Abstract
Emotional overeating (EOE) has been associated with increased obesity risk, while emotional undereating (EUE) may be protective. Interestingly, EOE and EUE tend to correlate positively, but it is unclear whether they reflect different aspects of the same underlying trait, or are distinct behaviours with different aetiologies. Data were from 2054 five-year-old children from the Gemini twin birth cohort, including parental ratings of child EOE and EUE using the Child Eating Behaviour Questionnaire. Genetic and environmental influences on variation and covariation in EUE and EOE were established using a bivariate Twin Model. Variation in both behaviours was largely explained by aspects of the environment completely shared by twin pairs (EOE: C = 90%, 95% CI: 89%-92%; EUE: C = 91%, 95% CI: 90%-92%). Genetic influence was low (EOE: A = 7%, 95% CI: 6%-9%; EUE: A = 7%, 95% CI: 6%-9%). EOE and EUE correlated positively (r = 0.43, p < 0.001), and this association was explained by common shared environmental influences (BivC = 45%, 95% CI: 40%-50%). Many of the shared environmental influences underlying EUE and EOE were the same (rC = 0.50, 95% CI: 0.44, 0.55). Childhood EOE and EUE are etiologically distinct. The tendency to eat more or less in response to emotion is learned rather than inherited.
Introduction
Emotional eating is the tendency to change one’s eating behaviour in response to negative emotions1. Research with adults has shown that some people tend to consume more in stressful situations (so-called emotional overeating, [EOE]), whereas others experience a decrease in appetite when distressed and eat less (so-called emotional under-eating, [EUE])1, 2. The tendency to either over- or under-eat in response to negative emotion appears to emerge in the preschool years3.
Understanding the aetiologies of these behaviours in early life is important because EOE has been hypothesized to play a causal role in overweight, and EUE in under-weight4. There has been some support for these hypotheses insofar as EOE has been associated with higher weight cross-sectionally5–10, and with weight gain longitudinally from 5–6 years to 6–8 years11 and from 4 to 8 years12. On the other hand, EUE has been negatively associated with weight9, 13, 14. However, null findings have sometimes been reported in cross-sectional studies for both EOE15, 16 and EUE9, 15.
Despite their differing associations with weight, EOE and EUE tend to be positively correlated9, 17, 18, indicating that some children have an underlying tendency to both under- and overeat in response to negative emotions. This raises the question as to whether these two behaviours reflect different aspects of the same underlying trait (i.e. a tendency to both over- and under-eat in response to negative emotion) with a common aetiology, or are distinct traits with different aetiologies. Twin studies offer a powerful method for establishing the extent to which behaviour is shaped by genes and environments; and can also elucidate shared aetiology by quantifying the extent to which different behaviours share common or distinct genetic and environmental influences. We have conducted the only paediatric twin study of EOE, finding this behaviour to be influenced largely by aspects of the environment completely shared by twin pairs, in both toddlerhood and early childhood; with genetic factors playing a minimal role19. To date there have been no twin studies of EUE in adults or children. The objectives of this study were to use a twin design to: (i) establish for the first time the relative genetic and environmental contributions to EUE in early childhood, and (ii) establish the extent to which EOE and EUE share a common genetic and environmental aetiology.
Methods
Participants
Participants were from Gemini, a population-based birth cohort of 2402 British families with twins born in 2007. Gemini is the largest twin study ever set up to study genetic and environmental influences on early growth, and has a particular focus on the role of early eating behaviour20. Between March and December 2007, the Office for National Statistics contacted all families in England and Wales with live twin births (N = 6725) for consent to pass on their contact details to the Gemini research team. 2402 families (n = 4804 individual twins) completed and returned the baseline questionnaire; they constitute the Gemini sample, and include 749 monozygotic pairs [MZs], 1616 dizygotic pairs [DZs], and 37 pairs of unknown zygosity. The Gemini families are representative of UK twins when compared on zygosity, sex, gestational age and birth weight. As with most cohort studies, compared to national UK statistics Gemini parents have below average BMIs and healthier eating habits, and white and married couples are overrepresented in the sample. The current study includes data on EOE and EUE collected when the twins were approximately five years old (n = 2054 individual children; mean, 5.15 years; SD, 0.13). Ethical approval was granted by the University College London Committee for the Ethics of non–National Health Service Human Research, and all aspects of data collection and storage were in accordance with the standards stipulated by this body. Informed consent for both study participation and publication were obtained from all participants.
Measurement of emotional over- and under-eating
Emotional over- and under-eating were measured using the Child Eating Behaviour Questionnaire (CEBQ), a parent report questionnaire consisting of 35 items capturing eight distinct eating behaviours hypothesized to play a causal role in childhood weight variation. The EOE (4 items; e.g.: “My child eats more when annoyed”) and EUE (4 items; e.g.: “My child eats less when upset”) scales were used in the present study17. Items were rated along a 5-point Likert scale (‘never’; ‘rarely’; ‘sometimes’; ‘often’; ‘always’) and mean scores were calculated for participants whose parents had completed a minimum of 3 out of 4 items for each subscale. EOE and EUE scores were available for a total of 2054 children, which included 346 MZ twin pairs and 681 DZ twin pairs. The CEBQ is the most widely used psychometric measure of children’s eating behaviour with good internal reliability (EOE: α = 0.72–79, EUE: α = 0.74–0.75), good test-retest reliability over a two week period17, and has been validated against laboratory-based measures of eating behaviour21. Both scales had good internal reliability in this sample (EOE: α = 0.71, EUE: α = 0.81).
Zygosity, age and gestational age
Parents reported the weight at birth of each twin, taken from the child’s personal health record, as well as gestational age. The exact age of the twins at data collection was calculated from the twins’ date of birth and the date the questionnaire was completed. At baseline, opposite sex twin pairs were classified as DZs. For same sex pairs, parents were asked to complete the 18-item Zygosity Questionnaire for Young Twins. This questionnaire was specifically developed to aid zygosity classification in young twin pairs and has been validated longitudinally and against DNA markers22. DNA from a random sample of 81 twin pairs was used to validate the zygosity questionnaire in Gemini. DNA confirmed 43 pairs as MZ and 38 as DZ; which exactly matched the results of the questionnaires (Herle et al., 2016).
Statistical analyses
Pearson’s correlation coefficient was used to examine the association between EOE and EUE prior to twin analyses. In line with common practice EOE and EUE scores were regressed on sex, gestational age and age at measurement, to ensure that factors shared completely by twin pairs did not contribute to similarity between pairs and inflate the shared environmental effect. Further to remove any positive skew, EOE and EUE scores were log transformed prior to analyses.
Twin analyses
The twin method exploits the natural occurrence of identical and non-identical twin pairs. Identical twins [MZ] share 100% of their genetic material, whereas non-identical [DZ] twins share approximately 50% of their segregating genes. However, both types of twins share their environments to a very similar extent (e.g. gestated in the same mother, born at the same time, raised in the same family). This means MZ and DZ twins can be compared, and greater similarity among MZ than DZ pairs indicates a genetic contribution to variation in a trait because the only real difference between the two types of twins is that MZ pairs are twice as similar genetically. Comparing MZ and DZ correlations (or covariation) enables variation in the trait to be decomposed into three latent factors: additive genetic effects (A); shared environmental influence (C); and non-shared environmental influence (E). The shared environmental influence comprises environmental factors that contribute to similarity between twins in a pair, such as socio-economic status; non-shared environmental influences contribute to differences within twin pairs, such as twins attending different schools or illness experienced by only one twin. The bigger the difference in resemblance between MZs and DZs, the greater the genetic contribution to variation in the trait; the greater the similarity between twin pairs regardless of zygosity the more important the contribution from the shared environment; and any differences between MZ twins (who share both their genetic and shared environmental factors completely) quantifies the extent of unique environmental influence (as well as measurement error)23.
This concept can be extended to understand the extent of common genetic and environmental influences underlying two different traits – i.e. shared aetiology. The basis of the bivariate method is to compare the correlation (or covariation) between two different phenotypes, across twin pairs (so-called a cross-twin cross-trait correlation, CT-CT); resemblance in CT-CTs for MZ and DZ pairs is then compared, using the same principles as the univariate method. A higher CT-CT correlation for MZ pairs relative to DZ pairs indicates that genetic factors contribute to the phenotypic association between the two traits; similar CT-CT correlations for both types of twins indicates that shared environmental effects are important in driving the phenotypic association; no CT-CT correlation indicates that unique environmental influences are driving the phenotypic association between the two traits. The bivariate method therefore decomposes covariation between two traits into A, C and E; while the univariate method decomposes variation in a single trait into A, C and E23. Genetic and environmental contributions to variation and covariation in EOE and EUE were estimated using intraclass correlations (ICCs) and Maximum Likelihood Structural Equation Modelling (MLSEM).
Intraclass correlation analyses
MZ and DZ intraclass correlations (ICCs) were calculated for EOE and EUE scores to examine patterns of resemblance for EUE and EOE. CT-CT correlations were calculated for MZ and DZ twins to observe patterns of resemblance for covariation between EOE and EUE. ICCs and CT-CT correlations were calculated using OpenMx in R24.
Maximum likelihood structural equation modelling
Maximum Likelihood Structural Equation Modelling (MLSEM) was used to provide reliable parameter estimates of A, C and E with 95% confidence intervals and goodness-of-fit statistics. First a saturated model was computed with no parameter constraints; i.e. it estimates all possible means, variances and covariances for both EOE and EUE, for MZs and DZs. The specified ACE model was then compared to this model for goodness-of-fit.
A bivariate Correlated Factors Model was used to estimate A, C and E underlying the variation in EOE and EUE, and the covariation between them. The Correlated Factors Model produces etiological correlations (additive genetic correlation [rA], shared environmental correlation [rC], and non-shared environmental correlation [rE]) that indicate the extent to which the A, C and E influences underlying the two behaviours are the same or different. The etiological correlations can be interpreted along similar lines to a Pearson’s correlation coefficient. For example, a high positive rA indicates that many of the same genetic factors that predispose to EOE also predispose to EUE, whereas a low rA suggests that different genetic factors influence the two behaviours. In addition, a high negative rA would indicate that many of the same genetic factors that make a child more likely to engage in EOE would also make a child less likely to engage in EUE.
Bivariate estimates of A, C and E are also produced; these indicate the proportion of the phenotypic association between EOE and EUE explained by common genetic (bivariate A), common shared environmental (bivariate C) or common unique environmental (bivariate E) influences underlying the two behaviours. The bivariate estimates are calculated by dividing the covariance of the latent factors (A, C and E) by the phenotypic correlation between the two variables. If all bivariate estimates are in the same direction, negative or positive, they can be expressed as a proportion of the phenotypic correlation between the two variables. Importantly, etiological correlations and bivariate estimates are independent of one another. It is therefore possible for the majority of the genetic influences underlying EOE and EUE to be the same, but for common shared environmental influences to play the most important role in driving the phenotypic association between them (e.g. a high rA, and a high bivariate C)25.
MLSEM was carried out using OpenMx software24. The Likelihood Ratio Test (LRT) was chosen as an appropriate model fit indicator. The LRT compares the fit value (−2 Log likelihood) of the specified ACE model, to the fit value of the saturated model (in which it is nested). The p-value indicates how well the specified ACE model fits, compared to a fully saturated model. A significant p-value (<0.05) indicates that the ACE model has a significantly worse fit to the data; a non-significant difference in model fit (p > 0.05) indicates that the ACE model provides an acceptable fit to the data26.
Sensitivity analyses
Prior to analyses full sex limitation models were conducted to test for the presence of sex differences between boys and girls. Fit statistics indicated some sex differences for EOE, however none of the parameter estimates were significantly different for boys and girls. There were no sex differences in the aetiology underlying EUE. A model combining males and females was therefore used. Estimates and fit statistics for the sex limitation models can be found in the supplementary information (Supplementary Information, Tables 1 to 4).
The subscales of the CEBQ are correlated with one another: food approach behaviours are positively correlated (Food Responsiveness (FR), Enjoyment of Food (EF), Desire to Drink (DD) and EOE), as are food avoidant behaviours (Food Fussiness (FF), Satiety Responsiveness (SR), Slowness of Eating (SE) and EUE); and food approach behaviours are negatively correlated with food avoidance behaviours. In order to establish the specific aetiology of EOE and EUE (independently of the other CEBQ eating behaviours), sensitivity analyses were conducted on EOE scores that had been regressed on all of the other food approach behaviours (FR, EF and DD) and on EUE that had been regressed on all of the other food avoidant behaviours (FF, SR and SE). There were no differences between the estimates derived from the original analyses and the sensitivity analyses. We therefore present only the original analyses in the manuscript, and provide the sensitivity analyses in the supplementary information (Supplementary Information, Table 5).
Results
Characteristics of the sample are shown in Table 1. EOE and EUE were significantly positively correlated (r = 0.43, p < 0.001), indicating that children who emotionally overeat tend also to emotionally under-eat.
Table 1.
Descriptive Statistics for sample included in these analyses.
| Twin pairs | N (%) or Mean (SD) |
|---|---|
| Total | 1027 pairs (2054 children) |
| Zygosity | |
| MZ1 pairs | 346 (34) |
| DZ1 pairs | 681 (66) |
| Sex of pairs | |
| MZ Male-Male | 176 (17.2) |
| DZ Male-Male | 165 (16.1) |
| MZ Female-Female | 169 (16.5) |
| DZ Female-Female | 199 (19.4) |
| DZ Opposite Sex | 316 (30.8) |
| Gestational age (weeks) | 36.26 (2.43) |
| Weight at birth (kg) | 2.46 (0.54) |
| Weight at birth SDS 1 | −0.58 (0.92) |
| BMI 1 SDS at 5 years | −0.22 (1.10) |
| Age at 5 year questionnaire (years) 1 | 5.15 (0.13) |
| Emotional Overeating at 5 years | 1.56 (0.51) |
| Emotional Undereating at 5 years | 2.66 (0.84) |
1MZ: Monozygotic; DZ: Di-zygotic; SDS: Standard Deviation Score; BMI: Body Mass Index Age at 5 year questionnaire: Exact age when questionnaire was filled in was calculated using the date of birth of the twins and the date when the questionnaire was filled in.
Intraclass correlations (ICCs)
ICCs for EOE and EUE were calculated for MZ and DZ twin pairs separately to examine the patterns of resemblance for each behaviour. As shown in Table 2, the ICCs were high and of similar magnitude for both MZs and DZs, for both EUE and EOE. This pattern of twin correlations suggests strong shared environmental factors underlying variation in both EUE and EOE. The cross-twin cross-trait (CT-CT) correlations showed a similar pattern to the univariate ICCs (also shown in Table 3). The CT-CT correlations for both MZ and DZ pairs were significant, of similar magnitude, and of a comparable effect size to the phenotypic correlation itself indicating that shared environmental influences are largely driving the observed phenotypic association between EOE and EUE.
Table 2.
Intraclass correlations (ICCs) and cross-twin cross-trait (CT-CT) correlations for EOE and EUE measured at 5 years.
| MZ 1 | DZ 1 | |
|---|---|---|
| EOE ICCs1 (95% CI1) | 0.98 (0.97–0.98) | 0.94 (0.92–0.94) |
| EUE ICCs (95% CI) | 0.98 (0.97–0.98) | 0.95 (0.94–0.95) |
| CT-CT (95% CI) | 0.43 (0.42–0.43) | 0.44 (0.39–0.49) |
1MZ: Monozygotic; DZ: Di-zygotic; EOE: Emotional Overeating; ICCs: Intraclass correlations; CI: Confidence intervals; CT-CT: Cross-twin cross-trait.
Table 3.
Model fit statistics for saturated and bivariate model.
| Model | parameters | −2LL1 | df1 | Δ χ² (df) | p-value | AIC1 |
|---|---|---|---|---|---|---|
| Sat1 | 28 | −1542.604 | 4078 | −9698.604 | ||
| ACE1 | 11 | −1518.404 | 4095 | 24.199 (17) | 0.11 | −9708.404 |
1Abbreviations: 2LL: −2 times log-likelihood of data; df: degrees of freedom; Δ χ²: change in chi-square; AIC: Akaike’s Information Criterion; Sat: Saturated model; ACE: Full bivariate Correlation Factors Model.
Maximum Likelihood Structural Equation Modelling (MLSEM)
The bivariate ACE Correlated Factors model (including all parameters; A, C, and E for EOE and EUE; rA, rC, and rE between EOE and EUE) was tested against the saturated model. The LRT indicated no significant difference in fit between the two models (Δ χ² = 24.199, p = 0.11), confirming that the bivariate ACE Correlated Factors Model fitted the data well. In line with the LRT, Aikaike’s Information Criterion (AIC) favored the bivariate Correlated Factors Model over the saturated model, indicated by the lower value. Fit statistics for the saturated and bivariate Correlated Factors Model are shown in Table 3.
Parameter estimates for A, C and E (and 95% confidence intervals, CIs) indicated the relative importance of genetic, shared environmental and unique environmental influences on variation in EOE and EUE. As suggested by the ICCs, variation in both EOE and EUE was largely explained by shared environmental influences (EOE: C = 0.90, 95% CI: 0.89, 0.92; EUE: C = 0.91, 95% CI: 0.90, 0.92). In contrast, genetic effects only played a minor role in explaining variation in either of the two behaviours (EOE: A = 0.07, 95% CI: 0.06, 0.09; EUE: A = 0.07, 95% CI: 0.06, 0.09). Contributions from non-shared environmental factors were also small (EOE: E = 0.02, 95% CI: 0.02, 0.03; EUE: E = 0.02, 95% CI: 0.02, 0.02).
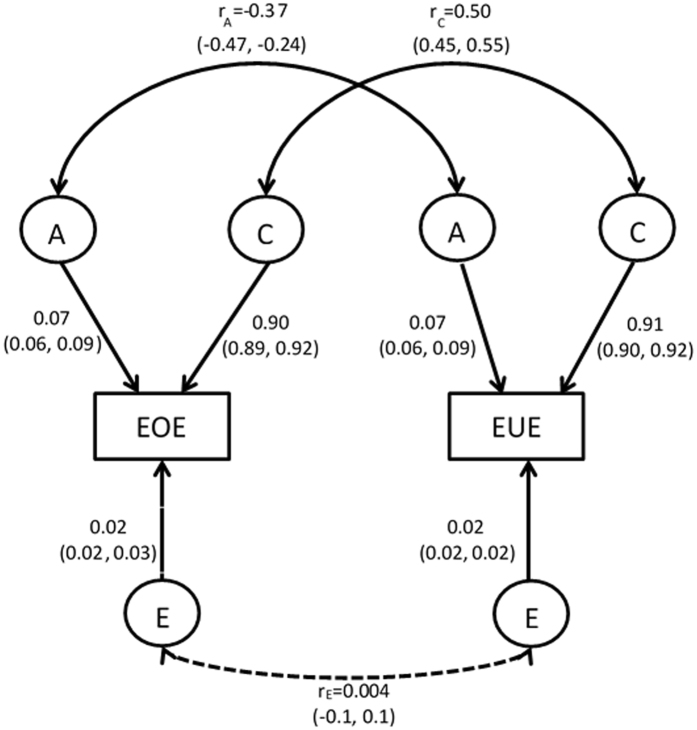
The etiological correlations are displayed in Fig. 1. The shared environmental correlation (rC) was significant, positive and moderate in effect size (rC = 0.50, 95% CI: 0.44, 0.55) indicating that a quarter (0.502) of the shared environmental influences that predispose a child to engage in EOE are the same as those that predispose a child to engage in EUE. There was also a significant negative genetic correlation which was moderate in effect size (rA = −0.38, 95% CI: −0.51, −0.26). However, because the genetic contributions to variation in EUE and EOE were so small (7%), the genetic correlation between them is difficult to interpret. The correlation for non-shared environmental effects was non-significant (rE = 0.02, 95% CI: −0.1, 0.1).
Figure 1.
Full bivariate Correlated Factors Model, including all parameters. The rectangular boxes represent the measured phenotype (emotional overeating, EOE and emotional under-eating, EUE) using the Child Eating Behaviour Questionnaire at five years of age. The circles indicate the latent factors: additive genetic effects (A), shared environmental effects (C) and non-shared environmental effects (E). The straight single-headed arrows reflect pathways with the variance explained by each latent factor (including 95% confidence intervals, CI). The etiological correlations are shown on the curved double-headed arrows. These indicate the extent of common genetic (rA), shared environmental (rC) and non-shared environmental (rE) influences across the two phenotypes. The non-significant etiological correlation (rE), with a 95% CI crossing 0, is represented as a dotted line. Bivariate estimates (not shown on the path diagram) quantify the proportion of the phenotypic association (r = 0.41, p < 0.001) attributable to genetic (bivariate A = −0.03, 95% CI: −0.004, −0.02), shared environmental (bivariate C = 0.44, 95% CI: 0.39, 0.48) and unique environmental factors (BivE: 0.00, 95% CI: −0.00, 0.00) that are common to both EOE and EUE.
The bivariate estimates indicated that common shared environmental factors underlying both EOE and EUE were largely driving the observed phenotypic association between them (BivC = 0.45, 95% CI: 0.40, 0.50). In contrast bivariate A was very small (BivA = −0.03, 95% CI: −0.04, −0.02); and bivariate E was estimated as zero (BivE = 0.00, 95% CI: −0.00, 0.00).
Discussion
Summary of findings
This is the first twin study to establish the relative importance of genetic and environmental influences on variation in EUE, and the extent to which emotional under- and overeating share their aetiology. In line with our previous study of EOE19, we found individual differences in EUE are largely explained by shared environmental factors (91%) in early childhood, while genetic influences play only a minor role (7%). The very low heritability estimates for both of these behaviours were somewhat surprising, and in stark contrast to the much higher heritability estimates observed for a range of other eating behaviours in both infants and children, captured using the CEBQ and the Baby Eating Behaviour Questionnaire, the infant version which includes four subscales: food responsiveness, satiety responsiveness, slowness in eating and enjoyment of food27, 28. In infancy, heritability is moderate to high for four eating behaviours (53–84%)27; in toddlers, heritability is high for ‘food fussiness’ (78%)29, and in 10-year-old children heritability is high for both ‘satiety responsiveness’ (63%) and ‘food responsiveness’ (75%)30.
EOE and EUE were moderately positively correlated, in line with previous studies9, 17, 18 indicating that children who tend to emotionally overeat tend also to emotionally under-eat. Common shared environmental influences largely explained the observed association between these two behaviours; in other words, the reason EOE and EUE are positively correlated is because there are a number of shared environmental factors that shape the development of both of these behaviours. Children’s eating behaviour is influenced by a multitude of factors - individual and environmental31 - and parenting behaviours have received most attention32. Although the present inquiry did not examine which environmental factors are at play, longitudinal research has shown that parents who use food to soothe their child when upset (so-called ‘emotional feeding’) encourage the development of emotional overeating in their school-aged children33; cross-sectional studies have also reported associations between emotional feeding and EOE34, 35. Cross-sectional studies have also reported associations between other parental feeding practices and higher EOE in two to five year-old children, including exerting high levels of pressure on a child to eat, and being overly restrictive about what or how much the child is allowed to eat14, 36. A recent study showed that five to seven year old children consumed more calories from snack foods when exposed to a mild stressor. Children were more likely to consume greater amounts when experiencing negative emotions if their parents reported using food as a reward and greater restriction of food for health reasons two years earlier37. The present findings indicate that many of the factors that shape EOE are the same as those that shape EUE. It is therefore reasonable to assume that parents’ feeding practices may affect EUE as well, although research is needed to confirm this assumption. For example, parents who exert high levels of pressure on their children to eat might induce stress during mealtimes, making it more likely that the child will become anxious around food, especially when upset. Parental pressure to eat is associated with lower food intake38, 39; EUE might be one of the behaviours that explains this.
Research has suggested that a lack of social support and a more negative family environment are associated with EUE, e.g. children whose parents have a more hostile relationship with each other were found to engage more in EUE40. Further evidence comes from a retrospective study of adult women diagnosed with anorexia nervosa, who reported both a lack of social support and having engaged in EUE during their childhood41. It is possible that lack of social support may be particularly important for the development of EUE but further research is needed.
To our knowledge no previous theory has been developed to explain why some individuals are more likely to both over- and under-eat in response to stress. Observational studies have shown that individuals differ substantially in their response to emotional stress, insofar as some increase their food intake, while others decrease it1. But this research does not address the issue of the same individuals responding differently on different occasions. Early animal research, however, suggested that stress intensity might contribute to response differences. The appetite and grooming behaviors of rats have been shown to differ in response to low and chronic42 or intense stress (electric shock)43. It may therefore be the case that the same individual may be prone to undereating in response to a certain type of stress (e.g. intense acute stress), but tends to overeat in response to low-level chronic stress (e.g. certain aspects of the family environment). However, even when exposed to the same stressors, rats showed large variation in appetite response, pointing towards the contribution of individual factors44. A child’s ability to regulate their emotion may be one of the common factors that explains the positive association between EOE and EUE. It might be the case that children who are less able to manage their own emotions through positive strategies, have a tendency for their eating behaviour to be affected under emotions of stress; and the intensity and type of stressor determines whether the child ends up over- or under-eating. Child emotion regulation has in fact been shown to predict a parents’ tendency to use food to sooth, and to predict the tendency of a child to engage in EOE; i.e. parents are more likely to emotionally feed their child if he or she has greater negative affectivity, and emotional feeding contributes to increasing EOE as time goes on (in a reciprocal, progressive manner)45. Less is known about the shapers of EUE, but parental feeding strategies may also play a role. A potential theoretical model of EOE and EUE may therefore include stress intensity, emotional regulation ability and parental feeding strategies. Future research needs to examine the expression of EOE and EUE in the context of different stressors of varying intensity, children’s ability to self-regulate their emotions, and parental feeding strategies. Novel theoretical models also need to account for EOE and EUE having opposite effects on weight and weight gain, in spite of their positive association.
This is the first twin study of EUE, so it is not possible to compare the findings with any other study. However, there have been three adult twin studies of EOE, although none of which observed any shared environmental effects, with all the variance in EOE being explained by genetic and non-shared environmental effects. Overall, these adult studies have also reported larger genetic influences on EOE than were found in the present study, although estimates varied widely (9%–60%) due to limited sample sizes and wide age ranges46–48. Outcomes from twin studies are age and sample specific and findings from adult studies cannot be extrapolated to children. This is of particular importance for twin studies because genetic influence tends to increase steadily with development for a variety of phenotypes (e.g: IQ49 and BMI50); something that may indicate active gene-environment correlation51 – seeking out the opportunity to act upon genetic predispositions, which increases as children mature and gain autonomy. It would be interesting to establish the heritability of EUE in an adult sample to ascertain if genetic influence is higher. The recent development of the Adult Eating Behaviour Questionnaire52, which includes both EOE and EUE scales, makes this possible.
This research has increased our understanding of the aetiology of EOE and EUE. Our findings suggest that emotional eating is a learned behaviour in early childhood, and that many of the underlying environmental factors are the same for EOE and EUE. This suggests that the same factors can be addressed to prevent both EOE and EUE in children. Preventive efforts are required as emotional eating increases the risk for developing over- and underweight in childhood9, 11 and is associated with later symptoms of eating disorders53–55.
Limitations
EOE and EUE were parent reported, and the measures could be subject to bias. Direct observations and laboratory tests would be advantageous, and have been used to validate some of the CEBQ subscales21. However such methods are time consuming and costly for large samples and therefore pose practical issues for large scale research. Nevertheless, it would be useful to apply the methods developed by a few other researchers who have been able to measure EOE objectively in a laboratory setting in order to validate the CEBQ measures of EOE and EUE. Additionally, the EOE and EUE subscales tap different emotions: EOE relates to annoyance, worry, anxiety and boredom; EUE to feeling upset, tired, angry, and unhappy. It is possible that parental feeding practices differ depending on the emotion in question, such as feeding to sooth in response to a child’s sadness but not in response to anger. Future research needs to investigate more thoroughly the complex relationships between parental feeding and child emotional eating, acknowledging the different negative emotional states. It is possible that the aetiology of eating in response to anger differs from the aetiology of overeating when feeling sad. Future research would therefore benefit from differentiating emotional over and under-eating by different emotional states. Parents are deemed to know their children better than anyone else and are arguably the best informants of their children’s eating behaviour. In addition, EOE and EUE scores may be influenced to some extent by the parents’ own tendencies to emotionally eat, or by their emotional feeding practices. Both might have resulted in parents scoring the two children more similarly, inflating the shared environmental effect (because both twins would be rated similarly regardless of their zygosity). However, this bias should apply equally to other parent-reported child eating behaviours (e.g. food fussiness), yet EOE and EUE show much higher shared environmental influence and much lower genetic influence in comparison27–29; suggesting that parents can and do distinguish between their child and their own behaviour. Lastly, this is the first twin study of EUE, replication is therefore necessary.
Conclusion
EOE and EUE in early childhood are learned, not inherited. The most important environmental shapers of these behaviours are those that are entirely shared by twin pairs, such as parental and family factors. The reason EOE and EUE are positively correlated is because many of the shared environmental influences that shape them are the same. Future research should aim to establish which environmental factors are associated with both EOE and EUE in childhood. High influence of the shared environment suggests emotional eating is modifiable in early life.
Electronic supplementary material
Acknowledgements
We thank the Gemini families who are participating in the study and the Office for National Statistics for their help in recruiting them. The authors would like to acknowledge Professor Jane Wardle who was the original Principal Investigator on Gemini, who sadly passed away in 2015. Furthermore we would like to thank the members of the London Behaviour Genetics Network (LBGN) for their helpful comments regarding the analyses. Cancer Research UK. Grant Number: C1418/A7974.
Author Contributions
The authors’ responsibilities were as follows – M.H., A.F. and C.L.: designed the research, M.H.: performed the statistical analyses; and all authors (M.H., A.F., F.R., S.S. and C.L.) wrote, read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-09519-0
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Cardi V, Leppanen J, Treasure J. The effects of negative and positive mood induction on eating behaviour: A meta-analysis of laboratory studies in the healthy population and eating and weight disorders. Neuroscience and biobehavioral reviews. 2015;57:299–309. doi: 10.1016/j.neubiorev.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft J, Semmler C, Carnell S, van Jaarsveld CH, Wardle J. Continuity and stability of eating behaviour traits in children. European journal of clinical nutrition. 2008;62:985–990. doi: 10.1038/sj.ejcn.1602855. [DOI] [PubMed] [Google Scholar]
- 4.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the children’s eating behaviour questionnaire. Journal of Child Psychology and Psychiatry. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 5.Joyce JL, Zimmer-Gembeck MJ. Parent feeding restriction and child weight. The mediating role of child disinhibited eating and the moderating role of the parenting context. Appetite. 2009;52:726–734. doi: 10.1016/j.appet.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Webber L, Hill C, Saxton J, Van Jaarsveld CH, Wardle J. Eating behaviour and weight in children. International journal of obesity. 2009;33:21–28. doi: 10.1038/ijo.2008.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spence JC, Carson V, Casey L, Boule N. Examining behavioural susceptibility to obesity among Canadian pre-school children: The role of eating behaviours. Int J Pediatr Obes. 2011;6:E501–E507. doi: 10.3109/17477166.2010.512087. [DOI] [PubMed] [Google Scholar]
- 8.dos Passos DR, Gigante DP, Maciel FV, Matijasevich A. [Children’s eating behaviour: comparison between normal and overweight children from a school in Pelotas, Rio Grande do Sul, Brazil] Revista paulista de pediatria: orgao oficial da Sociedade de Pediatria de Sao Paulo. 2015;33:42–49. doi: 10.1016/j.rpped.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domoff SE, Miller AL, Kaciroti N, Lumeng JC. Validation of the Children’s Eating Behaviour Questionnaire in a low-income preschool-aged sample in the United States. Appetite. 2015;95:415–420. doi: 10.1016/j.appet.2015.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez U, Weisstaub G, Santos JL, Corvalan C, Uauy R. GOCS cohort: children’s eating behavior scores and BMI. European journal of clinical nutrition. 2016 doi: 10.1038/ejcn.2016.18. [DOI] [PubMed] [Google Scholar]
- 11.Parkinson KN, Drewett RF, Le Couteur AS, Adamson AJ. & T, G. M. S. C. Do maternal ratings of appetite in infants predict later Child Eating Behaviour Questionnaire scores and body mass index? Appetite. 2010;54:186–190. doi: 10.1016/j.appet.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Steinsbekk S, Wichstrom L. Predictors of Change in BMI From the Age of 4 to 8. J Pediatr Psychol. 2015 doi: 10.1093/jpepsy/jsv052. [DOI] [PubMed] [Google Scholar]
- 13.Viana V, Sinde S, Saxton JC. Children’s eating behaviour questionnaire: associations with BMI in Portuguese children. Brit J Nutr. 2008;100:445–450. doi: 10.1017/S0007114508894391. [DOI] [PubMed] [Google Scholar]
- 14.Jansen PW, et al. Children’s eating behavior, feeding practices of parents and weight problems in early childhood: results from the population-based Generation R Study. The international journal of behavioral nutrition and physical activity. 2012;9 doi: 10.1186/1479-5868-9-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCarthy EK, et al. Eating behaviour and weight status at 2 years of age: data from the Cork BASELINE Birth Cohort Study. European journal of clinical nutrition. 2015 doi: 10.1038/ejcn.2015.130. [DOI] [PubMed] [Google Scholar]
- 16.Braden A, et al. Associations between child emotional eating and general parenting style, feeding practices, and parent psychopathology. Appetite. 2014;80:35–40. doi: 10.1016/j.appet.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 17.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. Journal of child psychology and psychiatry, and allied disciplines. 2001;42:963–970. doi: 10.1111/1469-7610.00792. [DOI] [PubMed] [Google Scholar]
- 18.Sleddens EF, Kremers SP, Thijs C. The children’s eating behaviour questionnaire: factorial validity and association with Body Mass Index in Dutch children aged 6-7. The international journal of behavioral nutrition and physical activity. 2008;5 doi: 10.1186/1479-5868-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herle, M., Fildes, A., Rijsdijk, F., Steinsbekk, S. & Llewellyn, C. The home environment shapes emotional eating. Child Dev. doi:10.1111/cdev.12799 (2017). [DOI] [PubMed]
- 20.van Jaarsveld CH, Johnson L, Llewellyn C, Wardle J. Gemini: a UK twin birth cohort with a focus on early childhood weight trajectories, appetite and the family environment. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2010;13:72–78. doi: 10.1375/twin.13.1.72. [DOI] [PubMed] [Google Scholar]
- 21.Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: Validation of the child eating behaviour questionnaire. Appetite. 2007;48:104–113. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- 22.Price TS, et al. Infant zygosity can be assigned by parental report questionnaire data. Twin research: the official journal of the International Society for Twin Studies. 2000;3:129–133. doi: 10.1375/twin.3.3.129. [DOI] [PubMed] [Google Scholar]
- 23.Rijsdijk FV, Sham PC. Analytic approaches to twin data using structural equation models. Briefings in bioinformatics. 2002;3:119–133. doi: 10.1093/bib/3.2.119. [DOI] [PubMed] [Google Scholar]
- 24.Boker S, et al. OpenMx: An Open Source Extended Structural Equation Modeling Framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Posthuma D, et al. Theory and practice in quantitative genetics. Twin research: the official journal of the International Society for Twin Studies. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- 26.Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of akaike information criterion and bayesian approaches over likelihood ratio tests. Systematic biology. 2004;53:793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- 27.Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S, Wardle J. Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. The American journal of clinical nutrition. 2010;91:1172–1179. doi: 10.3945/ajcn.2009.28868. [DOI] [PubMed] [Google Scholar]
- 28.Smith AD, et al. Food fussiness and food neophobia share a common etiology in early childhood. Journal of child psychology and psychiatry, and allied disciplines. 2016 doi: 10.1111/jcpp.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fildes A, van Jaarsveld CHM, Cooke L, Wardle J, Llewellyn CH. Common genetic architecture underlying young children’s food fussiness and liking for vegetables and fruit. American Journal of Clinical Nutrition. 2016;103:1099–1104. doi: 10.3945/ajcn.115.122945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. International journal of obesity. 2008;32:1468–1473. doi: 10.1038/ijo.2008.127. [DOI] [PubMed] [Google Scholar]
- 31.Story M, Neumark-Sztainer D, French S. Individual and environmental influences on adolescent eating behaviors. J Am Diet Assoc. 2002;102:S40–51. doi: 10.1016/S0002-8223(02)90421-9. [DOI] [PubMed] [Google Scholar]
- 32.Vaughn AE, et al. Fundamental constructs in food parenting practices: a content map to guide future research. Nutr Rev. 2016;74:98–117. doi: 10.1093/nutrit/nuv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinsbekk, S., Barker, D., Llewellyn, C., Fildes, A. & Wichstrom, L. Emotional Feeding and Emotional Eating: Reciprocal Processes and the Influence of Negative Affectivity. Child Dev (in press). [DOI] [PubMed]
- 34.Blissett J, Haycraft E, Farrow C. Inducing preschool children’s emotional eating: relations with parental feeding practices. The American journal of clinical nutrition. 2010;92:359–365. doi: 10.3945/ajcn.2010.29375. [DOI] [PubMed] [Google Scholar]
- 35.Tan CC, Holub SC. Emotion Regulation Feeding Practices Link Parents’ Emotional Eating to Children’s Emotional Eating: A Moderated Mediation Study. J Pediatr Psychol. 2015 doi: 10.1093/jpepsy/jsv015. [DOI] [PubMed] [Google Scholar]
- 36.Haycraft E, Blissett J. Predictors of Paternal and Maternal Controlling Feeding Practices with 2- to 5-year-old Children. J Nutr Educ Behav. 2012;44:390–397. doi: 10.1016/j.jneb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Farrow CV, Haycraft E, Blissett JM. Teaching our children when to eat: how parental feeding practices inform the development of emotional eating-a longitudinal experimental design. American Journal of Clinical Nutrition. 2015;101:908–913. doi: 10.3945/ajcn.114.103713. [DOI] [PubMed] [Google Scholar]
- 38.Webber L, Cooke L, Hill C, Wardle J. Child adiposity and maternal feeding practices a longitudinal analysis. American Journal of Clinical Nutrition. 2010;92:1423–1428. doi: 10.3945/ajcn.2010.30112. [DOI] [PubMed] [Google Scholar]
- 39.Ventura, A. K. & Birch, L. L. Does parenting affect children’s eating and weight status? Int J Behav Nutr Phy5, doi:10.1186/1479-5868-5-15 (2008). [DOI] [PMC free article] [PubMed]
- 40.Haycraft E, Blissett J. The role of parents’ romantic relationship warmth and hostility in child feeding practices and children’s eating behaviours. Maternal and Child Nutrition. 2010;6:266–274. doi: 10.1111/j.1740-8709.2009.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim YR, Lim SJ, Treasure J. Different Patterns of Emotional Eating and Visuospatial Deficits Whereas Shared Risk Factors Related with Social Support between Anorexia Nervosa and Bulimia Nervosa. Psychiat Invest. 2011;8:9–14. doi: 10.4306/pi.2011.8.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sampson D, Muscat R, Phillips G, Willner P. Decreased reactivity to sweetness following chronic exposure to mild unpredictable stress or acute administration of pimozide. Neuroscience and biobehavioral reviews. 1992;16:519–524. doi: 10.1016/S0149-7634(05)80193-9. [DOI] [PubMed] [Google Scholar]
- 43.Krebs H, Macht M, Weyers P, Weijers HG, Janke W. Effects of stressful noise on eating and non-eating behavior in rats. Appetite. 1996;26:193–202. doi: 10.1006/appe.1996.0015. [DOI] [PubMed] [Google Scholar]
- 44.Macht M, Krebs H, Weyers P, Janke W. Effect of stress on feeding behavior in rats: individual differences. Pers Indiv Differ. 2001;30:463–469. doi: 10.1016/S0191-8869(00)00037-4. [DOI] [Google Scholar]
- 45.Steinsbekk S, Belsky J, Wichstrom L. Parental Feeding and Child Eating: An Investigation of Reciprocal Effects. Child Dev. 2016;87:1538–1549. doi: 10.1111/cdev.12546. [DOI] [PubMed] [Google Scholar]
- 46.Keskitalo K, et al. The Three-Factor Eating Questionnaire, body mass index, and responses to sweet and salty fatty foods: a twin study of genetic and environmental associations. The American journal of clinical nutrition. 2008;88:263–271. doi: 10.1093/ajcn/88.2.263. [DOI] [PubMed] [Google Scholar]
- 47.Sung J, Lee K, Song YM, Lee MK, Lee DH. Heritability of Eating Behavior Assessed Using the DEBQ (Dutch Eating Behavior Questionnaire) and Weight-related Traits: The Healthy Twin Study. Obesity. 2010;18:1000–1005. doi: 10.1038/oby.2009.389. [DOI] [PubMed] [Google Scholar]
- 48.Tholin S, Rasmussen F, Tynelius P, Karlsson J. Genetic and environmental influences on eating behavior: the Swedish Young Male Twins Study. The American journal of clinical nutrition. 2005;81:564–569. doi: 10.1093/ajcn/81.3.564. [DOI] [PubMed] [Google Scholar]
- 49.Haworth CM, et al. The heritability of general cognitive ability increases linearly from childhood to young adulthood. Molecular psychiatry. 2010;15:1112–1120. doi: 10.1038/mp.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haworth CM, et al. Increasing heritability of BMI and stronger associations with the FTO gene over childhood. Obesity. 2008;16:2663–2668. doi: 10.1038/oby.2008.434. [DOI] [PubMed] [Google Scholar]
- 51.Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2007;10:423–433. doi: 10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- 52.Hunot C, et al. Appetitive traits and relationships with BMI in adults: Development of the Adult Eating Behaviour Questionnaire. Appetite. 2016;105:356–363. doi: 10.1016/j.appet.2016.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pearson CM, Riley EN, Davis HA, Smith GT. Research Review: Two pathways toward impulsive action: an integrative risk model for bulimic behavior in youth. Journal of Child Psychology and Psychiatry. 2014;55:852–864. doi: 10.1111/jcpp.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stice E. A prospective test of the dual-pathway model of bulimic pathology: Mediating effects of dieting and negative affect. J Abnorm Psychol. 2001;110:124–135. doi: 10.1037/0021-843X.110.1.124. [DOI] [PubMed] [Google Scholar]
- 55.Stice E, Presnell K, Spangler D. Risk factors for binge eating onset in adolescent girls: A 2-year prospective investigation. Health Psychology. 2002;21:131–138. doi: 10.1037/0278-6133.21.2.131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.