Summary
Background
Total domestic and international funding for malaria is inadequate to achieve WHO global targets in burden reduction by 2030. We describe the trends of investments in malaria-related research in sub-Saharan Africa and compare investment with national disease burden to identify areas of funding strength and potentially neglected populations. We also considered funding for malaria control.
Methods
Research funding data related to malaria for 1997–2013 were sourced from existing datasets, from 13 major public and philanthropic global health funders, and from funding databases. Investments (reported in US$) were considered by geographical area and compared with data on parasite prevalence and populations at risk in sub-Saharan Africa. 45 sub-Saharan African countries were ranked by amount of research funding received.
Findings
We found 333 research awards totalling US$814·4 million. Public health research covered $308·1 million (37·8%) and clinical trials covered $275·2 million (33·8%). Tanzania ($107·8 million [13·2%]), Uganda ($97·9 million [12·0%]), and Kenya ($92·9 million [11·4%]) received the highest sum of research investment and the most research awards. Malawi, Tanzania, and Uganda remained highly ranked after adjusting for national gross domestic product. Countries with a reasonably high malaria burden that received little research investment or funding for malaria control included Central African Republic (ranked 40th) and Sierra Leone (ranked 35th). Congo (Brazzaville) and Guinea had reasonably high malaria mortality, yet Congo (Brazzaville) ranked 38th and Guinea ranked 25th, thus receiving little investment.
Interpretation
Some countries receive reasonably large investments in malaria-related research (Tanzania, Kenya, Uganda), whereas others receive little or no investments (Sierra Leone, Central African Republic). Research investments are typically highest in countries where funding for malaria control is also high. Investment strategies should consider more equitable research and operational investments across countries to include currently neglected and susceptible populations.
Funding
Royal Society of Tropical Medicine and Hygiene and Bill & Melinda Gates Foundation.
Introduction
The current total domestic and international investments in malaria are considered grossly inadequate to meet the annual global target for investment of US$6 billion.1 The 2015 Global Burden of Disease study estimated that there were 731 000 malaria-associated deaths (a decline of about 37% since 2005, along with a decline in age-standardised mortality of 43%)2 and 296 million positive cases (a decline of about 30% since 2005) in 2015,3 with a high prevalence in sub-Saharan Africa.4 To address this burden, malaria is the focus of large and well funded programmes from influential global health actors such as The Global Fund5 and the Bill & Melinda Gates Foundation, both of which have targeted malaria for elimination.6
An estimated US$8·9 billion was disbursed globally for malaria control and elimination programmes between 2006 and 2010, with most of this funding targeted to Africa.7 As well as provision of finance from The Global Fund, substantial investment came from other actors, such as the World Bank and the President's Malaria Initiative. As investment specifically focused on malaria control increases, the burden of malaria decreases,8 with interventions estimated to have averted 663 million clinical cases of malaria globally since 2000. Insecticide-treated nets, the most widespread intervention, were responsible for 68% of the averted cases.9 However, a substantial burden still remains, requiring efficient allocation of scarce financial resources to address gaps in implementation and research.
The ten largest global health research funders, which include the US National Institutes of Health, the European Commission, the Wellcome Trust, and the Bill & Melinda Gates Foundation, collectively invest about $37·1 billion into research each year,10 and malaria is a research priority or part of a wider focus (eg, global health) for these organisations. Investments cover the full pipeline of research, from preclinical science, to clinical trial phases and product development, and on to implementation and operational research. However, few multi-funder analyses of the focus of these awards exist.
Research in context.
Evidence before this study
In July and August, 2016, we searched PubMed and the grey literature (via internet search engines and stakeholder websites, such as WHO) using the search terms “research investments”, “research funding”, “malaria investments”, “malaria funding”, and “malaria Africa” for articles written only in English. One author (MGH) also searched a personal Mendeley literature database that is built for the purpose of informing the Research Investments in Global Health (ResIn) study. Previous investment analyses include the study by Pigott and colleagues, ResIn publications, and the Policy Cures annual reports on product development research in infectious diseases.
Added value of this study
To the best of our knowledge, this is the first study to systematically describe the geography of public and philanthropic research funding for malaria in sub-Saharan Africa. The study combined and then re-analysed open data sources from numerous key global health investors, and categorised the awards via the classification system developed by the ResIn study. This strategy allowed us to provide a comprehensive overview of the investment landscape, with actionable data that can help inform equitable decisions around resource allocation.
Implications of all the available evidence
The findings show that much of the available resources are directed towards key global health hubs in sub-Saharan Africa—for example, Tanzania, Uganda, and Kenya. However, several countries, such as Chad and Central African Republic, receive little or no research and operational funding despite having high malaria-associated burdens and mortality. These countries have neglected populations, and the global health community should reconsider strategies around resource allocation to reduce inequality and improve equity.
The Research Investments in Global Health study (ResIn) has analysed funding trends in infectious disease research awarded to UK institutions11, 12 and has identified Africa as being the focus of much of the UK global health research portfolio.13 Here, we aimed to systematically analyse investments in research related to malaria from leading international donors, in particular when the focus of the project was in sub-Saharan Africa. We also aimed to locate the site of the research at the national level, describe the geography of investment trends, and compare investments with the local prevalence of malaria caused by Plasmodium falciparum and malaria burden, as measured by the sizes of at-risk populations.
Methods
Search strategy and selection criteria
The process of collating and categorising infection-related research awards to UK institutions for this systematic analysis has been described in detail elsewhere.11, 12, 13 Briefly, we extracted award data for studies of infectious diseases from funder's websites or requested award data directly from the funder. We also searched funding databases, such as the National Research Register, owned by the UK Department of Health, and clinicaltrials.gov, for infection-related awards. Each award was individually scrutinised and categorised under a number of diseases, disease areas (eg, global health, antimicrobial resistance), and by type of science (eg, phase 1–3 clinical trials, public health research). Award types included project grants, programme grants, fellowships, and pump-priming (development grants) or pilot projects that had a clear research component to the project.
We focused specifically on awards relating to malaria research in sub-Saharan Africa. We used the UK portfolio already collated by the ResIn study12 and further considered 28 leading funders of global health research (see appendix 1 for the full list of funders that were assessed, including those that did not have data that met the inclusion criteria). We used existing knowledge and data from the ResIn study, author knowledge, and healthresearchfunders.org to identify key funders who were likely to have provided research investment for malaria. Much of the newly collected data were sourced from the Dimensions for Funders database, UberResearch. When searching online databases for awards related to malaria, we used the search terms “malaria”, “plasmodium”, and “anopheles”. From the retrieved awards, we reviewed the title and abstract to ascertain whether the project had a focus in the 45 sub-Saharan African nations for which data were available. When information about the project was insufficient, we searched databases (including the UK Research Councils' Gateway to Research database, PubMed, and Europe PMC) for publications related to the original award and for information about the award on institutional or study-specific websites. We included awards for which the commitment to fund was dated between 1997 and 2013 (inclusive). The UK Department for International Development and the Bill & Melinda Gates Foundation fund both research and implementation activity; here, we only included the research projects. Using the malaria awards from the preexisting ResIn UK dataset as an example, we included awards of greater than $150 000 (the 10th percentile in the UK dataset). Awards solely related to preclinical science were excluded because they were unlikely to have a specific geographical focus; all other types of science along the research pipeline (from phase 1 studies through to public health and implementation research) were included.
Data analysis
We categorised the included awards as phase 1–3 trials, intervention and product development (including pharmacovigilance), public health research, or cross-disciplinary research. Public health research included epidemiology, statistics and modelling, and implementation and operational research. Cross-disciplinary research was defined as an award that clearly encompassed two types of science categories (ie, an award covering a phase 3 trial and pharmacovigilance research would be classified as cross-disciplinary). Awards were also categorised by tool or product, specifically diagnostics, vaccines, and therapeutics. Inter-author checks on categorisation yielded a fixed-marginal κ score of 0·88 (indicating a reasonably high level of agreement between authors). All awards were adjusted for 2013 inflation and, when required, were converted to US$ by use of the average exchange rate in the year of the award. South Sudan and north Sudan were unified as Sudan for most of the time period under consideration and were therefore counted together (no investments were specifically for South Sudan after their separation).
Some included awards focused on malaria in multiple sites in multiple countries. Because it was not possible to establish the exact proportion of each award that was invested in or for each site, we used a pragmatic approach to divide the total award size evenly by the number of sites of focus as an approximate measure of likely allocation of resources. For example, for an award of US$1·5 million for malaria research in two sites in Kenya and one site in Tanzania, $1 million would be allocated to Kenya and $500 000 to Tanzania. Similarly, when an award had a clear focus on both malaria and other disease(s), for simplicity of analysis, the total investment was divided by the number of diseases involved. For example, a $1 million award for malaria and tuberculosis in Kenya would have resulted in $500 000 being allocated to malaria research, and the $500 000 assumed to be for tuberculosis research would have been excluded. Awards were not split when the focus was explicitly consideration of malaria as a coinfection with other diseases (the assumption being that all of the funding was for malaria-related burdens).
National-level disbursements of funding for malaria control for 2006–11 were included. Data up to 2010 have been published previously,7 and an author (MGH) provided one further year (2011) of unpublished disaggregated information about funding for malaria control (appendix 2). Donors included were national governments, UNICEF, the Global Fund, President's Malaria Initiative, the World Bank, and the Development Assistance Committee. Levels of investment in funding for malaria control and research datasets were compared and ranked at the national level. World Bank data were used to compare 2013 national gross domestic product (GDP).14
Data for local prevalence of malaria caused by P falciparum were sourced from the Malaria Atlas Project,15 which had mapped variables including parasite prevalence and incidence of malaria between 2000 and 2015. We used age-standardised, gridded P falciparum parasite prevalence data for ages 2–10 years (PfPR2–10) at 5 km × 5 km spatial resolution. PfPR2–10 data were available for all included sub-Saharan African countries, with the exceptions of São Tomé and Príncipe and Comoros. The gridded PfPR2–10 data were summarised at the country level through integration with WorldPop gridded population data15 to construct population-weighted PfPR values for each country. All funding data for each country were combined, and maps were produced at the country level to display the total research investment per country between 1997 and 2013.
Role of the funding source
The funders had no role in the study design, data interpretation, or writing of the report. MGH had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
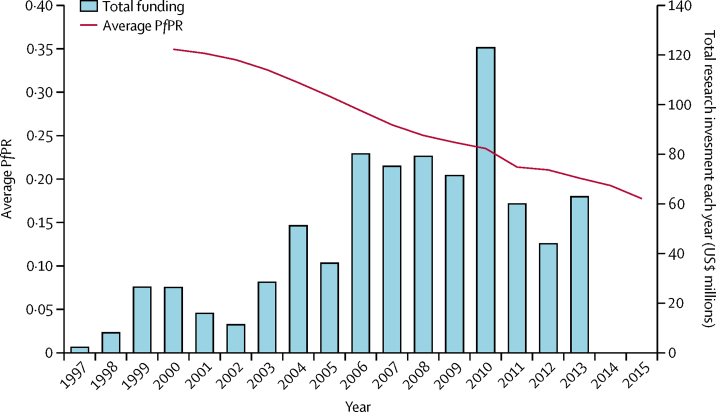
By research investment, 333 awards met the inclusion criteria, with a total inflation-adjusted amount for research funding of US$814·4 million (table 1). The mean award size was $2 445 591 (SD 4 054 664) and the median award size was $941 808 (IQR 419 529–$2 605 340). These findings show significant skew in the data, with a small number of very large awards driving the mean higher than the median. The total annual investment for malaria in sub-Saharan Africa varied substantially in the period of 1997–2015, although with a broadly increasing temporal trend alongside observed decreases in disease burden (figure 1). 317 (95%) research awards solely focused on malaria and 16 (5%) awards considered malaria alongside other infections. A total of 285 (86%) awards had just one country of focus.
Table 1.
Funding for malaria research in sub-Saharan Africa for 1997–2013 by product area and type of science
| Number of awards | Proportion of total awards (%) | Total investment (US$) | Proportion of total investment (%) | Mean award size (US$)* | Median award size (US$)† | ||
|---|---|---|---|---|---|---|---|
| Product area | |||||||
| Diagnostics | 29 | 9% | 41 010 386 | 5·0% | 14 14 151 (1 670 125) | 906 950 (409 574–1 430 935) | |
| Vaccines | 18 | 5% | 64 995 864 | 8·0% | 3 610 881 (3 394 021) | 2 909 619 (510 504–4 855 510) | |
| Therapeutics | 83 | 25% | 261 205 837 | 32·1% | 3 147 058 (5 731 588) | 991 048 (326 333–3 910 552) | |
| Type of science | |||||||
| Phase 1–3 clinical trials | 80 | 24% | 275 214 430 | 33·8% | 3 440 180 (4 787 761) | 1 317 954 (426 077–4 955 076) | |
| Intervention and product development | 35 | 11% | 87 580 346 | 10·8% | 2 502 296 (4 186 004) | 999 485 (293 800–2 605 340) | |
| Public health | 153 | 46% | 308 076 978 | 37·8% | 2 013 575 (4 053 184) | 666 164 (375 387–2 209 865) | |
| Cross-disciplinary | 65 | 20% | 143 510 172 | 17·6% | 2 207 849 (2 630 770) | 1 071 300 (552 164–2 517 231) | |
| Total | 333 | NA | 814 381 928 | NA | 2 445 591 (4 054 664) | 941 808 (419 529–2 605 340) | |
NA=not applicable.
Data are mean (SD).
Data are median (IQR).
Figure 1.
Sum of annual research investment for malaria in sub-Saharan Africa (1997–2013) and mean national-level PfPR for all countries in sub-Saharan Africa (2000–15)
PfPR=Plasmodium falciparum parasite prevalence.
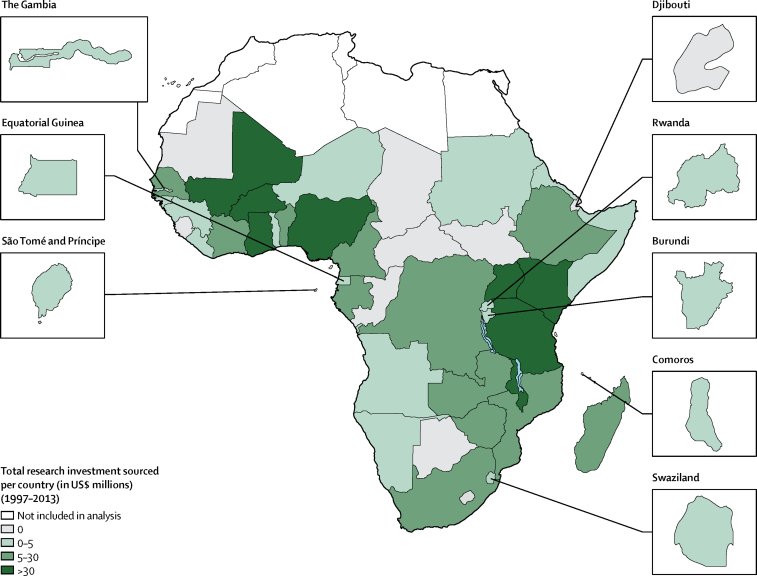
By country, Tanzania, Uganda, Kenya, Malawi, and Ghana were the top five nations to receive the greatest amount of research investment, and 18 nations received greater than US$10 million of research funding (figure 2, figure 3, and table 2). Eight countries were not allocated research investments: Botswana, Cape Verde, Central African Republic, Chad, Congo (Brazzaville), Djibouti, Mauritania, and Sierra Leone.
Figure 2.
Total research investment (US$ millions) for malaria sourced per country for 1997–2013
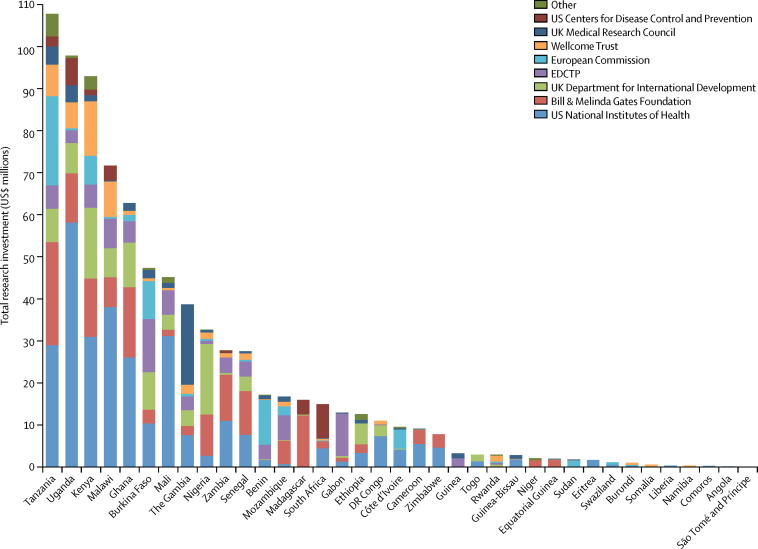
Figure 3.
Sum funding for malaria research in sub-Saharan Africa, by country and funder, for 1997–2013
Other included French National Research Agency, National Science Foundation, Research Council of Norway, Swedish Research Council, Swiss National Science Foundation, US Food and Drug Administration, and Economics and Social Research Council. EDCTP=The European & Developing Countries Clinical Trials Partnership. DRC=Democratic Republic of the Congo.
Table 2.
Total funding and rankings for malaria research and control investments by country
|
Research investment for malaria |
Funding for malaria control |
Combined rankings |
||||||
|---|---|---|---|---|---|---|---|---|
| Award numbers | Investment allocated (US$) | Ranking | Total funds (US$) | Ranking | Difference between funding and research rankings | Sum of funding and research rankings | Overall ranking* | |
| Tanzania | 170 | 107 769 388 | 1 | 749 985 362 | 2 | 1 | 3 | 1 |
| Kenya | 148 | 92 938 771 | 3 | 621 884 255 | 3 | 0 | 6 | 2 |
| Uganda | 115 | 97 865 177 | 2 | 390 362 646 | 6 | 4 | 8 | 3 |
| Malawi | 67 | 71 664 330 | 4 | 424 565 187 | 5 | 1 | 9 | 4 |
| Nigeria | 36 | 32 639 664 | 9 | 786 174 145 | 1 | −8 | 10 | 5 |
| Ghana | 83 | 62 725 691 | 5 | 345 841 459 | 8 | 3 | 13 | 6 |
| Mozambique | 19 | 16 358 722 | 13 | 350 860 934 | 7 | −6 | 20 | 7 |
| Ethiopia | 28 | 12 557 685 | 17 | 577 983 173 | 4 | −13 | 21 | 8 |
| Zambia | 34 | 27 775 658 | 10 | 253 054 196 | 14 | 4 | 24 | 9= |
| Madagascar | 22 | 15 944 015 | 14 | 309 559 262 | 10 | −4 | 24 | 9= |
| Mali | 42 | 45 135 562 | 7 | 175 217 824 | 18 | 11 | 25 | 11 |
| Senegal | 36 | 27 509 977 | 11 | 218 009 244 | 15 | 4 | 26 | 12 |
| Democratic Republic of the Congo | 13 | 10 930 149 | 18 | 331 377 636 | 9 | −9 | 27 | 13 |
| Benin | 20 | 17 095 744 | 12 | 203 545 688 | 16 | 4 | 28 | 14= |
| South Africa | 8 | 14 896 558 | 15 | 275 467 512 | 13 | −2 | 28 | 14= |
| Burkina Faso | 70 | 47 333 448 | 6 | 106 721 492 | 23 | 17 | 29 | 16 |
| Rwanda | 12 | 2 933 323 | 24 | 299 031 287 | 12 | −12 | 36 | 17 |
| The Gambia | 61 | 38 653 641 | 8 | 41 275 673 | 30 | 22 | 38 | 18 |
| Cote d'Ivoire | 11 | 9 535 162 | 19 | 129 028 300 | 20 | 1 | 39 | 19 |
| Togo | 4 | 2 965 449 | 23 | 163 947 976 | 19 | −4 | 42 | 20 |
| Zimbabwe | 6 | 7 726 559 | 21 | 114 130 493 | 22 | 1 | 43 | 21 |
| Cameroon | 15 | 9 133 108 | 20 | 99 854 472 | 24 | 4 | 44 | 22 |
| Sudan | 7 | 1 749 370 | 28 | 178 508 093 | 17 | −11 | 45 | 23 |
| Angola | 1 | 117 993 | 36 | 300 977 800 | 11 | −25 | 47 | 24 |
| Guinea | 4 | 3 224 682 | 22 | 44 039 001 | 28 | 6 | 50 | 25 |
| Gabon | 13 | 12 886 319 | 16 | 21 071 247 | 36 | 20 | 52 | 26 |
| Niger | 7 | 2 122 215 | 26 | 64 687 908 | 27 | 1 | 53 | 27 |
| Liberia | 1 | 297 205 | 33 | 121 097 057 | 21 | −12 | 54 | 28 |
| Burundi | 3 | 982 238 | 31 | 67 946 385 | 26 | −5 | 57 | 29 |
| Namibia | 4 | 292 409 | 34 | 74 777 578 | 25 | −9 | 59 | 30 |
| Equatorial Guinea | 4 | 1 908 866 | 27 | 34 065 864 | 33 | 6 | 60 | 31= |
| Eritrea | 2 | 1 662 342 | 29 | 39 739 873 | 31 | 2 | 60 | 31= |
| Guinea-Bissau | 5 | 2 772 862 | 25 | 15 118 865 | 38 | 13 | 63 | 33 |
| Somalia | 2 | 604 537 | 32 | 26 459 527 | 34 | 2 | 66 | 34 |
| Sierra Leone | 0 | 0 | 38 | 42 116 404 | 29 | −9 | 67 | 35 |
| Swaziland | 2 | 1 049 598 | 30 | 12 763 749 | 40 | 10 | 70 | 36= |
| Chad | 0 | 0 | 38 | 37 921 952 | 32 | −6 | 70 | 36= |
| Congo (Brazzaville) | 0 | 0 | 38 | 23 751 153 | 35 | −3 | 73 | 38 |
| Mauritania | 0 | 0 | 38 | 15 373 467 | 37 | −1 | 75 | 39 |
| Comoros | 1 | 224 619 | 35 | 7 254 263 | 42 | 7 | 77 | 40= |
| Central African Republic | 0 | 0 | 38 | 13 812 987 | 39 | 1 | 77 | 40= |
| São Tomé and Príncipe | 1 | 27 804 | 37 | 7 929 237 | 41 | 4 | 78 | 42 |
| Djibouti | 0 | 0 | 38 | 6 763 868 | 43 | 5 | 81 | 43 |
| Botswana | 0 | 0 | 38 | 5 870 021 | 44 | 6 | 82 | 44 |
| Cape Verde | 0 | 0 | 38 | 2 029 301 | 45 | 7 | 83 | 45 |
Countries are ordered by their overall ranking.
Overall ranking is the sum ranking in order.
By funder, the US National Institutes of Health provided the greatest investment of $292·0 million (36·4%), followed by the Bill & Melinda Gates Foundation, with a total investment of $144·1 million (17·9%); these two funders provided more than 40% of the research investments in all of the top five countries. Some nations were mostly reliant on investment from one funder—for example, the European & Developing Countries Clinical Trials Partnership provided 79% ($10·2 million) of the research investment and was the main funder in Gabon.
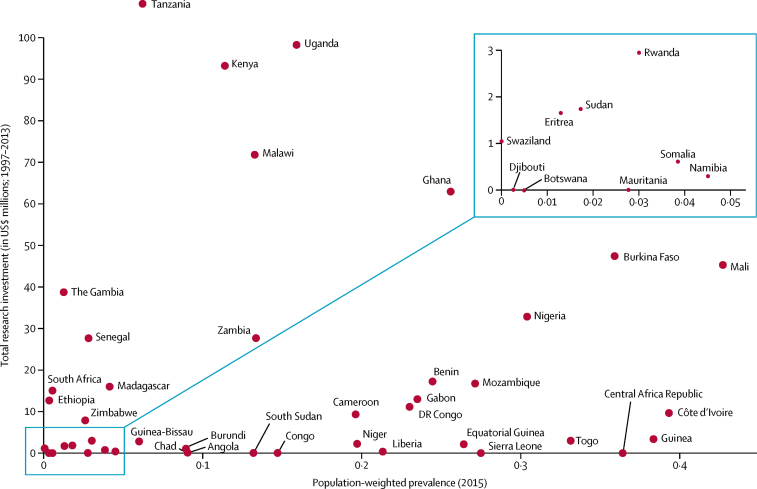
By type of science (table 1), investment for phase 1–3 clinical trials totalled $275·2 million (33·8%) across 80 (24%) awards. Public health investments totalled $308·1 million (37·8%) across 153 (46%) awards. Cross-disciplinary research covered 65 (20%) awards and product development covered 35 (11%) awards. By product area, 83 (25%) awards were related to research in antimalarial therapeutics, with a total investment of $261·2 million (32·1%). 18 (5%) awards were related to vaccine research ($65·0 million [8·0%]), and 29 (9%) awards focused on diagnostic development ($41·0 million [5·0%]). There was no obvious association between the national population-weighted PfPR and the total research investment or funding for malaria control, by country (figure 4; appendix 1). Investments were distributed across high-burden, medium-burden, and elimination settings.
Figure 4.
Comparison of 2015 population-weighed parasite prevalence and total research investment received for 1997–2013 by sub-Saharan African nation
DRC=Democratic Republic of the Congo.
$8·1 billion of funding for malaria control was disbursed in the period of 2006–11 to 45 sub-Saharan African countries (table 2, appendix), with the lowest annual funding in 2006 ($585·2 million, 7·2%) and the highest annual funding in 2010 ($1·9 billion, 23·2%, appendix 2). The top five countries to receive funding in that period were Nigeria ($786·2 million, 9th in malaria research rankings), Tanzania ($750·0 million, ranked 1st in the research rankings), Kenya ($621·9 million, also ranked 3rd in the research rankings), Ethiopia ($578·0 million, ranked 17th in the research rankings), and Malawi ($424·6 million, ranked 4th in the research rankings; table 2). Ghana ranked in the top ten for both level of funding for malaria control (8th) and research investments (5th). Of the eight nations with no allocated research investment, all were ranked in the lowest third (30th or lower out of 45 countries) when considering funding for malaria control, with the exception of Sierra Leone (ranked 29th). The Gambia ranked 30th for funding for malaria control, but 8th for research investments. Most nations ranked similarly for both research investments and funding for malaria control.
Combined rankings showed the top five nations to be Tanzania, Kenya, Uganda, Malawi, and Nigeria. When adjusting for GDP (appendix), the top five nations to rank in the top ten across both funding for malaria control and research investment were Malawi, Tanzania, Uganda, Kenya, and Madagascar.
Discussion
Many research investments for malaria were focused in sub-Saharan Africa. We found that the greatest investments were allocated to Tanzania, Uganda, and Kenya. Malawi, Ghana, and Nigeria also ranked highly across both research investment and funding for malaria control. Investments for research were typically highest in countries where funding for malaria control was also high (The Gambia and Nigeria being notable exceptions). Similarly, most nations receiving little or no research investment for malaria also received scarce funding for malaria control, despite typically having a reasonably high malaria-related burden of disease. About a third of research investments were related to antimalarial therapeutics. The US National Institutes of Health and the Bill & Melinda Gates Foundation provided about 60% of research funding for malaria in sub-Saharan Africa, and funding was disbursed to countries with high and low burdens of malarial disease, indicating that drivers for allocation of investments are likely to include the desire to both reduce existing high burdens and to pursue efforts towards elimination in relatively low-burden settings. There appeared to be inequalities in overall disbursement of funding for malaria research, which will probably help to accelerate elimination in some settings but might neglect other vulnerable populations in locations of high malarial burden. Investments in malaria control broadly reflected the trends in research investment.
Evidence that investments in malaria research contribute to improved health outcomes is scarce, in part because of the numerous and complex variables that must be considered when assessing resource allocation. Thus, the correlation between increased research investment and decreased burden cannot in itself be considered causal. Funding for malaria control also increased between 2006 and 2011, and interventions, including bednets and therapeutics, have clearly had substantial impacts.9 Case studies provide qualitative evidence to justify investments in global health research,16 and the findings presented here lend some weight to that conclusion. To qualify and address the gaps in evidence, WHO has established the Global Observatory on Health Research and Development,17 and is openly seeking evidence-based contributions to assimilate existing knowledge and inform global strategies.18 The ResIn study is also scheduled to report findings of a global research investments analysis for all infectious diseases in 2017.
Investing requires confidence on the part of the investor that they will see a return on their investment (for example, health gains from operational investments such as provision of insecticide-treated nets, or research investments that generate new knowledge or products such as vaccines and diagnostics). Therefore, particularly in environments where the logistics for research might be complex and challenging, the inclination is to fund governments and institutions with a track record of success and in locations where it is perceived that the investment will make a positive difference and where any research will be feasible. Thus, countries such as Tanzania, Uganda, and Kenya, which all have existing relatively good infrastructures for research (such as the Kenya Medical Research Institute sites in Nairobi and Kilifi), continue to receive steady sums of research investment and also benefit from relatively high levels of funding for malaria control. From these streams of funding, high-quality research that provides clarity on the research questions under investigation is likely. Conversely, funders do not have the incentive or confidence to invest in African nations such as Chad, Somalia, and the Central African Republic, which are considered politically fragile and have inadequate infrastructure and regulations surrounding business start-ups and trading.19 The USA and the UK are leading investors in global health and, specifically, in provision of funding for malaria, but the changeable political climates in both countries might have implications for global health investment and activity.
Other factors that influence resource allocation include political and socioeconomic factors such as conflict, corruption, and crime and economic considerations. Poorer nations with weak infrastructures are less likely to reap the benefits from research investment without improvements in health systems. For example, eight of the top ten nations considered most susceptible to infectious disease outbreaks are in sub-Saharan Africa, and the three most susceptible are Somalia (ranking 34th in our investment rankings), Central African Republic (ranking 40th in our investment rankings), and Chad (ranking 36th in our investment rankings).20 Corruption indices rank Somalia as the worst performer, with numerous sub-Saharan African nations ranked in the lower percentiles.21 In a systematic analysis22 of the geographical distribution of global health partnerships with the 100 highest-ranked universities worldwide, Kenya, Tanzania, and Uganda had 43 partnerships. Of the 12 countries that ranked lowest in our investment rankings, only Sierra Leone, with five partnerships, and Botswana, with three partnerships, had any recorded global health partnerships.22 The global health partnership analysis22 also described areas in northern and central Africa that had either few or no partnerships with high-ranking universities, despite these areas being in great need of health care and thus surely also in particular need of investment (institutions ranked outside of the top 100 will potentially have international partnerships that were undocumented in the global health partnership analysis and might include the lower-ranked nations here). Capacity building and training initiatives, and geographical priorities set by research funders, might help incentivise leading academic institutions to develop new partnerships with sub-Saharan African countries. Our findings correspond with other analyses23 in showing broadly low sum investments in central Africa, with resources mostly concentrated in western and eastern Africa. High malaria mortality and low coverage (<50%) of insecticide-treated bednets are seen in lower-ranked countries in these central regions, such as Congo (Brazzaville), Guinea, and Central African Republic.14 The five countries ranked lowest in terms of their likelihood to meet the targets set in the health-related UN Sustainable Development Goals were Central African Republic, Somalia, South Sudan, Niger, and Chad.24 There are huge differences in the amount of investment each high-income nation will allocate to global health operational and research investments and so, when the amount of resource allocated to each low-income or middle-income nation is linked to the wealthier nation's historical ties,13 there is the potential for inequalities in resource allocation to be introduced and for health issues to be inadequately addressed.
Research outputs can translate across national borders, but might not do so without appropriate consideration of the context in which research findings are used. Substantial advantages exist in investment in local research, particularly with regards to ownership of the results, trust, inter-sector sharing of expertise between researchers and policy makers, and increased contextualisation of findings.25 If little or no local track record in research exists, then inter-sector expertise might be less likely.26 Decision-makers will assess research findings by taking into account the political context,27 the capacity of local health systems to absorb the knowledge and put the innovation into practice, and the appropriateness of the proposed intervention.28 The Lancet's Commission on Global Health 203529 highlighted how low-income nations such as Chad or Somalia could experience substantial health gains with an “enhanced R&D investment scenario” deployed at a national level, alongside societal benefits. Investment in biomedical science research and development in the UK yields a 17% return to the economy, and each pound of public investment stimulates the same investment from the private sector.30 New investment in research and development in low-income settings could potentially be the catalyst for improved health systems and confidence in the business and academic sectors.
In 2015, WHO announced a new global strategy for addressing malaria between 2016 and 2030, for which the target is to reduce the global malaria burden by 90% before 2030.4 Innovation and research is one of the three major pillars of that strategy. WHO estimates that additional funding of $673 million annually is needed for malaria research,4 a substantial increase compared with current funding, and that $6·4 billion is needed every year for funding of malaria control.1 Research recommendations include analysis of transmission-blocking medicines in high-transmission settings, investigation of the short-term and long-term effects of administration of effective antimalarials, implementation research that refines approaches to application of existing interventions to make them more effective and more efficient in local contexts, diagnostics that detect low-level parasitaemia in asymptomatic carriers, and research to develop more effective vaccines. The African Union health strategy for years 2016–30 aims to “increase investments in health, improve equity and address social determinants of health”.31 One of the strategic areas is to “end malaria”,31 although little specific detail is available on how this could be achieved. Further tracking of resource allocation, such as with research portfolios, is essential to inform strategies that will allow WHO's ambitious 90% reduction target to be met.
Limitations of the ResIn analyses have been previously described in full.11, 12 The research funding data included here were sourced from probably the largest public and philanthropic contributors to global health and malaria-related research; however, resource constraints prevented inclusion and analysis of other national funders who might provide further investment in their countries of focus. We also excluded preclinical science and malaria research focused on nations outside of sub-Saharan Africa, although findings from such studies might eventually inform sub-Saharan-African-focused strategies. The Global Fund and President's Malaria Initiative typically do not consider their investments as research but as operational activity; thus, they were not included in the research analyses but were included in the funding for malaria control dataset.7 The Bill & Melinda Gates Foundation provides investments for both research and operational activity; however, scarce information was openly available on the Foundation's grants database, and categorisation of these awards was therefore sometimes difficult. Private sector investments were not available for detailed systematic analyses and thus were not included, leading to a clear data gap, particularly in investment directed towards research for new products. However, almost all financing for malaria vaccine research and drug development comes from public and philanthropic sources.32 Some of the research investments not included here in the public, philanthropic, or private sectors might have been focused on countries that we found to be neglected in terms of receipt of investment in our analysis. Although we found correlations between temporal levels of investment and disease burden, causality could not be inferred from those results. The categorisation process was subjective and we made assumptions about allocations of awards, athough we took care to reduce subjectivity; the high κ score on inter-observer concordance indicates a high level of agreement between authors and inter-author checks reduced the likelihood of systematic error.
To the best of our knowledge, this is the first study to systematically analyse both research and operational investments for malaria in sub-Saharan Africa. Reasonable consistency in amounts of FMC and research investment was seen in those African nations that benefit from receipt of significant shares of available resources and consistent inequalities were seen in those that do not; many countries receiving little in the way of investment are those with the highest burden of malaria and the weakest health systems and infrastructures. Evidence suggests that ownership of research findings increases their uptake and that, although doing research in some settings is extremely challenging because of negative political and socioeconomic factors, these areas contain some of the world's most vulnerable populations. Thus, careful investment strategies should consider how these populations can best be reached.
Acknowledgments
Acknowledgments
We thank the funders, who provided research investments data for this research, and ÜberResearch, who allowed access to their Dimensions database. We also thank David Pigott for providing further disaggregated data on funding for malaria control.
Contributors
MGH and JRF designed the Research Investments in Global Health study. SCC, AJT, RA, JAGS, and M-LN contributed to the design and conception of this study. YG, SG, MGH, and RJB did data collection, cleaning, and analysis. VA, AJT, and SSP provided comment on geospatial methodology and use of the malaria burden datasets. SCC, SSP, M-LN, RA, JAGS, and JRF provided comment on the methods and findings. All authors commented on and approved the draft and final manuscripts.
Declaration of interests
Funding included a small grant (£5000) from the Royal Society of Tropical Medicine and Hygiene (GR000551). MGH and RJB are funded by the Bill & Melinda Gates Foundation (OPP1127615). JRF is currently employed by the Bill & Melinda Gates Foundation. JAGS works on the KEMRI-Wellcome Trust Research Programme in Kilifi, Kenya, and is supported by a Wellcome Trust Fellowship (098532). AJT was supported by funding from the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (U19AI089674), the Bill & Melinda Gates Foundation (OPP1106427, OPP1134076, OPP1094793), the Clinton Health Access Initiative, and the Wellcome Trust (106866/Z/15/Z), outside of this work. SG, YG, VA, SCC, M-LN, RA, and SPP declare no competing interests.
Supplementary Material
References
- 1.WHO . World Malaria Report 2016. World Health Organization; Geneva: 2016. http://www.who.int/malaria/publications/world-malaria-report-2016/report/en (accessed Dec 15, 2016). [Google Scholar]
- 2.GBD 2015 Mortality and Causes of Death Collaborators Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1545–1602. doi: 10.1016/S0140-6736(16)31678-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO . Global technical strategy for malaria 2016–2030. World Health Organization; Geneva: 2016. http://apps.who.int/iris/bitstream/10665/176712/1/9789241564991_eng.pdf?ua=1&ua=1 (accessed Oct 3, 2016). [Google Scholar]
- 5.Zelman B, Melgar M, Larson E, Phillips A, Shretta R. Global fund financing to the 34 malaria-eliminating countries under the new funding model 2014–2017: an analysis of national allocations and regional grants. Malar J. 2016;15:118. doi: 10.1186/s12936-016-1171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gates Bill. We can eradicate malaria—within a generation. 2014. https://www.gatesnotes.com/Health/Eradicating-Malaria-in-a-Generation (accessed June 22, 2017).
- 7.Pigott DM, Atun R, Moyes CL, Hay SI, Gething PW. Funding for malaria control 2006–2010: a comprehensive global assessment. Malar J. 2012;11:246. doi: 10.1186/1475-2875-11-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan I, Korenromp E, Bendavid E. Mortality changes after grants from the Global Fund to Fight AIDS, tuberculosis and malaria: an econometric analysis from 1995 to 2010. BMC Public Health. 2015;15:977. doi: 10.1186/s12889-015-2305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt S, Weiss DJ, Cameron E. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viergever RF, Hendriks TCC. The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds. Health Res Policy Syst. 2016;14:12. doi: 10.1186/s12961-015-0074-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Head MG, Fitchett JR, Cooke MK, Wurie FB, Hayward AC, Atun R. UK investments in global infectious disease research 1997–2010: a case study. Lancet Infect Dis. 2013;13:55–64. doi: 10.1016/S1473-3099(12)70261-X. [DOI] [PubMed] [Google Scholar]
- 12.Head MG, Fitchett JR, Nageshwaran V, Kumari N, Hayward AC, Atun R. Research investments in global health: a systematic analysis of UK infectious disease research funding and global health metrics, 1997–2013. EBioMedicine. 2016;3:180–190. doi: 10.1016/j.ebiom.2015.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitchett JR, Head MG, Atun R. Infectious disease research investments follow colonial ties: questionable ethics. Int Health. 2014;6:74–76. doi: 10.1093/inthealth/iht036. [DOI] [PubMed] [Google Scholar]
- 14.The World Bank GDP per capita (current US$) http://data.worldbank.org/indicator/NY.GDP.PCAP.CD (accessed June 22, 2017).
- 15.Gething PW, Casey DC, Weiss DJ. Mapping Plasmodium falciparum mortality in Africa between 1990 and 2015. N Engl J Med. 2016;375:2435–2445. doi: 10.1056/NEJMoa1606701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatem AJ, Tatem AJ, Gilbert M. WorldPop, open data for spatial demography. Sci Data. 2017;4:170004. doi: 10.1038/sdata.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King's College London and Digital Science . The nature, scale and beneficiaries of research impact. King's College London; London: 2015. http://www.kcl.ac.uk/sspp/policy-institute/publications/Analysis-of-REF-impact.pdf (accessed Dec 21, 2015). [Google Scholar]
- 18.Kieny MP, Viergever RF, Adam T, Boerma T, Røttingen J-A. Global platform to inform investments for health R&D. Lancet. 2016;387:1157. doi: 10.1016/S0140-6736(16)00705-4. [DOI] [PubMed] [Google Scholar]
- 19.Adam T, Røttingen J-A, Kieny M-P. Informing the establishment of the WHO Global Observatory on Health Research and Development: a call for papers. Health Res Policy Syst. 2015;13:9. doi: 10.1186/1478-4505-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The World Bank Doing business 2016: measuring regulatory quality and efficiency. 2015. http://www.doingbusiness.org/reports/global-reports/doing-business-2016 (accessed June 22, 2017).
- 21.Moore M, Gelfeld B, Okunogbe A, Paul C. Identifying future disease hot spots: Infectious Disease Vulnerability Index. RAND Corporation; Santa Monica, CA: 2016. https://www.rand.org/pubs/research_reports/RR1605.html (accessed Oct 3, 2016). [PMC free article] [PubMed] [Google Scholar]
- 22.Transparency International Corruption Perception Index. 2015. http://www.transparency.org/cpi2015 (accessed Dec 1, 2016).
- 23.Herrick C, Reades J. Mapping university global health partnerships. Lancet Glob Health. 2016;4:e694. doi: 10.1016/S2214-109X(16)30213-3. [DOI] [PubMed] [Google Scholar]
- 24.European & Developing Countries Clinical Trials Partnership Current state of health research on poverty-related and neglected infectious diseases in sub-Saharan Africa. 2015. http://www.edctp.org/web/app/uploads/2015/01/Report_on_the_current_state_of_health_research_-_Africa.pdf (accessed Dec 1, 2016).
- 25.GBD 2015 SDG Collaborators Measuring the health-related Sustainable Development Goals in 188 countries: a baseline analysis from the Global Burden of Disease Study 2015. Lancet. 2016;388:1813–1850. doi: 10.1016/S0140-6736(16)31467-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orem JN, Mafigiri DK, Marchal B, Ssengooba F, Macq J, Criel B. Research, evidence and policymaking: the perspectives of policy actors on improving uptake of evidence in health policy development and implementation in Uganda. BMC Public Health. 2012;12:109. doi: 10.1186/1471-2458-12-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corluka A, Cohen M, Lanktree E, Larocque R. Uptake and impact of research for evidence-based practice: lessons from the Africa Health Systems Initiative Support to African Research Partnerships. BMC Health Serv Res. 2014;14(suppl 1):I1. doi: 10.1186/1472-6963-14-S1-I1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panisset U, Koehlmoos TP, Alkhatib AH. Implementation research evidence uptake and use for policy-making. Health Res Policy Syst. 2012;10:20. doi: 10.1186/1478-4505-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atun R, De Jongh T, Secci F, Ohiri K, Adeyi O. A systematic review of the evidence on integration of targeted health interventions into health systems. Health Policy Plan. 2010;25:1–14. doi: 10.1093/heapol/czp053. [DOI] [PubMed] [Google Scholar]
- 30.Jamison DT, Summers LH, Alleyne G. Global health 2035: a world converging within a generation. Lancet. 2013;382:1898–1955. doi: 10.1016/S0140-6736(13)62105-4. [DOI] [PubMed] [Google Scholar]
- 31.Sussex J, Feng Y, Mestre-Ferrandiz J. Quantifying the economic impact of government and charity funding of medical research on private research and development funding in the United Kingdom. BMC Med. 2016;14:32. doi: 10.1186/s12916-016-0564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.African Union Africa Health Strategy 2016–2030. 2016. https://www.au.int/web/en/document/africa-health-strategy-2016-%E2%80%93-2030 (accessed June 22, 2016).
Uncited Reference
- 33.Årdal C, Røttingen J-A, Gosling R, Feachem R, Fisher K. An open source business model for malaria. PLoS One. 2015;10:e0117150. doi: 10.1371/journal.pone.0117150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.