Abstract
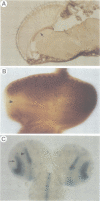
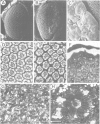
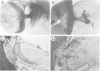
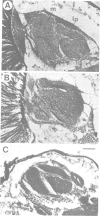
Mutations in the Drosophila gene giant lens (gil) affect ommatidial development, photoreceptor axon guidance and optic lobe development. We have cloned the gene using an enhancer trap line. Molecular analysis of gil suggests that it encodes a secreted protein with an epidermal-growth-factor-like motif. We have generated mutations at the gil locus by imprecise excision of the enhancer trap P-element. In the absence of gil, additional photoreceptors develop at the expense of pigment cells, suggesting an involvement of gil in cell determination during eye development. In addition, gil mutants show drastic effects on photoreceptor axon guidance and optic lobe development. In wildtype flies, photoreceptor axons grow from the eye disc through the optic stalk into the larval brain hemisphere, where retinal innervation is required for the normal development of the lamina and distal medulla. The projection pattern of these axons in the developing lamina and medulla is highly regular and reproducible. In gil, photoreceptor axons enter the larval brain but fail to establish proper connections in the lamina or medulla. We propose that gil encodes a new type of signalling molecule involved in the process of axon pathfinding and cell determination in the visual system of Drosophila.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Brayton K., Dixon J. E. Precursors to regulatory peptides: their proteolytic processing. Experientia. 1987 Jul 15;43(7):784–790. doi: 10.1007/BF01945356. [DOI] [PubMed] [Google Scholar]
- Baker N. E., Mlodzik M., Rubin G. M. Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science. 1990 Dec 7;250(4986):1370–1377. doi: 10.1126/science.2175046. [DOI] [PubMed] [Google Scholar]
- Banerjee U., Zipursky S. L. The role of cell-cell interaction in the development of the Drosophila visual system. Neuron. 1990 Feb;4(2):177–187. doi: 10.1016/0896-6273(90)90093-u. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Fong N. M., Stempien M. M., Wormsted M. A., Caput D., Ku L. L., Urdea M. S., Rall L. B., Sanchez-Pescador R. Human epidermal growth factor precursor: cDNA sequence, expression in vitro and gene organization. Nucleic Acids Res. 1986 Nov 11;14(21):8427–8446. doi: 10.1093/nar/14.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellen H. J., O'Kane C. J., Wilson C., Grossniklaus U., Pearson R. K., Gehring W. J. P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev. 1989 Sep;3(9):1288–1300. doi: 10.1101/gad.3.9.1288. [DOI] [PubMed] [Google Scholar]
- Bier E., Vaessin H., Shepherd S., Lee K., McCall K., Barbel S., Ackerman L., Carretto R., Uemura T., Grell E. Searching for pattern and mutation in the Drosophila genome with a P-lacZ vector. Genes Dev. 1989 Sep;3(9):1273–1287. doi: 10.1101/gad.3.9.1273. [DOI] [PubMed] [Google Scholar]
- Brunner A., Wolf R., Pflugfelder G. O., Poeck B., Heisenberg M. Mutations in the proximal region of the optomotor-blind locus of Drosophila melanogaster reveal a gradient of neuroanatomical and behavioral phenotypes. J Neurogenet. 1992 Feb;8(1):43–55. doi: 10.3109/01677069209167271. [DOI] [PubMed] [Google Scholar]
- Cagan R. L., Ready D. F. Notch is required for successive cell decisions in the developing Drosophila retina. Genes Dev. 1989 Aug;3(8):1099–1112. doi: 10.1101/gad.3.8.1099. [DOI] [PubMed] [Google Scholar]
- Cavener D. R. Comparison of the consensus sequence flanking translational start sites in Drosophila and vertebrates. Nucleic Acids Res. 1987 Feb 25;15(4):1353–1361. doi: 10.1093/nar/15.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach K. F. Neural cell types surviving congenital sensory deprivation in the optic lobes of Drosophila melanogaster. Dev Biol. 1983 Jan;95(1):1–18. doi: 10.1016/0012-1606(83)90002-7. [DOI] [PubMed] [Google Scholar]
- Fischbach K. F., Technau G. Cell degeneration in the developing optic lobes of the sine oculis and small-optic-lobes mutants of Drosophila melanogaster. Dev Biol. 1984 Jul;104(1):219–239. doi: 10.1016/0012-1606(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Fleming R. J., Scottgale T. N., Diederich R. J., Artavanis-Tsakonas S. The gene Serrate encodes a putative EGF-like transmembrane protein essential for proper ectodermal development in Drosophila melanogaster. Genes Dev. 1990 Dec;4(12A):2188–2201. doi: 10.1101/gad.4.12a.2188. [DOI] [PubMed] [Google Scholar]
- Haefliger J. A., Tschopp J., Vial N., Jenne D. E. Complete primary structure and functional characterization of the sixth component of the human complement system. Identification of the C5b-binding domain in complement C6. J Biol Chem. 1989 Oct 25;264(30):18041–18051. [PubMed] [Google Scholar]
- Hafen E., Basler K., Edstroem J. E., Rubin G. M. Sevenless, a cell-specific homeotic gene of Drosophila, encodes a putative transmembrane receptor with a tyrosine kinase domain. Science. 1987 Apr 3;236(4797):55–63. doi: 10.1126/science.2882603. [DOI] [PubMed] [Google Scholar]
- Hafen E. Patterning by cell recruitment in the Drosophila eye. Curr Opin Genet Dev. 1991 Aug;1(2):268–274. doi: 10.1016/s0959-437x(05)80081-4. [DOI] [PubMed] [Google Scholar]
- Kopczynski C. C., Alton A. K., Fechtel K., Kooh P. J., Muskavitch M. A. Delta, a Drosophila neurogenic gene, is transcriptionally complex and encodes a protein related to blood coagulation factors and epidermal growth factor of vertebrates. Genes Dev. 1988 Dec;2(12B):1723–1735. doi: 10.1101/gad.2.12b.1723. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lawrence P. A., Green S. M. Cell lineage in the developing retina of Drosophila. Dev Biol. 1979 Jul;71(1):142–152. doi: 10.1016/0012-1606(79)90088-5. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Baker N. E., Rubin G. M. Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev. 1990 Nov;4(11):1848–1861. doi: 10.1101/gad.4.11.1848. [DOI] [PubMed] [Google Scholar]
- Mlodzik M., Hiromi Y., Weber U., Goodman C. S., Rubin G. M. The Drosophila seven-up gene, a member of the steroid receptor gene superfamily, controls photoreceptor cell fates. Cell. 1990 Jan 26;60(2):211–224. doi: 10.1016/0092-8674(90)90737-y. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ready D. F. A multifaceted approach to neural development. Trends Neurosci. 1989 Mar;12(3):102–110. doi: 10.1016/0166-2236(89)90166-5. [DOI] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Renfranz P. J., Benzer S. Monoclonal antibody probes discriminate early and late mutant defects in development of the Drosophila retina. Dev Biol. 1989 Dec;136(2):411–429. doi: 10.1016/0012-1606(89)90267-4. [DOI] [PubMed] [Google Scholar]
- Robertson H. M., Preston C. R., Phillis R. W., Johnson-Schlitz D. M., Benz W. K., Engels W. R. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics. 1988 Mar;118(3):461–470. doi: 10.1093/genetics/118.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg J. M., Hartley D. A., Walther Z., Artavanis-Tsakonas S. slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988 Dec 23;55(6):1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Signal transduction and the fate of the R7 photoreceptor in Drosophila. Trends Genet. 1991 Nov-Dec;7(11-12):372–377. doi: 10.1016/0168-9525(91)90258-r. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selleck S. B., Steller H. The influence of retinal innervation on neurogenesis in the first optic ganglion of Drosophila. Neuron. 1991 Jan;6(1):83–99. doi: 10.1016/0896-6273(91)90124-i. [DOI] [PubMed] [Google Scholar]
- Simon M. A., Bowtell D. D., Dodson G. S., Laverty T. R., Rubin G. M. Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell. 1991 Nov 15;67(4):701–716. doi: 10.1016/0092-8674(91)90065-7. [DOI] [PubMed] [Google Scholar]
- Steller H., Fischbach K. F., Rubin G. M. Disconnected: a locus required for neuronal pathway formation in the visual system of Drosophila. Cell. 1987 Sep 25;50(7):1139–1153. doi: 10.1016/0092-8674(87)90180-2. [DOI] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Theres C., Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990 Jun 1;61(5):787–799. doi: 10.1016/0092-8674(90)90189-l. [DOI] [PubMed] [Google Scholar]
- Tomlinson A. Cellular interactions in the developing Drosophila eye. Development. 1988 Oct;104(2):183–193. doi: 10.1242/dev.104.2.183. [DOI] [PubMed] [Google Scholar]
- Tomlinson A., Kimmel B. E., Rubin G. M. rough, a Drosophila homeobox gene required in photoreceptors R2 and R5 for inductive interactions in the developing eye. Cell. 1988 Dec 2;55(5):771–784. doi: 10.1016/0092-8674(88)90133-x. [DOI] [PubMed] [Google Scholar]
- Vässin H., Bremer K. A., Knust E., Campos-Ortega J. A. The neurogenic gene Delta of Drosophila melanogaster is expressed in neurogenic territories and encodes a putative transmembrane protein with EGF-like repeats. EMBO J. 1987 Nov;6(11):3431–3440. doi: 10.1002/j.1460-2075.1987.tb02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wilson C., Pearson R. K., Bellen H. J., O'Kane C. J., Grossniklaus U., Gehring W. J. P-element-mediated enhancer detection: an efficient method for isolating and characterizing developmentally regulated genes in Drosophila. Genes Dev. 1989 Sep;3(9):1301–1313. doi: 10.1101/gad.3.9.1301. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Yoon J., Shortridge R. D., Bloomquist B. T., Schneuwly S., Perdew M. H., Pak W. L. Molecular characterization of Drosophila gene encoding G0 alpha subunit homolog. J Biol Chem. 1989 Nov 5;264(31):18536–18543. [PubMed] [Google Scholar]
- Zinsmaier K. E., Hofbauer A., Heimbeck G., Pflugfelder G. O., Buchner S., Buchner E. A cysteine-string protein is expressed in retina and brain of Drosophila. J Neurogenet. 1990 Nov;7(1):15–29. doi: 10.3109/01677069009084150. [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Venkatesh T. R., Teplow D. B., Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984 Jan;36(1):15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]