi: 10.1053/ctrv.1999.0151. [DOI] [PubMed] [Google Scholar]
- 21.Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786–4800. doi: 10.1158/1078-0432.CCR-14-1190. [DOI] [PubMed] [Google Scholar]
- 22.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat Clin Pract Oncol. 2005;2:416–422. [PubMed] [Google Scholar]
- 23.Ellis P, Barrett-Lee P, Johnson L, Cameron D, Wardley A, O'Reilly S, Verrill M, Smith I, Yarnold J, Coleman R, Earl H, Canney P, Twelves C, Poole C, Bloomfield D, Hopwood P, Johnston S, Dowsett M, Bartlett JM, Ellis I, Peckitt C, Hall E, Bliss JM, TACT Trial Management Group; TACT Trialists Sequential docetaxel as adjuvant chemotherapy for early breast cancer (TACT): an open-label, phase III, randomised controlled trial. Lancet. 2009;373:1681–1692. doi: 10.1016/S0140-6736(09)60740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bliss JM, Ellis P, Kilburn L, Bartlett J, Bloomfield D, Cameron D, Canney P, Coleman RE, Dowsett M, Earl H, Verrill M, Wardley A, Yarnold J, Ahern R, Atkins N, Fletcher M, McLinden M, Barrett-Lee P. Mature analysis of UK Taxotere as Adjuvant Chemotherapy (TACT) trial (CRUK 01/001); effects of treatment and characterisation of patterns of breast cancer relapse. Cancer Res Suppl. 2012;72 Abstract P1-13-03. [Google Scholar]
- 25.Muller I, Giani C, Zhang L, Grennan-Jones FA, Fiore E, Belardi V, Rosellini V, Funel N, Campani D, Giustarini E, Lewis MD, Bakhsh AD, Roncella M, Ghilli M, Vitti P, Dayan CM, Ludgate ME. Does thyroid peroxidase provide an antigenic link between thyroid autoimmunity and breast cancer? Int J Cancer. 2014;134:1706–1714. doi: 10.1002/ijc.28493. [DOI] [PubMed] [Google Scholar]
- 26.Vanderpump MP, Tunbridge WM, French JM, et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 1995;43:55–68. doi: 10.1111/j.1365-2265.1995.tb01894.x. [DOI] [PubMed] [Google Scholar]
- 27.Bulow Pedersen I, Knudsen N, Carle A, Vejbjerg P, Jorgensen T, Perrild H, Ovesen L, Banke Rasmussen L, Laurberg P. A cautious iodization program bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin Endocrinol (Oxf) 2011;75:120–126. doi: 10.1111/j.1365-2265.2011.04008.x. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, Clark GM, Ruby SG, O'Malley F, Simpson JF, Connolly JL, Hayes DF, Edge SB, Lichter A, Schnitt SJ. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 29.Brandt J, Garne JP, Tengrup I, Manjer J. Age at diagnosis in relation to survival following breast cancer: a cohort study. World J Surg Oncol. 2015;13:33. doi: 10.1186/s12957-014-0429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87:489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 31.Tunbridge WM, Brewis M, French JM, Appleton D, Bird T, Clark F, Evered DC, Evans JG, Hall R, Smith P, Stephenson J, Young E. Natural history of autoimmune thyroiditis. Br Med J (Clin Res Ed) 1981;282:258–262. doi: 10.1136/bmj.282.6260.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franzke A, Peest D, Probst-Kepper M, Buer J, Kirchner GI, Brabant G, Kirchner H, Ganser A, Atzpodien J. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J Clin Oncol. 1999;17:529–533. doi: 10.1200/JCO.1999.17.2.529. [DOI] [PubMed] [Google Scholar]
- 33.Kumar N, Allen KA, Riccardi D, Bercu BB, Cantor A, Minton S, Balducci L, Jacobsen PB. Fatigue, weight gain, lethargy and amenorrhea in breast cancer patients on chemotherapy: is subclinical hypothyroidism the culprit? Breast Cancer Res Treat. 2004;83:149–159. doi: 10.1023/B:BREA.0000010708.99455.e1. [DOI] [PubMed] [Google Scholar]
- 34.de Groot S, Janssen LGM, Charehbili A, Dijkgraaf EM, Smit VTHBM, Kessels LW, van Bochove A, van Laarhoven HWM, Meershoek-Klein Kranenbarg E, van Leeuwen-Stok AE, van de Velde CJH, Putter H, Nortier JWR, van der Hoeven JJM, Pijl H, Kroep JR. Thyroid function alters during neoadjuvant chemotherapy in breast cancer patients: results from the NEOZOTAC trial (BOOG 2010-01). Breast Cancer Res Treat. 2015;149:461–466. doi: 10.1007/s10549-014-3256-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bruning P, Bonfrer J, De Jong-Bakker M, Nooyen W, Burgers M. Primary hypothyroidism in breast cancer patients with irradiated supraclavicular lymph nodes. Br J Cancer. 1985;51:659–663. doi: 10.1038/bjc.1985.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cutuli B, Quentin P, Rodier JF, Barakat P, Grob JC. Severe hypothyroidism after chemotherapy and locoregional irradiation for breast cancer. Radiother Oncol. 2000;57:103–105. doi: 10.1016/s0167-8140(00)00183-3. [DOI] [PubMed] [Google Scholar]
- 37.Smith GL, Smith BD, Giordano SH, Shih YC, Woodward WA, Strom EA, Perkins GH, Tereffe W, Yu TK, Buchholz TA. Risk of hypothyroidism in older breast cancer patients treated with radiation. Cancer. 2008;112:1371–1379. doi: 10.1002/cncr.23307. [DOI] [PubMed] [Google Scholar]
- 38.Anker GB, Lonning PE, Aakvaag A, Lien EA. Thyroid function in postmenopausal breast cancer patients treated with tamoxifen. Scand J Clin Lab Invest. 1998;58:103–107. doi: 10.1080/00365519850186670. [DOI] [PubMed] [Google Scholar]
- 39.Zidan J, Rubenstein W. Effect of adjuvant tamoxifen therapy on thyroid function in postmenopausal women with breast cancer. Oncology. 1999;56:43–45. doi: 10.1159/000011928. [DOI] [PubMed] [Google Scholar]
- 40.Boomsma MF, Garssen B, Slot E, Berbee M, Berkhof J, Meezenbroek Ede J, Slieker W, Visser A, Meijer S, Beelen RH. Breast cancer surgery-induced immunomodulation. J Surg Oncol. 2010;102:640–648. doi: 10.1002/jso.21662. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Supplementary data