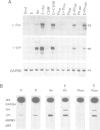
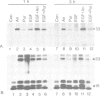
Abstract
Protein synthesis inhibitors strongly augment and prolong the usually transient induction of c-fos and c-jun by growth factors, phorbol esters etc., a phenomenon termed superinduction which is conventionally regarded as a secondary consequence of translational arrest. Our recent demonstration that some inhibitors can act positively as nuclear signalling agonists compromises this view and necessitates a re-evaluation of superinduction. First, we show that labile repressors, widely postulated to act negatively on diverse superinducible genes, are not involved in regulating c-fos and c-jun. Secondly, two components of c-fos and c-jun superinduction, namely the delay in shutting off transcription and stabilization of their mRNAs, arise from translational arrest and are common to all protein synthesis inhibitors. Thirdly, the recently described capacity to act positively as nuclear signalling agonists to stimulate pp33/pp15 phosphorylation is restricted to compounds such as anisomycin and cycloheximide; these, but not emetine or puromycin, will induce c-fos/c-jun on their own. Fourthly, the translational arrest-related components of superinduction are dissociable from the signalling agonist effects at sub-inhibitory concentrations of anisomycin, under which conditions a new type of c-fos/c-jun superinduction with 'spike' kinetics is observed. Finally, we show that in response to EGF plus anisomycin, the nuclear signalling responses are themselves augmented and prolonged in a manner that corresponds to c-fos/c-jun superinduction under these conditions.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Vazquez D. Ribosome changes during translation. J Mol Biol. 1975 Apr 25;93(4):449–463. doi: 10.1016/0022-2836(75)90239-9. [DOI] [PubMed] [Google Scholar]
- Bonnieu A., Rech J., Jeanteur P., Fort P. Requirements for c-fos mRNA down regulation in growth stimulated murine cells. Oncogene. 1989 Jul;4(7):881–888. [PubMed] [Google Scholar]
- Bravo R. Growth factor-responsive genes in fibroblasts. Cell Growth Differ. 1990 Jun;1(6):305–309. [PubMed] [Google Scholar]
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Dangy J. P., Mechta F., Hirai S., Yaniv M., Samarut J., Lassailly A., Brun G. Overexpression of avian or mouse c-jun in primary chick embryo fibroblasts confers a partially transformed phenotype. Oncogene. 1990 Oct;5(10):1541–1547. [PubMed] [Google Scholar]
- Chen T. A., Allfrey V. G. Rapid and reversible changes in nucleosome structure accompany the activation, repression, and superinduction of murine fibroblast protooncogenes c-fos and c-myc. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5252–5256. doi: 10.1073/pnas.84.15.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. A., Sterner R., Cozzolino A., Allfrey V. G. Reversible and irreversible changes in nucleosome structure along the c-fos and c-myc oncogenes following inhibition of transcription. J Mol Biol. 1990 Apr 5;212(3):481–493. doi: 10.1016/0022-2836(90)90327-I. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Tourkine N., Belin D., Vassalli P., Jeanteur P., Blanchard J. M. c-fos gene transcription in murine macrophages is modulated by a calcium-dependent block to elongation in intron 1. Mol Cell Biol. 1991 May;11(5):2826–2831. doi: 10.1128/mcb.11.5.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Denhardt D. T. A study of mitochondrial and nuclear transcription with cloned cDNA probes. Changes in the relative abundance of mitochondrial transcripts after stimulation of quiescent mouse fibroblasts. Exp Cell Res. 1985 Mar;157(1):127–143. doi: 10.1016/0014-4827(85)90157-0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fort P., Rech J., Vie A., Piechaczyk M., Bonnieu A., Jeanteur P., Blanchard J. M. Regulation of c-fos gene expression in hamster fibroblasts: initiation and elongation of transcription and mRNA degradation. Nucleic Acids Res. 1987 Jul 24;15(14):5657–5667. doi: 10.1093/nar/15.14.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hann S. R., King M. W., Bentley D. L., Anderson C. W., Eisenman R. N. A non-AUG translational initiation in c-myc exon 1 generates an N-terminally distinct protein whose synthesis is disrupted in Burkitt's lymphomas. Cell. 1988 Jan 29;52(2):185–195. doi: 10.1016/0092-8674(88)90507-7. [DOI] [PubMed] [Google Scholar]
- Laird-Offringa I. A., de Wit C. L., Elfferich P., van der Eb A. J. Poly(A) tail shortening is the translation-dependent step in c-myc mRNA degradation. Mol Cell Biol. 1990 Dec;10(12):6132–6140. doi: 10.1128/mcb.10.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Tourkine N., Jeanteur P., Blanchard J. M. Demonstration in living cells of an intragenic negative regulatory element within the rodent c-fos gene. Cell. 1990 May 4;61(3):485–496. doi: 10.1016/0092-8674(90)90530-r. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Edwards D. R. Signalling and superinduction. Nature. 1991 Feb 28;349(6312):747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Heath J. K., Leichtfried F. E., Cumming D. V., Hirst E. M., Foulkes J. G. Rapid appearance of novel phosphoproteins in the nuclei of mitogen-stimulated fibroblasts. Oncogene. 1988 Mar;2(3):249–257. [PubMed] [Google Scholar]
- Mahadevan L. C., Targett K., Heath J. K. 2-Aminopurine abolishes epidermal growth factor-stimulated phosphorylation of complexed and chromatin-associated forms of a 33 kDa phosphoprotein. Oncogene. 1989 Jun;4(6):699–706. [PubMed] [Google Scholar]
- Mahadevan L. C., Willis A. C., Barratt M. J. Rapid histone H3 phosphorylation in response to growth factors, phorbol esters, okadaic acid, and protein synthesis inhibitors. Cell. 1991 May 31;65(5):775–783. doi: 10.1016/0092-8674(91)90385-c. [DOI] [PubMed] [Google Scholar]
- Mahadevan L. C., Wills A. J., Hirst E. A., Rathjen P. D., Heath J. K. 2-Aminopurine abolishes EGF- and TPA-stimulated pp33 phosphorylation and c-fos induction without affecting the activation of protein kinase C. Oncogene. 1990 Mar;5(3):327–335. [PubMed] [Google Scholar]
- Mechti N., Piechaczyk M., Blanchard J. M., Jeanteur P., Lebleu B. Sequence requirements for premature transcription arrest within the first intron of the mouse c-fos gene. Mol Cell Biol. 1991 May;11(5):2832–2841. doi: 10.1128/mcb.11.5.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Morello D., Lavenu A., Babinet C. Differential regulation and expression of jun, c-fos and c-myc proto-oncogenes during mouse liver regeneration and after inhibition of protein synthesis. Oncogene. 1990 Oct;5(10):1511–1519. [PubMed] [Google Scholar]
- Pontecorvi A., Tata J. R., Phyillaier M., Robbins J. Selective degradation of mRNA: the role of short-lived proteins in differential destabilization of insulin-induced creatine phosphokinase and myosin heavy chain mRNAs during rat skeletal muscle L6 cell differentiation. EMBO J. 1988 May;7(5):1489–1495. doi: 10.1002/j.1460-2075.1988.tb02967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quantin B., Breathnach R. Epidermal growth factor stimulates transcription of the c-jun proto-oncogene in rat fibroblasts. Nature. 1988 Aug 11;334(6182):538–539. doi: 10.1038/334538a0. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Ryseck R. P., Hirai S. I., Yaniv M., Bravo R. Transcriptional activation of c-jun during the G0/G1 transition in mouse fibroblasts. Nature. 1988 Aug 11;334(6182):535–537. doi: 10.1038/334535a0. [DOI] [PubMed] [Google Scholar]
- Rüther U., Komitowski D., Schubert F. R., Wagner E. F. c-fos expression induces bone tumors in transgenic mice. Oncogene. 1989 Jul;4(7):861–865. [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Verma I. M. Modulation of c-fos gene transcription by negative and positive cellular factors. Nature. 1987 Apr 2;326(6112):507–510. doi: 10.1038/326507a0. [DOI] [PubMed] [Google Scholar]
- Schuh A. C., Keating S. J., Monteclaro F. S., Vogt P. K., Breitman M. L. Obligatory wounding requirement for tumorigenesis in v-jun transgenic mice. Nature. 1990 Aug 23;346(6286):756–760. doi: 10.1038/346756a0. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Büscher M., Angel P., Rahmsdorf H. J., Ponta H., Hattori K., Chiu R., Karin M., Herrlich P. The Fos and Jun/AP-1 proteins are involved in the downregulation of Fos transcription. Oncogene. 1989 May;4(5):629–636. [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Shyu A. B., Belasco J. G., Greenberg M. E. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991 Feb;5(2):221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Shyu A. B., Greenberg M. E., Belasco J. G. The c-fos transcript is targeted for rapid decay by two distinct mRNA degradation pathways. Genes Dev. 1989 Jan;3(1):60–72. doi: 10.1101/gad.3.1.60. [DOI] [PubMed] [Google Scholar]
- Subramaniam M., Schmidt L. J., Crutchfield C. E., 3rd, Getz M. J. Negative regulation of serum-responsive enhancer elements. Nature. 1989 Jul 6;340(6228):64–66. doi: 10.1038/340064a0. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Wall R., Briskin M., Carter C., Govan H., Taylor A., Kincade P. A labile inhibitor blocks immunoglobulin kappa-light-chain-gene transcription in a pre-B leukemic cell line. Proc Natl Acad Sci U S A. 1986 Jan;83(2):295–298. doi: 10.1073/pnas.83.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson T., Treisman R. Removal of poly(A) and consequent degradation of c-fos mRNA facilitated by 3' AU-rich sequences. Nature. 1988 Nov 24;336(6197):396–399. doi: 10.1038/336396a0. [DOI] [PubMed] [Google Scholar]
- Wisdom R., Lee W. The protein-coding region of c-myc mRNA contains a sequence that specifies rapid mRNA turnover and induction by protein synthesis inhibitors. Genes Dev. 1991 Feb;5(2):232–243. doi: 10.1101/gad.5.2.232. [DOI] [PubMed] [Google Scholar]