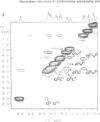
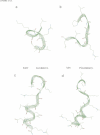
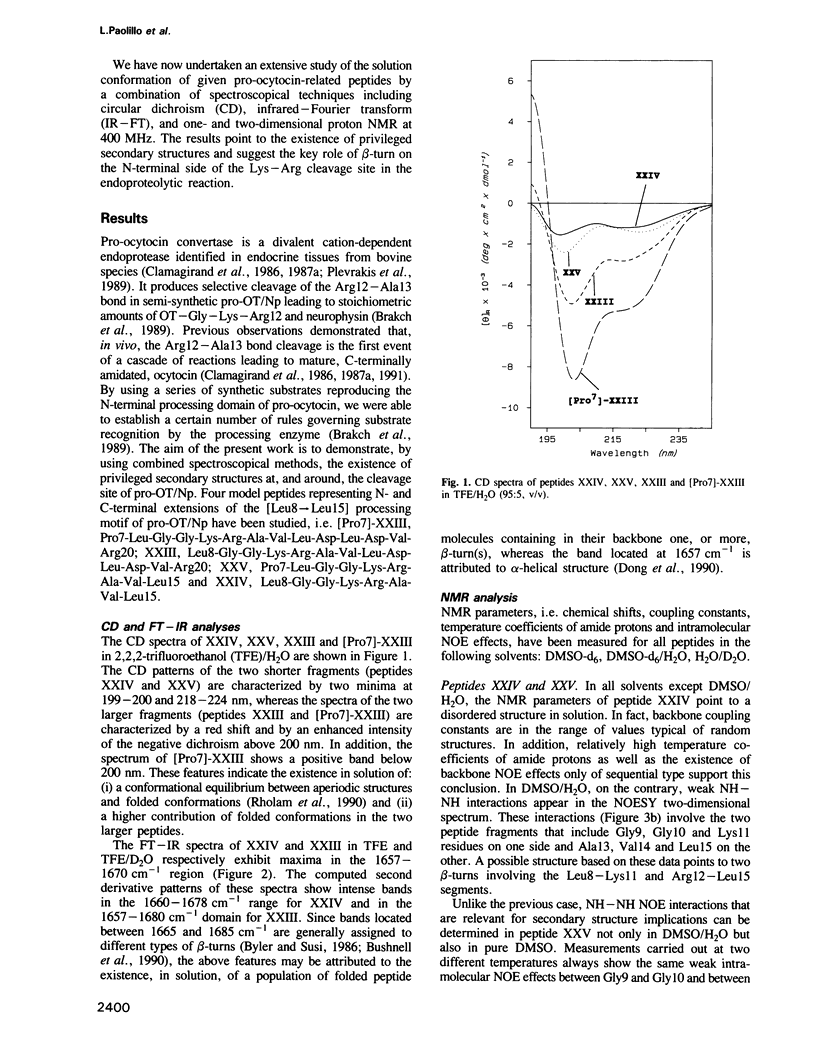
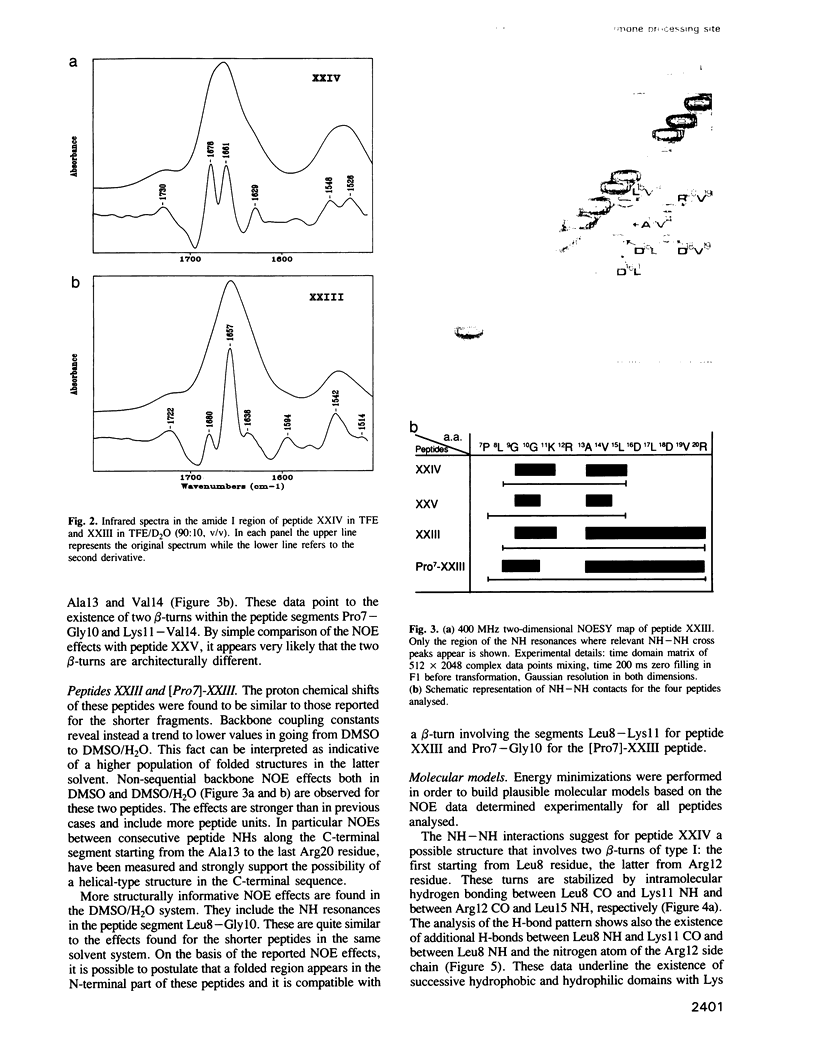
Abstract
Bioactivation of pro-proteins by limited proteolysis is a general mechanism in the biosynthesis of hormones, receptors and viral protein precursors. This proceeds by cleavage of peptide bonds at the level of single or pairs of basic residues in the proforms. Examination of a number of cleavage loci in various precursors failed to reveal any consensus primary sequence around the dibasic cleavage sites. Thus it has been proposed, on the basis of secondary structure predictions [Rholam, M., Nicolas, P. and Cohen, P. (1986) FEBS Lett., 207, 1-6], that those basic residues which operate as signal loci for the proteolytic enzyme machinery are situated in, or next to, privileged precursor regions most often constituted by flexible and exposed motifs, e.g. beta-turns and/or loops. Peptides reproducing the N-terminal processing domain of the hormone precursor, pro-ocytocin-neurophysin, were examined by a combination of spectroscopical techniques including circular dichroism, infrared Fourier transform and one- and two-dimensional proton NMR. The results indicate that: (i) the region situated on the N terminus of the Lys-Arg doublet is organized as a beta-turn in solution; (ii) the sequential organization of the residues participating in the beta-turn determines the privileged relative orientation of the basic amino acid side chains and the subtype of turn; (iii) the peptide segment situated on the C-terminal side of the dibasic, corresponding to the N-terminal octapeptide of neurophysin, is organized as an alpha-helix.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aumelas A., Audousset-Puech M. P., Heitz A., Bataille D., Martinez J. 1H n.m.r. conformational studies on the C-terminal octapeptide of oxyntomodulin, a beta-turn locked by a salt bridge. Int J Pept Protein Res. 1989 Oct;34(4):268–276. doi: 10.1111/j.1399-3011.1989.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Bek E., Berry R. Prohormonal cleavage sites are associated with omega loops. Biochemistry. 1990 Jan 9;29(1):178–183. doi: 10.1021/bi00453a024. [DOI] [PubMed] [Google Scholar]
- Benatan E., Rose J., Breslow E., Wang B. C. Crystals of a bovine neurophysin II tripeptide complex. J Mol Biol. 1991 Nov 5;222(1):23–25. doi: 10.1016/0022-2836(91)90733-m. [DOI] [PubMed] [Google Scholar]
- Bourdais J., Cohen P. Sur la maturation protéolytique post-traductionnelle de la prosomatostatine. Une approche cellulaire et moléculaire. Ann Endocrinol (Paris) 1991;52(5):339–347. [PubMed] [Google Scholar]
- Bourdais J., Pierotti A. R., Boussetta H., Barre N., Devilliers G., Cohen P. Isolation and functional properties of an arginine-selective endoprotease from rat intestinal mucosa. A putative prosomatostatin convertase. J Biol Chem. 1991 Dec 5;266(34):23386–23391. [PubMed] [Google Scholar]
- Brakch N., Boussetta H., Rholam M., Cohen P. Processing endoprotease recognizes a structural feature at the cleavage site of peptide prohormones. The pro-ocytocin/neurophysin model. J Biol Chem. 1989 Sep 25;264(27):15912–15916. [PubMed] [Google Scholar]
- Brakch N., Rholam M., Nault C., Boileau G., Cohen P. Differential processing of hormone precursor. Independent production of somatostatins 14 and 28 in transfected neuroblastoma 2A cells. FEBS Lett. 1991 May 6;282(2):363–367. doi: 10.1016/0014-5793(91)80514-4. [DOI] [PubMed] [Google Scholar]
- Bushnell G. W., Louie G. V., Brayer G. D. High-resolution three-dimensional structure of horse heart cytochrome c. J Mol Biol. 1990 Jul 20;214(2):585–595. doi: 10.1016/0022-2836(90)90200-6. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Camier M., Benveniste D., Barré N., Brakch N., Cohen P. Synthesis and processing of pro-ocytocin in bovine corpus luteum and granulosa cells. Mol Cell Endocrinol. 1991 May;77(1-3):141–147. doi: 10.1016/0303-7207(91)90068-4. [DOI] [PubMed] [Google Scholar]
- Clamagirand C., Camier M., Boussetta H., Fahy C., Morel A., Nicolas P., Cohen P. An endopeptidase associated with bovine neurohypophysis secretory granules cleaves pro-ocytocin/neurophysin peptide at paired basic residues. Biochem Biophys Res Commun. 1986 Feb 13;134(3):1190–1196. doi: 10.1016/0006-291x(86)90376-1. [DOI] [PubMed] [Google Scholar]
- Clamagirand C., Camier M., Fahy C., Clavreul C., Créminon C., Cohen P. C-terminally extended ocytocin and pro-ocytocin: neurophysin peptide converting enzyme in bovine corpus luteum. Biochem Biophys Res Commun. 1987 Mar 13;143(2):789–796. doi: 10.1016/0006-291x(87)91423-9. [DOI] [PubMed] [Google Scholar]
- Clamagirand C., Creminon C., Fahy C., Boussetta H., Cohen P. Partial purification and functional properties of an endoprotease from bovine neurosecretory granules cleaving proocytocin/neurophysin peptides at the basic amino acid doublet. Biochemistry. 1987 Sep 22;26(19):6018–6023. doi: 10.1021/bi00393a011. [DOI] [PubMed] [Google Scholar]
- Créminon C., Rholam M., Boussetta H., Marrakchi N., Cohen P. Synthetic peptide substrates as models to study a pro-ocytocin/neurophysin converting enzyme. J Chromatogr. 1988 May 25;440:439–448. doi: 10.1016/s0021-9673(00)94547-3. [DOI] [PubMed] [Google Scholar]
- Darby N. J., Smyth D. G. Endopeptidases and prohormone processing. Biosci Rep. 1990 Feb;10(1):1–13. doi: 10.1007/BF01116845. [DOI] [PubMed] [Google Scholar]
- Devi L. Consensus sequence for processing of peptide precursors at monobasic sites. FEBS Lett. 1991 Mar 25;280(2):189–194. doi: 10.1016/0014-5793(91)80290-j. [DOI] [PubMed] [Google Scholar]
- Dong A., Huang P., Caughey W. S. Protein secondary structures in water from second-derivative amide I infrared spectra. Biochemistry. 1990 Apr 3;29(13):3303–3308. doi: 10.1021/bi00465a022. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Brake A., Thorner J. Yeast prohormone processing enzyme (KEX2 gene product) is a Ca2+-dependent serine protease. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1434–1438. doi: 10.1073/pnas.86.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluschankof P., Gomez S., Morel A., Cohen P. Enzymes that process somatostatin precursors. A novel endoprotease that cleaves before the arginine-lysine doublet is involved in somatostatin-28 convertase activity of rat brain cortex. J Biol Chem. 1987 Jul 15;262(20):9615–9620. [PubMed] [Google Scholar]
- Gomez S., Boileau G., Zollinger L., Nault C., Rholam M., Cohen P. Site-specific mutagenesis identifies amino acid residues critical in prohormone processing. EMBO J. 1989 Oct;8(10):2911–2916. doi: 10.1002/j.1460-2075.1989.tb08440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez S., Gluschankof P., Lepage A., Cohen P. Relationship between endo- and exopeptidases in a processing enzyme system: activation of an endoprotease by the aminopeptidase B-like activity in somatostatin-28 convertase. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5468–5472. doi: 10.1073/pnas.85.15.5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuks P. F., Créminon C., Leseney A. M., Bourdais J., Morel A., Cohen P. Xenopus laevis skin Arg-Xaa-Val-Arg-Gly-endoprotease. A highly specific protease cleaving after a single arginine of a consensus sequence of peptide hormone precursors. J Biol Chem. 1989 Sep 5;264(25):14609–14612. [PubMed] [Google Scholar]
- Maret G. E., Fauchère J. L. Purification of an endopeptidase from bovine adrenal medulla granules which cleaves in vitro at paired but not at single basic residues. Anal Biochem. 1988 Jul;172(1):248–258. doi: 10.1016/0003-2697(88)90439-3. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Matsuo H. A novel protease from yeast with specificity towards paired basic residues. Nature. 1984 Jun 7;309(5968):558–560. doi: 10.1038/309558a0. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Delfour A., Boussetta H., Morel A., Rholam M., Cohen P. Solid phase synthesis of somatostatin-28 II. A new biologically active octacosapeptide from anglerfish pancreatic islets. Biochem Biophys Res Commun. 1986 Oct 30;140(2):565–573. doi: 10.1016/0006-291x(86)90769-2. [DOI] [PubMed] [Google Scholar]
- Plevrakis I., Clamagirand C., Créminon C., Brakch N., Rholam M., Cohen P. Proocytocin/neurophysin convertase from bovine neurohypophysis and corpus luteum secretory granules: complete purification, structure-function relationships, and competitive inhibitor. Biochemistry. 1989 Mar 21;28(6):2705–2710. doi: 10.1021/bi00432a051. [DOI] [PubMed] [Google Scholar]
- Rholam M., Cohen P., Brakch N., Paolillo L., Scatturin A., Di Bello C. Evidence for beta-turn structure in model peptides reproducing pro-ocytocin/neurophysin proteolytic processing site. Biochem Biophys Res Commun. 1990 May 16;168(3):1066–1073. doi: 10.1016/0006-291x(90)91138-i. [DOI] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Precursors for peptide hormones share common secondary structures forming features at the proteolytic processing sites. FEBS Lett. 1986 Oct 20;207(1):1–6. doi: 10.1016/0014-5793(86)80002-3. [DOI] [PubMed] [Google Scholar]
- Thomas G., Thorne B. A., Thomas L., Allen R. G., Hruby D. E., Fuller R., Thorner J. Yeast KEX2 endopeptidase correctly cleaves a neuroendocrine prohormone in mammalian cells. Science. 1988 Jul 8;241(4862):226–230. doi: 10.1126/science.3291117. [DOI] [PubMed] [Google Scholar]
- Tinker D. A., Krebs E. A., Feltham I. C., Attah-Poku S. K., Ananthanarayanan V. S. Synthetic beta-turn peptides as substrates for a tyrosine protein kinase. J Biol Chem. 1988 Apr 15;263(11):5024–5026. [PubMed] [Google Scholar]
- Wise R. J., Barr P. J., Wong P. A., Kiefer M. C., Brake A. J., Kaufman R. J. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zollinger L., Racine C., Crine P., Boileau G., Germain D., Thomas D. Y., Gossard F. Intracellular proteolytic processing of proopiomelanocortin in heterologous COS-1 cells by the yeast KEX2 endoprotease. Biochem Cell Biol. 1990 Mar;68(3):635–640. doi: 10.1139/o90-090. [DOI] [PubMed] [Google Scholar]