Abstract
Background and aims
Carotid plaque size and the mean common carotid intima-media thickness measured in plaque-free areas (PF CC-IMTmean) have been identified as predictors of vascular events (VEs), but their complementarity in risk prediction and stratification is still unresolved. The aim of this study was to evaluate the independence of carotid plaque thickness and PF CC-IMTmean in cardiovascular risk prediction and risk stratification.
Methods
The IMPROVE-study is a European cohort (n = 3703), where the thickness of the largest plaque detected in the whole carotid tree was indexed as cIMTmax. PF CC-IMTmean was also assessed. Hazard Ratios (HR) comparing the top quartiles of cIMTmax and PF CC-IMTmeanversus their respective 1–3 quartiles were calculated using Cox regression.
Results
After a 36.2-month follow-up, there were 215 VEs (125 coronary, 73 cerebral and 17 peripheral). Both cIMTmax and PF CC-IMTmean were mutually independent predictors of combined-VEs, after adjustment for center, age, sex, risk factors and pharmacological treatment [HR (95% CI) = 1.98 (1.47, 2.67) and 1.68 (1.23, 2.29), respectively]. Both variables were independent predictors of cerebrovascular events (ischemic stroke, transient ischemic attack), while only cIMTmax was an independent predictor of coronary events (myocardial infarction, sudden cardiac death, angina pectoris, angioplasty, coronary bypass grafting). In reclassification analyses, PF CC-IMTmean significantly adds to a model including both Framingham Risk Factors and cIMTmax (Integrated Discrimination Improvement; IDI = 0.009; p = 0.0001) and vice-versa (IDI = 0.02; p < 0.0001).
Conclusions
cIMTmax and PF CC-IMTmean are independent predictors of VEs, and as such, they should be used as additive rather than alternative variables in models for cardiovascular risk prediction and reclassification.
Keywords: Carotid intima-media thickness, Cardiovascular risk factors, Cardiovascular clinical research, Atherosclerosis, Prevention, Coronary artery disease
Graphical abstract
Highlights
-
•
Taken by themselves, both cIMTmax and PF CC-IMTmean are associated with the incidence of cardiovascular disease.
-
•
Complementarity of cIMTmax and PF CC-IMTmean in cardiovascular risk prediction/reclassification is still under debate.
-
•
In our study, both cIMTmax and PF CC-IMTmean were mutually independent predictors of cerebral and coronary vascular events.
-
•
In reclassification analyses, PF CC-IMTmean adds to a model including Framingham Risk Factors and cIMTmax and vice-versa.
-
•
cIMTmax and PF CC-IMTmean should be used as additive rather than alternative variables in risk prediction/reclassification.
1. Introduction
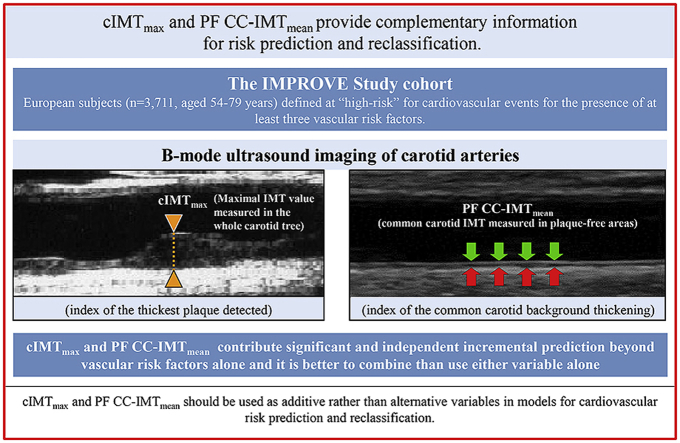
The measurement of carotid plaque thickness, rather than the mean of common carotid intima-media thickness measured in plaque-free areas (PF CC-IMTmean), is an important yet controversial issue for cardiovascular risk prediction and/or risk refinement. Both variables have been associated with vascular events (VEs), independently of conventional vascular risk factors (VRFs) [1]. However, the decision to use one or the other in models for risk prediction or risk stratification is often based on methodological issues such as accuracy and ease of measurement [2], [3], or relative power in risk prediction [4], [5], [6], [7], [8]. Carotid plaque thickness and PF CC-IMTmean are correlated, yet they differ considerably from a histological point of view and they can better be considered as distinct phenotypes [9] describing two different phenomena, being mainly due to atherosclerosis [10] and hypertrophy/hyperplasia of smooth muscle cells, respectively [2]. Several observational studies have focused on either carotid IMT (cIMT) or plaque, but few studies [5], [8], [11], [12] have examined whether carotid plaque thickness and PF CC-IMTmean can be used as additive rather than alternative variables in models for cardiovascular risk prediction and reclassification. With the aim of gaining further insight into this issue, we evaluated the independence of carotid plaque thickness (indexed in terms of cIMTmax i.e. the maximal carotid IMT detected in the whole carotid tree) and PF CC-IMTmean, in cardiovascular risk prediction and/or risk refinement in a large, multicenter, prospective cohort study of high-risk individuals [Carotid Intima–Media Thickness (IMT) and IMT–Progression as Predictors of Vascular Events in a High–Risk European Population (acronym: IMPROVE)].
2. Patients and methods
2.1. Subjects
A complete description of the IMPROVE-study design, objectives, sampling strategy and methods for clinical and haematological evaluation has been reported in the text and Online Materials of Baldassarre et al. [13], [14]. Briefly, a total of 3711 individuals (age 54–79 years) were recruited, with at least three VRFs but free of any cardio- or cerebro-VEs prior to enrolment. The participants were enrolled at 7 centers in 5 European countries: Finland (Kuopio, 2 centers), France (Paris), Italy (Milan and Perugia), The Netherlands (Groningen) and Sweden (Stockholm).
The occurrence of VEs (myocardial infarction (MI), sudden cardiac death, angina pectoris, ischemic stroke, transient ischemic attack, new diagnosis of intermittent claudication, or any surgical intervention or revascularization of coronary or peripheral arteries) was assessed at months 15 and 30 by regular visits, and at the end of follow-up (36 months in average) by phone interview. The sample size considered for this report is 3703 since the carotid walls were not properly visualized in 8 subjects.
2.2. Ultrasonographic assessment
The ultrasound procedure in the IMPROVE study has been described [13], [14]. Briefly, 7 identical scanners (Technos System, Esaote, Genoa, Italy) equipped with 5–10 Mhz linear array probes were used and the images were recorded on sVHS videotapes by trained sonographers. The cIMT was measured centrally by trained readers at the ultrasound reading center in Milan. cIMT was assessed in the entire length of the common carotid, in the carotid artery bifurcation (1 cm proximal to the flow divider) and in the internal carotid artery (1 cm immediately distal to the flow divider) of both left and right carotids. At each of these segments, the mean and maximal values of IMT were measured on the far wall from three angles (anterior, lateral and posterior) by means of a specific software (M'Ath).
In this study, we also considered the mean of common carotid IMT measured in plaque-free areas (PF CC-IMTmean), i.e. areas with a cIMTmax <1 mm. This variable is the average of all plaque-free mean IMT values obtained from the left and right CC visualized in their entire length (excluding the 1st cm) with sequential 1 cm-long probe movements according to the 3 aforementioned scan angles. The total number of segments averaged for assessing PF CC-IMTmean ranged from 6 to 24, according to the subject's neck length and according to the number of segments with cIMTmax ≥1, which were excluded from the average calculation. The precision of cIMTmax has been reported [13]. Details on precision of PF CC-IMTmean are provided in Supplementary Data.
2.3. Ethical considerations
The Ethics Committees of all participating institutions approved the IMPROVE study, which complied with the Declaration of Helsinki. Written informed consent was obtained from all subjects.
2.4. Statistical analysis
Cox models were used to estimate crude and adjusted hazard ratios (HRs) and to compute adjusted Kaplan-Meier survival curves over 36 months of follow-up. The HRs comparing the top quartiles of cIMTmax (2.5 mm) and PF CC-IMTmean (0.76 mm) to their respective 1–3 quartiles were calculated. We decided a priori to use these cut offs because the ASE consensus statement described PF CC-IMTmean values ≥ 75th percentile as indicative of increased cardiovascular risk [15]. Regarding plaques, we decided to use cIMTmax values ≥ 75th percentile because most large longitudinal studies showed that the risk is mainly increased in the top quartiles or quintiles [16]. As a sensitivity analysis, we also tested models where cIMTmax and PF CC-IMTmean were included as continuous variables. Cox models were stratified for center (Model-1), then further adjusted for age and sex (Model-2) and then for risk factors and pharmacological treatment (Model-3). Departure from the proportional hazard assumption was assessed by the Kolmogorov-type supremum test computed on 1000 Monte-Carlo simulations. Area under the ROC curves (AUC), Integrated Discrimination Improvement (IDI), and Net Reclassification Improvement (NRI) were used for assessing the potential of the PF CC-IMTmean in improving risk prediction based on cIMTmax and risk factors included in the Framingham Risk Score (age, sex, total cholesterol, HDL-cholesterol, systolic blood pressure, diabetes, current smoking and antihypertensive treatments) and vice-versa. As in our previous study [14], we included risk factors contained in the Framingham Risk Score as separated variables in all models, instead of the Framingham Risk Score, as this algorithm is not specifically calibrated for a European population. To assess the impact of cIMTmax and PF CC-IMTmean on the risk reclassification of subjects located in the so called “gray-zone” of risk prediction, we calculated the clinical NRI, i.e. the NRI only considering subjects at intermediate-risk (10% < Framingham Risk Score <20%). Positive and negative predictive values (PPV and NPV) were also computed.
All statistical tests were two-sided at a level of significance of 0.05. All analyses were performed using the SAS statistical package v. 9.4 (SAS Institute Inc., Cary, NC, USA). Reclassification statistics were assessed with the SAS macros published by Cook and Ridker [17].
3. Results
The baseline characteristics of IMPROVE study participants were described [13], [14]. Briefly, the mean age was 64.2 years and 47.9% of subjects were males. The participants were followed-up for a median of 36.2 months (interquartile range: 35.8 to 37.4) and 215 suffered a first VE, (incidence: 19.9/1000 person-years). Among these, 125 had a coronary event [34 had a MI (7 fatal), 3 suffered sudden cardiac death, 49 experienced symptoms of angina pectoris, 26 underwent angioplasty and 13 coronary bypass grafting]; 73 had a cerebrovascular event [32 had an ischemic stroke (0 fatal), 41 had a transient ischemic attack], and 17 had a peripheral VE (4 subjects underwent revascularization due to peripheral artery disease and 13 had a new diagnosis of intermittent claudication). Eighty participants had more than one VE during follow-up, but only the first event was used for the analysis of the primary combined endpoint.
3.1. cIMTmax, PF CC-IMTmean and risk of combined VEs
In Cox regression models with mutual adjustment for cIMTmax and PF CC-IMTmean, both variables (top quartiles vs. quartiles 1–3) were significantly and independently associated with the risk of combined-VEs, after stratifying for center (Table 1, Model-1), as well as with further adjustment for age and sex (Model-2) and for risk factors and pharmacological treatment (Model-3). These results were virtually unchanged when cIMTmax and PF CC-IMTmean were analysed as continuous variables (data not shown). For both cIMTmax and PF CC-IMTmean, no significant departure from the assumption of proportionality of the hazards was observed (p = 0.42 and p = 0.46, respectively).
Table 1.
Hazard Ratios (95% CI) and p values of combined, cerebro- and cardio-vascular endpoints comparing top quartiles of both cIMTmax and PF CC-IMTmeanvs. quartiles 1–3.
| Model-1 | Model-2 | Model-3 | |
|---|---|---|---|
| Combined endpoints (n = 215)a | |||
| cIMTmax | 2.05 (1.55, 2.72); <0.0001 | 1.88 (1.41, 2.51); <0.0001 | 1.98 (1.47, 2.67); <0.0001 |
| PF CC-IMTmean | 1.89 (1.41, 2.53); <0.0001 | 1.69 (1.26, 2.27); 0.0005 | 1.68 (1.23, 2.29); 0.0011 |
| Cerebrovascular endpoints (n = 73) (Ischemic stroke, transient ischemic attack)a | |||
| cIMTmax | 2.7 (1.67, 4.36); 0.0001 | 2.55 (1.57, 4.14); 0.0002 | 2.76 (1.66, 4.6); 0.0001 |
| PF CC-IMTmean | 2.25 (1.38, 3.68); 0.0012 | 2.07 (1.26, 3.4); 0.004 | 2.13 (1.26, 3.61); 0.005 |
| Coronary endpoints (n = 125) (myocardial infarction, sudden cardiac death, angina pectoris, angioplasty, coronary bypass grafting)a | |||
| cIMTmax | 1.69 (1.16, 2.47); 0.006 | 1.51 (1.03, 2.21); 0.036 | 1.58 (1.06, 2.37); 0.025 |
| PF CC-IMTmean | 1.70 (1.15, 2.5); 0.007 | 1.47 (0.99, 2.17); 0.056 | 1.49 (0.99, 2.26); 0.057 |
Model-1: cIMTmax, and PF CC-IMTmean stratified by center; Model-2: as model-1 plus age and sex; Model-3: as model-2 plus Framingham risk factors, family history of diabetes, family history of hypertension, pack-years, and pharmacological treatments (statins, beta-blockers, ACE-inhibitors, diuretics and calcium-antagonists).
Among the 215 combined endpoints, 17 were peripheral VEs (4 subjects underwent revascularization due to peripheral artery disease and 13 had a new diagnosis of intermittent claudication) and, as such, included neither in the analysis on cerebrovascular endpoints nor in the one on coronary endpoints.
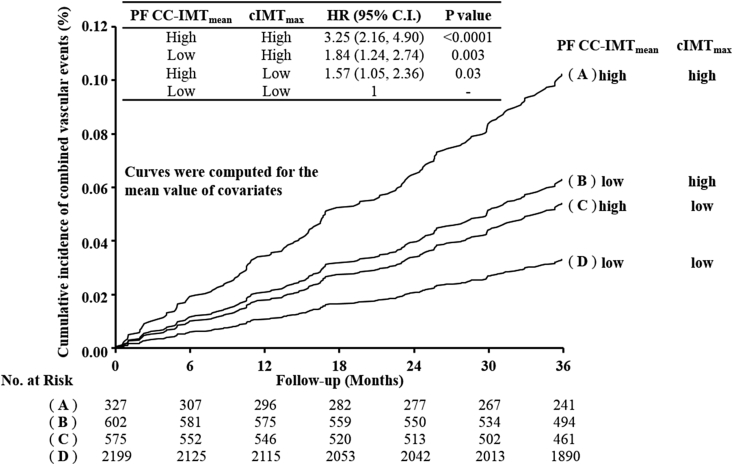
Fig. 1 shows the Kaplan-Meier incidence curves adjusted for Model-3 covariates and stratified into four groups according to cIMTmax and PF CC-IMTmean above or below their respective top quartiles (2.5 mm and 0.76 mm). The independent effect of the two variables is clearly shown.
Fig. 1.
Framingham risk factors-adjusted Kaplan-Meier incidence curves.
The study population was stratified according to cIMTmax and PF-CC-IMTmean values above or below their respective 75th percentiles (2.5 and 0.76 mm), respectively. Curves were computed for the mean value of each covariate used in Table 1, Model-3 (i.e. center, age, sex, Framingham risk factors, family history of diabetes, family history of hypertension, pack-years and pharmacological treatments (statins, beta-blockers, ACE-inhibitors, diuretics and calcium-antagonists)). IMT, intima-media thickness; PF CC-IMTmean, mean common carotid IMT measured in plaque-free areas; cIMTmax, measure of the thickest plaque detected in the whole carotid tree.
Table 1 also shows that both cIMTmax and PF CC-IMTmean were independent predictors of cerebrovascular and coronary events in all models.
Supplemental Table 1 shows the same analyses as Table 1 restricted to “hard clinical events”. While no significant association with hard coronary events (myocardial infarction, sudden cardiac death) was detected, the measures of cIMTmax and PF CC-IMTmean remained significantly and independently associated with hard cerebrovascular events (ischemic strokes), even after adjusting for center, age, sex, Framingham risk factors (FRFs) and pharmacological treatments (Model 3).
3.2. Incremental predictive value of cIMTmax and PF CC-IMTmean in reclassification analysis
Table 2 shows the reclassification statistics for the combined endpoints. In the first line, AUC, NRI and IDI values were obtained after adding PF CC-IMTmean to a reference model that included FRFs and cIMTmax. In the second line, AUC, NRI and IDI values were obtained after adding cIMTmax to a reference model that included FRFs and PF CC-IMTmean. cIMTmax appears to improve the classification of cases and controls more effectively than PF CC-IMTmean (NRI: 8.2% vs. 2.4% and IDI: 0.02 vs. 0.009).
Table 2.
Reclassification statistics for PF CC-IMTmean above or below top quartile as compared to classification based on Framingham Risk Factors (FRFs) and cIMTmax and vice-versa in risk models with combined vascular endpoints.
| New model | Reference model | AUC ref. model |
AUC new model |
p value |
NRI (95% CI) |
p value |
IDI (99% CI) |
p value |
|---|---|---|---|---|---|---|---|---|
| FRFs + cIMTmax + PF CC-IMTmean | FRFs + cIMTmax | 0.661 | 0.671 | 0.15 | 2.4% (−3.5, 8.3) | 0.42 | 0.009 (0.003, 0.016) | 0.0004 |
| FRFs + PF CC-IMTmean + cIMTmax | FRFs + PF CC-IMTmean | 0.657 | 0.671 | 0.054 | 8.2% (0.1, 16.3) | 0.047 | 0.02 (0.010, 0.029) | <0.0001 |
When NRI and/or IDI values are positive with a p < 0.01, the new model is better than the reference model, which includes FRFs and cIMTmax and vice-versa. AUC, area under the ROC curve.
Supplemental Tables 2 and 3 and Table 3 show the estimated 10-year VE risk categories according to FRFs before and after adding cIMTmax (Supplemental Table 2), PF CC-IMTmean (Supplemental Table 3) and the combination of the two variables (Table 3). In Supplemental Table 2, the overall NRI was 10% (p = 0.02) and 32% of subjects at intermediate risk were reclassified. The addition of PF CC-IMTmean to FRFs (Supplemental Table 3) resulted in the reclassification of only 23% of subjects at intermediate risk and the overall NRI was lower (5.3%) and not statistically significant (p = 0.19). However, when both variables were added (Table 3), the overall NRI increased to 13.9% (p = 0.003) and the percentage of subjects at intermediate-risk reclassified reached approximately 41%. Among these subjects, 30 cases and 425 non-cases were correctly reclassified, and 6 cases and 239 non-cases were wrongly reclassified, yielding a clinical NRI of 45.1%, compared to 29.6% using only cIMTmax and 27.5% using only PF CC-IMTmean.
Table 3.
Risk reclassification comparing the extrapolated 10-years risk according to Framingham Risk factors (FRFs) before and after adding both cIMTmax and PF CC-IMTmean in the prediction of combined vascular events.
| 10-year risk categories for FRFs | 10-year risk categories for FRFs plus cIMTmax plus PF CC-IMTmean |
|||
|---|---|---|---|---|
| <10% | 10–20% | >20% | N (%) reclassified | |
| <10% | ||||
| N = 678 (20%) | 556 (82%) | 113 (16.7%) | 9 (1.3%) | 122 (18%) |
| Observed-risk (95% C.I.) | 6.4 (3.2, 10.8) | 11.6 (3.2, 25.4) | No events | |
| 10–20% | ||||
| N = 1715 (52%) | 431 (25.1%) | 1015 (59.2%) | 269 (15.7%) | 700 (41%) |
| Observed-risk (95% C.I.) | 4.5 (1.7, 8.8) | 11.1 (7.7, 15) | 38 (25.6, 52.7) | |
| >20% | ||||
| N = 920 (28%) | 0 (0%) | 322 (35%) | 598 (65%) | 322 (35%) |
| Observed-risk (95% C.I.) | 24.2 (15.5, 34.7) | 36.7 (28.4, 46) | ||
| NRI: 13.9%; p = 0.003a | ||||
| Clinical NRI 45.1%; p < 0.0001b | ||||
NRI: 11.0% (3.1, 18.9); p = 0.007; when statins are added to FRFs.
Clinical NRI: 26.7% (13.0, 40.3); p = 0.0001; when statins are added to FRFs.
PPVs and NPVs for those with a high Framingham risk score (FRS>20) as well as for top quartile values of PF CC-IMTmean, cIMTmax or both, assessed considering combined-, coronary- or cerebrovascular events, are shown in Supplementary Fig. 1. As expected because of the low incidence of VEs (19.9/1000 person-years), the PPVs were rather low, (always <21%). However, even the PPVs of the FRS, the most widely accepted predictor of VEs, were about half (9.5%, 3.6%, and 5.9% for combined-, coronary- or cerebrovascular events, respectively) of those obtained with the combination of PF CC-IMTmean and cIMTmax (18.5%, 7.9%, and 10.9%). The best PPVs were obtained when all three variables (FRS, PF CC-IMTmean and cIMTmax) were in the high risk categories.
4. Discussion
This study shows that cIMTmax (an index of the thickest plaque detected in the whole carotid tree) and PF CC-IMTmean (an index of the common carotid background thickening) are both independent predictors of VEs, and that they independently add to risk reclassification in intermediate risk subjects. It is well known that, taken by themselves, carotid IMT and the presence/thickness of carotid plaques are both prognostic predictors of CV events, as reported by Naqvi and collaborators [1] in a “state-of-the-art” paper that examined many large cohort studies. Several studies have also investigated which of these two variables is the strongest predictor of VEs. Several meta-analyses [6], [7] have unequivocally reported that plaques are more accurate in predicting VEs than CC-IMT. In addition, studies evaluating whether carotid ultrasonographic measurements provide additional prognostic information over and above VRFs have been strongly positive when based on carotid plaques, and/or on cIMT variables incorporating plaques in their measurements [5], [8], [18], [19], [20], [21], [22], [23], and weaker or even negative when based on cIMT measured in plaque-free areas [5], [8], [16], [20], [21], [23], [24], [25], [26]. Hence, it is quite clear and widely accepted that if one must choose between CC-IMT measured in plaque-free areas or plaques (presence or thickness) for risk prediction and/or reclassification, the latter is the best choice.
It is important to emphasise that, instead of assessing whether plaques or PF CC-IMTmean are good and/or equipotent representations of the atherosclerotic process, the present study focuses on whether the two ultrasonographic measures represent complementary prognostic information when used together. To date, the potential complementarity of plaque and cIMT in risk prediction and reclassification has been addressed in only four large prospective cohort studies [5], [8], [11], [12], but with conflicting results. While Plichart [5] and Gardin [12] showed that adding plaques to cIMT does not result in a statistically significant improvement in risk prediction, Nambi [11] and Gepner [8], in agreement with our data, found that the prediction of coronary artery disease improves when cIMT and plaques are combined, compared with each measurement alone.
Some methodological differences in plaque definition and targets/modality of carotid IMT measurements between our study and the studies mentioned above should be mentioned. In the study of Plichart [5] and Gepner [8], plaques were defined as localized echo-structures for which the wall thickening was at least 50% greater than surrounding vessel walls. Thus, even a lesion with a thickness <1 mm was considered as “plaque” if the thickness of surrounding walls was <0.5 mm. By contrast, in our study, we used as cut-off the top quartile of cIMTmax (2.5 mm), so only real atherosclerotic plaques were considered. The other two studies [11], [12] evaluated the utility of adding a measure of plaque burden to cIMT variables which neither focused strictly on the common carotid artery nor on plaque-free areas. For example, the cIMT variable used in Gardin's analysis [12] was the mean of maximum IMT measurements of several carotid segments (which we define as IMTmean-max), a variable whose values are directly affected by the presence/absence of plaques. A similar comparison previously performed in the IMPROVE cohort [14] produced similar results by showing that the presence/absence of plaque did not add to reclassification when used on top of ultrasonographic variables which incorporate plaques, yet added to reclassification when combined with variables measured in plaque-free areas. Moreover, our data also show that the NRI and IDI, provided by the combination of the two variables used on top of FRFs, are not inferior to those obtained by using IMTmean-max on top of FRFs (Supplemental Table 4). These data agree with another study of Nambi et al. [27], who showed that the evaluation of the carotid artery for plaque presence and measurement of CC-IMT (which is easier and more precise than considering IMTmean or IMTmean-max in the whole carotid tree) provide a good alternative to the measurement of cIMT in all segments of the carotid tree for risk prediction and reclassification.
In this study, we, for three reasons, decided a priori to analyse the complementarity of cIMTmax and PF CC-IMTmean: (1) literature indications according to published data [15], [16], (2) these are the two variables most frequently used in clinical settings, and (3) there is evidence that, when taken by themselves, measurements of both variables can be performed in a reproducible way in the clinical setting [28], [29].
Our results distinctly support the concept that these two measures are complementary in risk prediction. Indeed, at the end of the follow-up period, FRF-adjusted Kaplan Meier curves (Fig. 1) shows a substantial increase of event risk in the stratum where both cIMTmax and PF CC-IMTmean indicate the presence of subclinical disease, compared with the strata where only one of the two variables were in the top quartile range.
When Cox analyses were restricted to cerebrovascular or coronary endpoints (regardless of whether “hard” or not), the strength of association between top quartile values and risk of disease was always greater with cerebrovascular than with coronary endpoints, and this was true even after the analyses were adjusted for center, pharmacological treatments and FRFs. A potential explanation is that FRFs are predominantly a tool for prediction of coronary events [30], whereas cerebrovascular events are related to a broader array of causes [31], including embolism from cardiac arrhythmias and/or valvular disease or hypertension giving rise to small vessel disease [32]. Another possible explanation is that the presence of atherosclerosis in the carotid arteries is both a marker and a cause of cerebrovascular events, whereas it is merely a marker of coronary events.
The complementarity of cIMTmax and PF CC-IMTmean is confirmed by the reclassification analysis, particularly in the intermediate-risk category. With such analysis, several authors have reported that the improvement of risk stratification over traditional VRFs provided by PF CC-IMTmean alone [5], [20], [21], [24], [25] is less consistent than that provided by plaques alone [5], [18], [19], [20], [21], [22]. As well as confirming this finding (Supplemental Tables 2 and 3), we show here a substantial improvement of risk stratification over FRFs when both cIMTmax and PF CC-IMTmean are used (Table 3), with a 3.9% (13.9% minus 10%) increase of NRI and a 15.5% (45.1% minus 29.6%) increase of clinical NRI when compared with the model including FRFs and cIMTmax (Supplemental Table 2). Specifically, Table 3 shows that the observed risk (38%; 95% CI 25.6, 52.7) of individuals reclassified to a higher risk category was actually much higher than the threshold of 20% estimated by FRFs only, and that the observed risk of individuals reclassified to a lower risk category was actually much lower (4.5%; 95% CI 1.7, 8.8) than the original 10–20% risk estimated by FRFs. By contrast, Table 3 shows that reclassification of subjects originally classified by FRFs at low or at high risk has to be viewed as inappropriate. For example, individuals who moved from the high-to intermediate-risk category had an observed risk of 24.2% (95% CI 15.5, 34.7), i.e. a risk greater than the threshold of 20%.
Despite this, at least in the intermediate-risk category, the benefits gained from the improvement in risk classification seem to easily offset the negligible additional costs required for measuring not only cIMTmax but also CC-IMT in plaque-free areas. Supporting the results obtained with Cox and reclassification analyses, when the two ultrasonographic variables were both in the top quartile, the improvements over the best performing single variable were consistent (+36%, +27% and +40% for composite, cerebrovascular and coronary-endpoints, respectively). Of note, the PPVs considering the single ultrasonographic variables were higher than the PPV of the FRS>20 and the addition of FRS>20 to the test with both the ultrasonographic variables in the top quartile resulted in a minor PPV improvement (ranging from 6 to 11%).
Another evidence supporting measurement of PF CC-IMTmean comes from 1) studies showing that the incidence of stroke [33] and coronary events [34] is related to cIMT even in the absence of plaques, 2) case-control studies showing that it is preferable to combine carotid IMT measurement with plaque assessment rather than using either measurement alone as screening tests for CHD [35], and 3) studies showing that the associations between cIMT and stroke remained significant even after adjusting for the presence of carotid plaques [33]. Moreover, a meta-analysis including eight relevant studies with cIMT assessment showed that for each 0.10 mm increase in CC-IMT, the estimated incidence of MI increases by 5% (from 12 to 17%) [36], thus suggesting that, even when measured in plaque-free areas, CC-IMT measurements still contain additional information for risk prediction regardless of the presence or absence of atherosclerotic plaques.
The complementary prognostic value of cIMTmax and PF CC-IMTmean has scientific support also from a biological/pathophysiological perspective. Carotid IMTmax is a plaque marker [23] and reflects a focal phenomenon mainly related to atherosclerotic processes such as inflammation, oxidation, endothelial dysfunction, foam cell proliferation and/or thrombosis [10], [37]. PF CC-IMTmean, instead, mainly reflects diffuse, non-atherosclerotic, adaptive changes to increased shear stress mediated by aging [37] or hypertension [2], [38]. In addition, variants in genes involved in pathways leading to atherosclerosis (e.g. inflammation, oxidative stress, and diabetes) were differentially associated with the two variables [9], [39], [40], [41], [42], [43]. Taken together, these pieces of evidences support the concept that, even if the processes underlying cIMTmax and PF CC-IMTmean formation may share some common mechanisms for initiation and progression [9], [37], [44], the two phenotypes represent biologically distinct aspects - or stages - of atherosclerosis [45]. Their overlap is only partial [37] and, consequently, they have different, independent and complementary prognostic value [2], [11], [19], [36], [46].
4.1. Strengths and limitations
The study has several strengths. Firstly, this is the first report evaluating the complementarity of cIMTmax and PF CC-IMTmean, in terms of prediction and reclassification, in European subjects at high risk of cardiovascular disease. The second strength is the tight control of the methodology for carotid image acquisition and measurement of ultrasonographic variables. Thirdly, all sonographers involved in the study were trained and certified, and all scans were read blindly in the same reading center. Other advantages are the large sample size and the tight standardizations of all methods across all recruitment units. There are also potential limitations: firstly, extrapolation of the findings to the general European population or to patients with fewer than 3 VRFs should be done with caution. However, the HRs observed are similar to those reported in other large population studies [1]. Secondly, the low number of VEs restricted the precision of estimates especially in subgroup analyses (coronary and cerebrovascular events). Thirdly, a further stratification according to number of plaques (i.e. number of segments with a cIMTmax>1 mm), as recently suggested [35], was not considered because of the limited number of VEs, and because almost all subjects (92.1%) in our “high risk” population have more than two plaques. However, repeating the analysis shown in Table 1 (Model 3) after including the number of plaques among covariates did not change the results substantially (data not shown). Fourthly, the prevalence of subjects treated with statins (40%) may have affected our reclassification analyses. It should be emphasized, however, that results did not change when statins were added to the FRFs (see footnotes of Supplemental Tables 2 and 3 and Table 3) or when the analysis was limited to statin-naïve individuals. In the latter case, for example, compared with a model including FRFs only, the IDI values of models including “FRFs + cIMTmax” or “FRFs + PF CC-IMTmean” or their combination (FRFs + cIMTmax + PF CC-IMTmean) were 0.014 (95% CI 0.005, 0.024), p < 0.0001; 0.008 (95% CI 0.001, 0.015), p = 0.005; and 0.019 (95% CI 0.008, 0.030), p < 0.0001, respectively.
We are aware that, based on concerns about quality of cIMT measurements and on the results of three review/meta-analyses [47], [48], [49], the working group on the 2013 ACC/AHA Cardiovascular Risk Guidelines [50] decided to advise against measuring cIMT in routine clinical practice for risk assessment for a first cardiovascular event in the general population. Nonetheless, our study has shown that cIMTmax and PF CC-IMTmean contribute significant and independent incremental prediction beyond FRFs alone, and that it is better to combine than use either measure alone.
4.2. Conclusions
Bearing in mind the almost negligible costs required for adding measurements of PF CC-IMTmean in scans devoted to cIMTmax measurements, we conclude that a risk stratification strategy based on the concomitant measurement of cIMTmax and PF CC-IMTmean, as an adjunct to FRFs, is a rational approach for better identifying subjects who need to be treated with pharmacological and/or lifestyle intervention (diet, smoking cessation etc.).
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Financial support
This work was supported by the European Commission [Contract number: QLG1- CT- 2002- 00896 to E.T., D.B., A.H., S.E.H., R.R., U.dF., A.J.S., P.G., S.K., E.M.], Ministero della Salute Ricerca Corrente, Italy [RC 2016 Cod 2622841 BIO23 to E.T., D.B.], the Swedish Heart-Lung Foundation (20140433), the Swedish Research Council [projects 8691 to A.H. and 0593 to U.dF.], the Foundation for Strategic Research, the Stockholm County Council [project 562183 to A.H.], and the British Heart Foundation [RG2008/008 to S.E.H.]. None of the aforementioned funding organizations or sponsors has had a specific role in design or conduct of the study, collection, management, analysis, or interpretation of the data, or preparation, review, or approval of the manuscript.
Author contributions
Study conception and design: Amato, Veglia, Baldassarre.
Substantial contributions to the acquisition, analysis, or interpretation of data for the work: Amato, Veglia, Ravani, Frigerio, Sansaro, Bonomi, Tedesco, Castelnuovo, Baldassarre.
Drafting of the manuscript: Amato, Veglia, Baldassarre.
Critical revision of the manuscript for important intellectual content: Amato, Veglia, de Faire, Giral, Rauramaa, Smit, Kurl, Ravani, Frigerio, Sansaro, Bonomi, Tedesco, Castelnuovo, Mannarino, Humphries, Hamsten, Tremoli, Baldassarre.
Final approval of the manuscript submitted: Amato, Veglia, de Faire, Giral, Rauramaa, Smit, Kurl, Ravani, Frigerio, Sansaro, Bonomi, Tedesco, Castelnuovo, Mannarino, Humphries, Hamsten, Tremoli, Baldassarre.
Acknowledgements
The authors wish to thank all the members of the IMPROVE group for their time and extraordinary commitment.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.atherosclerosis.2017.05.023.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Naqvi T.Z., Lee M.S. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. 2014;7:1025–1038. doi: 10.1016/j.jcmg.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Touboul P.J., Hennerici M.G., Meairs S. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). an update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany. Cerebrovasc. Dis. 2011;2012(34):290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bots M.L., Evans G.W., Tegeler C.H., Meijer R. Carotid intima-media thickness measurements: relations with atherosclerosis, risk of cardiovascular disease and application in randomized controlled trials. Chin. Med. J. Engl. 2016;129:215–226. doi: 10.4103/0366-6999.173500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnsen S.H., Mathiesen E.B. Carotid plaque compared with intima-media thickness as a predictor of coronary and cerebrovascular disease. Curr. Cardiol. Rep. 2009;11:21–27. doi: 10.1007/s11886-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 5.Plichart M., Celermajer D.S., Zureik M. Carotid intima-media thickness in plaque-free site, carotid plaques and coronary heart disease risk prediction in older adults. the three-city study. Atherosclerosis. 2011;219:917–924. doi: 10.1016/j.atherosclerosis.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Inaba Y., Chen J.A., Bergmann S.R. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. 2012;220:128–133. doi: 10.1016/j.atherosclerosis.2011.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Spence J.D. Carotid plaque measurement is superior to IMT Invited editorial comment on: carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis-Yoichi Inaba, M.D., Jennifer A. Chen M.D., Steven R. Bergmann M.D., Ph.D. Atherosclerosis. 2012;220:34–35. doi: 10.1016/j.atherosclerosis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Gepner A.D., Young R., Delaney J.A. Comparison of coronary artery calcium presence, carotid plaque presence, and carotid intima-media thickness for cardiovascular disease prediction in the multi-ethnic study of atherosclerosis. Circ. Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rundek T., Gardener H., Della-Morte D. The relationship between carotid intima-media thickness and carotid plaque in the Northern Manhattan Study. Atherosclerosis. 2015;241:364–370. doi: 10.1016/j.atherosclerosis.2015.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spence J.D. Measurement of intima-media thickness vs. carotid plaque: uses in patient care, genetic research and evaluation of new therapies. Int. J. Stroke. 2006;1:216–221. doi: 10.1111/j.1747-4949.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 11.Nambi V., Chambless L., Folsom A.R. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk in Communities) study. J. Am. Coll. Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardin J.M., Bartz T.M., Polak J.F., O'Leary D.H., Wong N.D. What do carotid intima-media thickness and plaque add to the prediction of stroke and cardiovascular disease risk in older adults? the cardiovascular health study. J. Am. Soc. Echocardiogr. 2014;27:998–1005. doi: 10.1016/j.echo.2014.06.013. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldassarre D., Nyyssonen K., Rauramaa R. Cross-sectional analysis of baseline data to identify the major determinants of carotid intima-media thickness in a European population: the IMPROVE study. Eur. Heart J. 2010;31:614–622. doi: 10.1093/eurheartj/ehp496. [DOI] [PubMed] [Google Scholar]
- 14.Baldassarre D., Hamsten A., Veglia F. Measurements of carotid intima-media thickness and of interadventitia common carotid diameter improve prediction of cardiovascular events: results of the IMPROVE (Carotid Intima Media Thickness [IMT] and IMT-progression as predictors of vascular events in a high risk European population) study. J. Am. Coll. Cardiol. 2012;60:1489–1499. doi: 10.1016/j.jacc.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 15.Stein J.H., Korcarz C.E., Hurst R.T. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography carotid intima-media thickness task force. endorsed by the society for vascular medicine. J. Am. Soc. Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Simon A., Megnien J.L., Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2010;30:182–185. doi: 10.1161/ATVBAHA.109.196980. [DOI] [PubMed] [Google Scholar]
- 17.Cook N.R., Ridker P.M. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann. Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnsen S.H., Mathiesen E.B., Joakimsen O. Carotid atherosclerosis is a stronger predictor of myocardial infarction in women than in men: a 6-year follow-up study of 6226 persons: the Tromso Study. Stroke. 2007;38:2873–2880. doi: 10.1161/STROKEAHA.107.487264. [DOI] [PubMed] [Google Scholar]
- 19.Mathiesen E.B., Johnsen S.H., Wilsgaard T. Carotid plaque area and intima-media thickness in prediction of first-ever ischemic stroke: a 10-year follow-up of 6584 men and women: the Tromso Study. Stroke. 2011;42:972–978. doi: 10.1161/STROKEAHA.110.589754. [DOI] [PubMed] [Google Scholar]
- 20.Polak J.F., Pencina M.J., Pencina K.M. Carotid-wall intima-media thickness and cardiovascular events. N. Engl. J. Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roman M.J., Kizer J.R., Best L.G. Vascular biomarkers in the prediction of clinical cardiovascular disease: the strong heart study. Hypertension. 2012;59:29–35. doi: 10.1161/HYPERTENSIONAHA.111.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polak J.F., Szklo M., Kronmal R.A. The value of carotid artery plaque and intima-media thickness for incident cardiovascular disease: the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2013;2:e000087. doi: 10.1161/JAHA.113.000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ershova A.I., Balakhonova T.V., Meshkov A.N., Rozhkova T.A., Boytsov S.A. Ultrasound markers that describe plaques are more sensitive than mean intima-media thickness in patients with familial hypercholesterolemia. Ultrasound Med. Biol. 2012;38:417–422. doi: 10.1016/j.ultrasmedbio.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz M.W., Schaefer C., Steinmetz H., Sitzer M. Is carotid intima media thickness useful for individual prediction of cardiovascular risk? Ten-year results from the Carotid Atherosclerosis Progression Study (CAPS) Eur. Heart J. 2010;31:2041–2048. doi: 10.1093/eurheartj/ehq189. [DOI] [PubMed] [Google Scholar]
- 25.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bots M.L., Groenewegen K.A., Anderson T.J. Common carotid intima-media thickness measurements do not improve cardiovascular risk prediction in individuals with elevated blood pressure: the USE-IMT collaboration. Hypertension. 2014;63:1173–1181. doi: 10.1161/HYPERTENSIONAHA.113.02683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nambi V., Chambless L., He M. Common carotid artery intima-media thickness is as good as carotid intima-media thickness of all carotid artery segments in improving prediction of coronary heart disease risk in the Atherosclerosis Risk in Communities (ARIC) study. Eur. Heart J. 2012;33:183–190. doi: 10.1093/eurheartj/ehr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baldassarre D., Amato M., Pustina L. Measurement of carotid artery intima-media thickness in dyslipidemic patients increases the power of traditional risk factors to predict cardiovascular events. Atherosclerosis. 2007;191:403–408. doi: 10.1016/j.atherosclerosis.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Polak J.F., O'Leary D.H. Edge-detected common carotid artery intima-media thickness and incident coronary heart disease in the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. 2015;4:e001492. doi: 10.1161/JAHA.114.001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias-Smale S.E., Wieberdink R.G., Odink A.E. Burden of atherosclerosis improves the prediction of coronary heart disease but not cerebrovascular events: the Rotterdam Study. Eur. Heart J. 2011;32:2050–2058. doi: 10.1093/eurheartj/ehr125. [DOI] [PubMed] [Google Scholar]
- 31.Naghavi M., Falk E., Hecht H.S. From vulnerable plaque to vulnerable patient–Part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) task force report. Am. J. Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Adams H.P. Principles of cerebrovascular disease. McGraw-Hill Prof. 2006 ISBN 13: 9780071416535, 1–564. [Google Scholar]
- 33.Rosvall M., Janzon L., Berglund G., Engstrom G., Hedblad B. Incidence of stroke is related to carotid IMT even in the absence of plaque. Atherosclerosis. 2005;179:325–331. doi: 10.1016/j.atherosclerosis.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Rosvall M., Janzon L., Berglund G., Engstrom G., Hedblad B. Incident coronary events and case fatality in relation to common carotid intima-media thickness. J. Intern Med. 2005;257:430–437. doi: 10.1111/j.1365-2796.2005.01485.x. [DOI] [PubMed] [Google Scholar]
- 35.Xie W., Liang L., Zhao L. Combination of carotid intima-media thickness and plaque for better predicting risk of ischaemic cardiovascular events. Heart. 2011;97:1326–1331. doi: 10.1136/hrt.2011.223032. [DOI] [PubMed] [Google Scholar]
- 36.Lorenz M.W., Markus H.S., Bots M.L., Rosvall M., Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115:459–467. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 37.Finn A.V., Kolodgie F.D., Virmani R. Correlation between carotid intimal/medial thickness and atherosclerosis: a point of view from pathology. Arterioscler. Thromb. Vasc. Biol. 2010;30:177–181. doi: 10.1161/ATVBAHA.108.173609. [DOI] [PubMed] [Google Scholar]
- 38.Spence J.D. Technology Insight: ultrasound measurement of carotid plaque–patient management, genetic research, and therapy evaluation. Nat. Clin. Pract. Neurol. 2006;2:611–619. doi: 10.1038/ncpneuro0324. [DOI] [PubMed] [Google Scholar]
- 39.Dong C., Della-Morte D., Wang L. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid plaque. PLoS One. 2011;6:e27157. doi: 10.1371/journal.pone.0027157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Della-Morte D., Dong C., Bartels S. Association of the sirtuin and mitochondrial uncoupling protein genes with carotid intima-media thickness. Transl. Res. 2012;160:389–390. doi: 10.1016/j.trsl.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacco R.L., Blanton S.H., Slifer S. Heritability and linkage analysis for carotid intima-media thickness: the family study of stroke risk and carotid atherosclerosis. Stroke. 2009;40:2307–2312. doi: 10.1161/STROKEAHA.109.554121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., Beecham A., Zhuo D. Fine mapping study reveals novel candidate genes for carotid intima-media thickness in dominican republican families. Circ. Cardiovasc Genet. 2012;5:234–241. doi: 10.1161/CIRCGENETICS.111.961763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gardener H., Beecham A., Cabral D. Carotid plaque and candidate genes related to inflammation and endothelial function in Hispanics from Northern Manhattan. Stroke. 2011;42:889–896. doi: 10.1161/STROKEAHA.110.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bis J.C., Kavousi M., Franceschini N. Meta-analysis of genome-wide association studies from the CHARGE consortium identifies common variants associated with carotid intima media thickness and plaque. Nat. Genet. 2011;43:940–947. doi: 10.1038/ng.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spence J.D., Hegele R.A. Noninvasive phenotypes of atherosclerosis: similar windows but different views. Stroke. 2004;35:649–653. doi: 10.1161/01.STR.0000116103.19029.DB. [DOI] [PubMed] [Google Scholar]
- 46.Rundek T., Arif H., Boden-Albala B. Carotid plaque, a subclinical precursor of vascular events: the Northern Manhattan study. Neurology. 2008;70:1200–1207. doi: 10.1212/01.wnl.0000303969.63165.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Den Ruijter H.M., Peters S.A., Anderson T.J. Common carotid intima-media thickness measurements in cardiovascular risk prediction: a meta-analysis. JAMA. 2012;308:796–803. doi: 10.1001/jama.2012.9630. [DOI] [PubMed] [Google Scholar]
- 48.Helfand M., Buckley D.I., Freeman M. Emerging risk factors for coronary heart disease: a summary of systematic reviews conducted for the U.S. preventive services task force. Ann. Intern Med. 2009;151:496–507. doi: 10.7326/0003-4819-151-7-200910060-00010. [DOI] [PubMed] [Google Scholar]
- 49.Peters S.A., den Ruijter H.M., Bots M.L., Moons K.G. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart. 2012;98:177–184. doi: 10.1136/heartjnl-2011-300747. [DOI] [PubMed] [Google Scholar]
- 50.Goff D.C., Jr., Lloyd-Jones D.M., Bennett G. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129:S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.