Abstract
Pulmonary arterial hypertension (PAH) is a progressive disease that culminates in right heart failure and death. Prostacyclin (PGI2) and its derivatives are effective treatments for PAH when administered as continuous parenteral infusions. This treatment paradigm requires medical sophistication, and patients are at risk for complications from an indewelling catheter; drug interruptions may result in rebound pulmonary hypertension and death. We hypothesized that the salivary gland can be repurposed into an endogenous production site for circulating PGI2 through the expression of a fusion protein embodying cyclooxygenase-1 (Cox1) and prostacyclin synthase (PGIS) domains. We utilized ultrasound-assisted gene transfer, a nonviral gene transfer strategy that achieves robust gene transfer to the salivary gland. We initially found that Cox1-PGIS expression in livers of mice using an adenoviral vector dramatically increased circulating PGI2 relative to untreated rats or rats treated with PGIS alone. We then utilized ultrasound-assisted gene transfer to express Cox1-PGIS in the submandibular glands of rats and showed a significant elevation of circulating PGI2 that corresponded to approximately 30% of that seen in humans undergoing intravenous infusion therapy for PAH. These results suggest the feasibility of gene therapy to drive endogenous biosynthesis of PGI2 as a therapeutic strategy for the treatment of PAH.
Keywords: : salivary gland, sonoporation, prostacyclin, pulmonary hypertension
Introduction
Pulmonary arterial hypertension (PAH) is a chronic and uniformly deadly disease characterized by extensive narrowing of the pulmonary vasculature leading to progressive increases in pulmonary vascular resistance, right ventricular failure, and death. Survival rates in PAH at 1, 3, and 5 years are estimated at 85%, 68%, and 57%, respectively, and there is an average survival from onset of symptoms of 2.8 years if left untreated.1,2 Prostacyclin (PGI2), an endogenous substance produced by the vascular endothelium, has potent vasodilatory, antiplatelet, and antiproliferative properties. Notably, a reduction in PGI2 urinary metabolites and an increase in thromboxane A2 urinary metabolites have been demonstrated in patients with PAH, suggesting a role for deficits in circulating PGI2 in the pathophysiology of PAH, and prostacyclin therapy continues to be considered the most effective treatment for PAH, particularly for advanced disease.3 Normalization of circulating PGI2, either exogenously or endogenously, is an active area of current research.4 Unfortunately, the complicated delivery systems and potential side effects associated with parenteral prostacyclins have deterred some patients and caregivers from using them.5,6
The salivary glands are an intriguing endogenous production site for biotherapeutics6,7 and could potentially be used to manufacture molecules such as prostacyclin (PGI2). Intriguingly, the salivary glands of multiple animal models have shown the ability to secrete transgene in both endocrine and exocrine pathways.8 We have recently developed ultrasound-assisted gene transfer (UAGT), a nonviral gene delivery technology, promoting expression of therapeutic transgenes in the salivary gland,6 obviating the need for viral vectors, which suffer from limitations related to host immune response. UAGT involves bloodless cannulation of the submandibular duct via the mouth, a simple process that takes less than 5 min to perform. The carrier solution contains a plasmid vector and lipid-shelled microbubbles. The microbubbles are locally destroyed by application of high mechanical index ultrasound energy. This results in local cavitation and disruption of cell membranes, allowing the gene transfer vector to enter cells. Using UAGT avoids permanent damage or immune response in the salivary glands. This confers the major advantage of allowing redosing of the transgene as needed, theoretically allowing lifelong gene expression through periodic boosters.
We hypothesized that by expressing a fusion protein embodying both prostacyclin synthase (PGIS) and cyclooxygenase-1 (Cox1) domains in the salivary glands of rats we could boost circulating PGI2 levels as an initial proof of principle. This fusion protein has previously been shown to produce high levels of prostacyclin (PGI2) when expressed in mammalian cells in vitro and in vivo.9 This manuscript describes salivary biosynthesis and endocrine secretion of PGI2 following UAGT of the Cox1-PGIS fusion protein to the salivary glands of rats, suggesting the feasibility of this novel gene therapy as a treatment strategy for PAH.
Methods
Protection of human subjects
All studies described in this manuscript were approved by the institutional review boards of the Allegheny Health Network or the Cleveland Clinic Foundation. All patients enrolled in this study signed informed consent and were de-identified using an assigned a study number. Participation in this study involved donation of a sample of peripheral blood. The only patient information collected for each patient was the dose of intravenous epoprostenol the patient was currently receiving.
Animals, husbandry, and experimental group design
The Institutional Animal Care and Use Committee of Allegheny Health Network Research Institute approved all animal experimentation described herein. A total of 46 male, Sprague-Dawley rats, aged roughly 2 months, were utilized in this study. Table 1 shows the experimental group design. One additional group of mice (n = 3) was utilized for the Western blot experiments (shown in Fig. 1B).
Table 1.
Experimental group design
| Species | Treatment Site | Treatment | Endpoint | n |
|---|---|---|---|---|
| Mice | None | Untreated controls | 24 hours | 2 |
| Mice | IV | AdCox1-PGIS | 24 hours | 1 |
| Rats | None | Untreated controls | 48 hours | 3 |
| Rats | IV | 1E9 AdPGIS | 48 hours | 5 |
| Rats | IV | 1E9 AdCox1-PGIS | 48 hours | 4 |
| Rats | IV | 1E10 AdCox1-PGIS | 48 hours | 8 |
| Rats | IV | 1E11 AdCox1-PGIS | 48 hours | 4 |
| Rats | SMG | Normal rats – UAGT/Luc | 48 hours | 2 |
| Rats | SMG | Normal rats – UAGT/Cox1-PGIS | 48 hours | 11 |
| Rats | SMG | MCT rats – 2 × UAGT/Luc | 21 days post-MCT | 4 |
| Rats | SMG | MCT rats – 2 × UAGT/Cox1-PGIS | 21 days post-MCT | 5 |
MCT rats received two UAGT treatments (2 × UAGT), on days 7 and 14 post MCT.
Cox, cyclooxygenase-1; IV, tail vein (intravenous injection); MCT, monocrotaline ; PGIS, prostacyclin synthase; SMG, submandibular gland; UAGT, ultrasound-assisted gene transfer.
Figure 1.
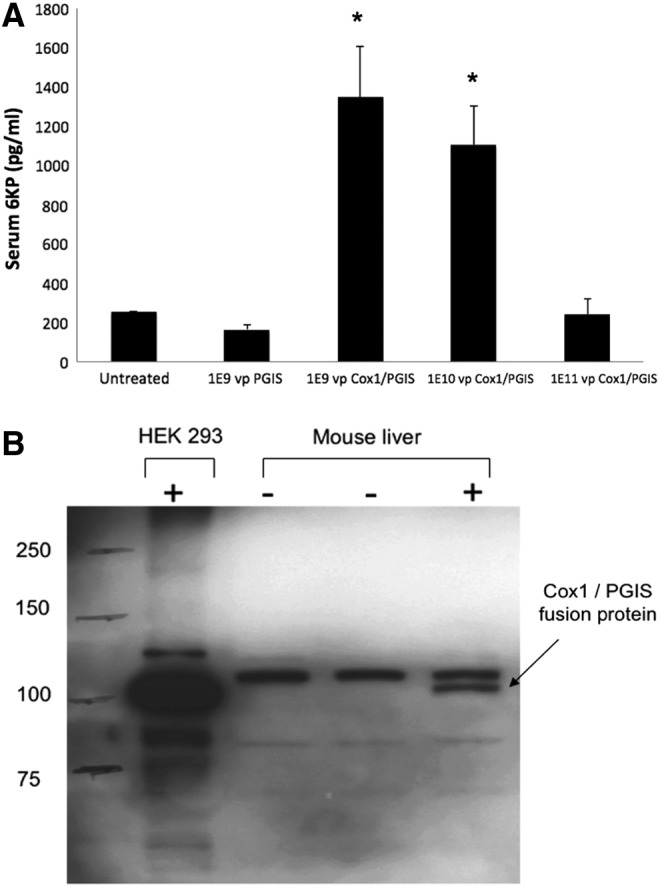
(A) Serum 6-keto-prostaglandin F1α (6KP) levels of mice treated with tail vein injection of adenovirus vectors at the doses indicated. Untreated animals did not undergo a tail vein injection. Measured values of 6KP below 50 pg/mL were recorded as 50 pg/mL. Error bars are ± standard error of the mean. p-Values were as follows: overall difference between groups, p = 0.002; 1E9vpPGIS vs. 1E9vpCOX1/PGIS, p = 0.008; 1E9vpPGIS vs. 1E10 vpCOX1/PGIS, p = 0.007. (B) Western blot analysis of liver homogenates from two untreated mice (−, −), and one mouse treated with 1E9vp of Cox1-PGIS by tail vein injection (+). Cell lysates from HEK293 cells transfected with pCMV-Cox1-PGIS (see Fig. 2B) were used as a positive control. Cox1, cyclooxygenase-1; PGIS, prostacyclin synthase.
Experimental PAH model in rats
Monocrotaline injury was used to induce experimental PAH in rats as previously described.10 Rats received a single subcutaneous injection of monocrotaline dosed at 60 mg/kg of body weight.
Vector design and preparation
The open reading frame encoding the Cox1-PGIS fusion protein, including the 10 amino acid hydrophobic linker (Fig. 2A) was synthesized by Genecopoeia, Inc. (Rockville, MD) based upon the design previously reported by Ruan et al.11 and sequence verified. The PGIS domain was amplified from the full Cox1-PGIS sequence for construction of the PGIS-alone vector. The plasmid vectors pCMV-GL3 (Luc) has been previously described by our group12 and was used to generate the pCMV-Cox1-PGIS vector, ensuring both vectors were isogenic with respect to the backbone. The adenoviral vectors expressing Cox1-PGIS or PGIS alone were constructing by cloning the respective cDNA sequences into the pShuttle-CMV vector (Agilent Technologies, Carlsbad, CA) using the In-Fusion cloning system (Clontech, Mountain View, CA). The resulting pShuttle-pAQP1 was linearized by digestion with PmeI and transformed into Escherichia coli BJ 5183-AD-1 strain cells (Agilent Technologies). Recombinant clones were selected by kanamycin resistance and confirmed by PacI endonuclease restriction analysis. Finally, the linearized recombinant plasmid was transfected into HEK293 cells by polyethylenimine transfection. The primary viral stock was prepared by freeze/thaw cycles of cells 15 days post transfection. Rescued vectors were upscaled and purified using standard methods previously described by our group.6
Figure 2.
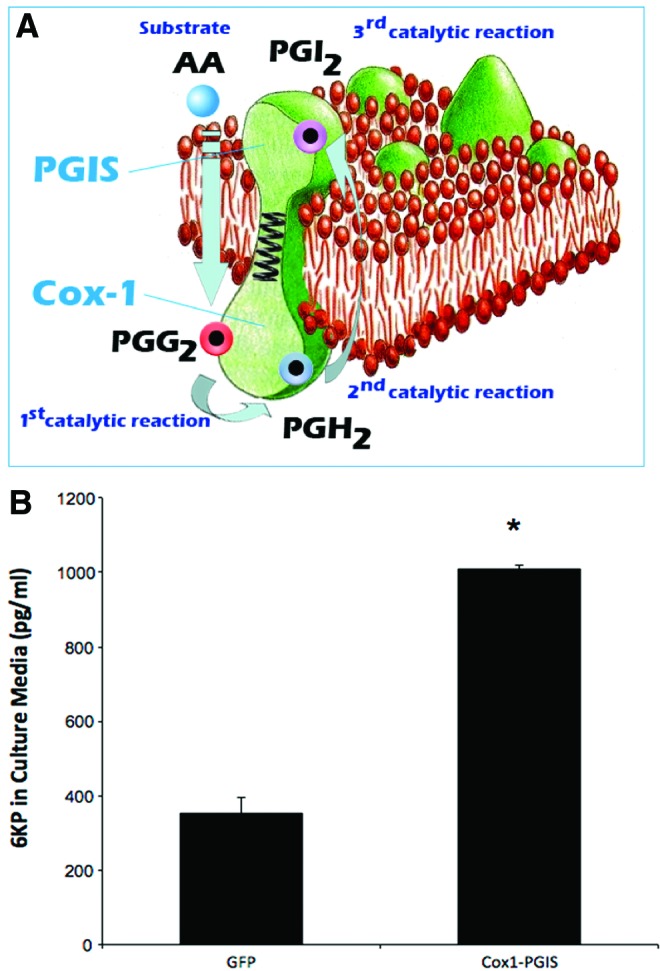
(A) Artist's rendering of the Cox1-PGIS fusion protein and its biological activity within the endoplasmic reticulum. (B) 6KP levels measured by ELISA in culture medium 24 h after transfection with pCMV-Cox1-PGIS or GFP (p < 0.05). Color images available online at www.liebertpub.com/hum
Gene transfer
Tail vein injections of adenovirus were carried out using standard methods in mice. UAGT to the submandibular glands of rats was performed as we have previously described12 in mice, but using a 150 μL infusate volume to account for the larger gland in rats. Briefly, animals were anesthetized with a mixture of ketamine and xylazine and the submandibular ducts were cannulated bilaterally; 150 μL of solution containing 15% v/v Definity microbubbles and 1 μg/μL of plasmid vector (the vectors used are listed in Table 1) in normal saline was infused to both the left and right submandibular ducts. Bubbles were destroyed using four 30 s bursts from a Sonigene device (Visualsonics, Inc., Toronto, Canada) set for 1 MHz, 50% duty cycle and 2 W/cm2, with 10 s between pulses. Following the four pulses, the transducer was withdrawn and the animal was allowed to rest for 10 min before the catheters were removed.
Cox1-PGIS transcript detection in submandibular glands
Forty-eight hours after UAGT of Cox1-PGIS, rats were sacrificed by isoflurane overdose, and submandibular glands were removed and homogenized in RNALater. Total RNA was isolated from homogenized tissue using the RNEasy protocol (Qiagen, Netherlands) and cDNA synthesized using a first strand synthesis kit (Roche Life Sciences, United States). Cox1-PGIS transcript levels were determined by CYBR green quantitative real-time reverse transcription PCR using mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) transcription level as an internal control on a Roche LightCycler® 480. Primers against human Cox1 and mouse GAPDH were used in the gPCR assay and primer sequences are as follows: human Cox1 forward primer, 5′-CTCTCAGGCATCACCTCCTC-3′, and reverse primer 5′- GGAGGGTCCCGATGATCT-3′. Mouse GAPDH forward primer, 5′- CAT GGG TGT GAA CCA TGA GAA-3′, and reverse primer 5′- CGTCCCGTAGACAAAATGGT −3′. The relative level of Cox1-PGIS in each sample was determined as a ratio to GAPDH (reference gene) and the nontreated gland samples were set as 1 to offer an index for all other samples. Amplification conditions were as follows: 95°C for 5 min followed by 45 cycles at 95°C for 10 s, 60°C for 10 s, and 72°C for 10 s.
Western blot of mouse livers
Forty-eight hours after tail vein injections, mice were sacrificed by cervical dislocation under general anesthesia and livers dissected and homogenized. Protein concentrations were evaluated using the Bradford method, and protein concentrations of samples were adjusted to 1 μg/μL. Twenty micrograms of each individual sample were separated by 10% SDS-PAGE and transferred onto a nitrocellulose membrane. The membrane was blocked in 5% milk in phosphate-buffered saline/Tween 20 (PBST) for 1 h and incubated with anti-Cox1 monoclonal antibody (1:1000, Cayman Chemical). After washing with PBST, the membrane was incubated with horseradish peroxidase–conjugated goat anti-mouse antibody (1:10000, Jackson ImmunoResearch) for 1 h at room temperature. Thereafter, the membrane was washed three times for 10 min each wash, and signals were visualized using Pierce ECL Plus Western Blotting Substrate.
Quantitation of 6-keto-prostaglandin F1α
Blood was collected at the endpoints indicated in Table 1 by exsanguination under general anesthesia. Plasma levels of 6-keto-prostaglandin F1α (6KP) were determined by ultra-performance liquid chromatography and tandem mass spectrometry (UPLC-MS/MS). A 250 μL aliquot of plasma was spiked with 250 pg 6-keto-prostaglandin F1α-d4 internal standard. An equal volume of 4% H3PO4 in water was added to acidify the samples prior to solid phase extraction. The extraction was performed on an Oasis HLB 96-well μElution plate (2 mg, 30 μm; Waters Corp., Milford, MA). The acidified samples were loaded onto the plate and washed with 5% methanol in water. A two-step elution (25 μL × 2) using methanol was dispensed into a 96-well plate containing 50 μL of water. The plate was gently vortexed and subsequently analyzed on a Waters Acquity UPLC–Xevo TQS Micro. Chromatographic separation was achieved using a Waters UPLC BEH C8, 2.1 mm × 100 mm, 1.7-μm particle size column. The mobile phases consisted of 1% formic acid in water (A) and 1% formic acid in acetonitrile (B). The initial mobile phase consisted of 15% B, which was linearly ramped to 50% B over 12 min at a flow rate of 400 μL/min. The triple quadrupole mass spectrometer was set to monitor m/z transitions of 369.3 > 163.1 (6KP) and 373.3 > 167.1 (6-keto-prostaglandin F1α-d4) in negative electrospray ionization mode. A lower limit of quantitation was established at 50 pg/mL. Pooled rat plasma was tested in quadruplicate to determine endogenous levels of 6KP prior to calibration curve preparation. The endogenous plasma levels were calculated to contain less than 20% of the 6KP lower limit of quantitation and were therefore deemed suitable for generation of the curve. The dynamic range of the assay was 50 pg/mL–3,200 pg/mL. All values obtained below 50 pg/mL were recorded as 50 pg/mL. All (6KP) blood levels reported in this manuscript used this 6KP quantitation method.
Statistical analysis
An unpaird, two-tailed t-test was used to compare the results in Fig. 2. A nonparametric ANOVA (Kruskal Wallis) test was used on group comparisons of serum levels of 6KP (pg/mL). Comparisons with more than 2 groups (Figs. 1A and 3B) Bonferroni post hoc tests were performed for multiple pairwise comparisons. All analyses were performed using Stata (v.12.1, StataCorp, LP, College Station, TX). The level of statistical significance was set at a two-sided p-value of <0.05.
Figure 3.
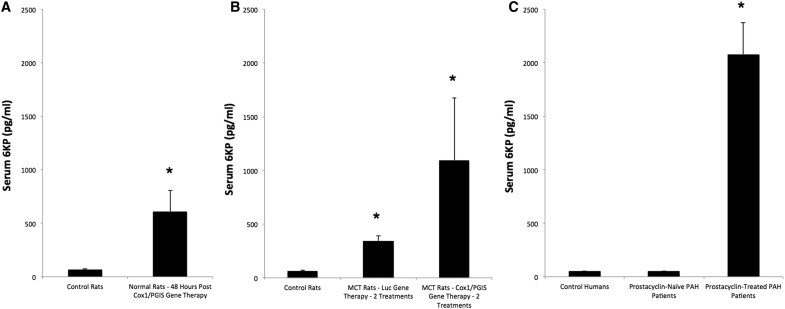
(A) Serum 6KP levels obtained from control rats and rats 48 h after salivary gland gene transfer of Cox1-PGIS (p = 0.006). (B) Serum 6KP levels obtained from control rats and monocrotaline-injured (i.e., “MCT”) rats after two treatments (2 × ) of salivary gland UAGT with either GL3 (Luc) or Cox1-PGIS. Control rats were not manipulated. Treatments were given on days 7 and 14 post MCT, and serum samples were taken on day 21 post MCT. Both MCT groups were significantly different from controls (UAGT/Luc, p = 0.012; UAGT/Cox1-PGIS, p = 0.001) but were not significantly different from one another (p = 0.27). (C) Serum 6KP levels obtained from control humans (n = 4), pulmonary arterial hypertension PAH patients naïve to prostacyclin therapy (n = 4) and PAH patients undergoing continuous prostacyclin therapy for PAH (n = 7). Adjusted p-values were as follows: overall, p = 0.008; for PAH patients on prostacyclin therapy vs. control humans, p = 0.006; and for PAH patients on prostacyclin therapy vs. PAH patients naïve to prostacyclin therapy, p = 0.006. Error bars are ± standard error of the mean. UAGT, ultrasound-assisted gene transfer.
Results
Construction and functional testing of Cox1-PGIS fusion protein cDNA
After receipt and sequence verification of the Cox1-PGIS cDNA (Fig. 2A), the open reading frame was cloned into pCMV-MCS and transfected into HEK 293 cells to confirm the ability of the transgene to synthesize prostacyclin. PGIS produces PGI2 (prostacyclin) directly from PGH2 (prostacyclin H), but PGI2 has a very short half-life (42 s) in serum13 and is thus virtually impossible to measure directly with analytical chemistry. The stable metabolite of PGI2, 6KP, has typically been used as an indirect measure of circulating prostacyclin.14 We initially measured 6KP using ELISA, an approach15 that has previously been widely utilized. Twenty-four hours after transfection, 6KP levels were elevated in culture supernatant from cells transfected with Cox1-PGIS relative to GFP transfection (Fig. 2B). 6KP in culture media was elevated ∼2.5 × relative to GFP transfection, demonstrating the functionality of the fusion enzyme, but the limitations of the 6KP ELISA with respect to accuracy are well appreciated. While this study was being carried out, an improved method for absolute quantitation of 6KP was developed, based upon ultra-performance liquid chromatography and tandem mass spectrometry, described above in methods. We adopted UPLC-MS/MS for all experiments involving plasma samples.
Cox1-PGIS expression in vivo results in PGI2 synthesis that is dramatically higher than PGIS expression alone
In order to test the in vivo prostacyclin synthesis capacity of our Cox1-PGIS fusion enzyme, we constructed a serotype 5 adenoviral vector (Ad5) to express the fusion enzyme and a second virus expressing PGIS alone, both under the control of the canonical CMV promoter. It is a well-established principle that tail vein injection of Ad5 in the rodent results in robust vector-mediated transgene expression in the liver, allowing us to measure PGI2 synthesis across a presumably wide range of transgene expression. Figure 1A shows the results of this experiment. We observed that PGIS alone had no discernible effect upon serum 6KP, presumably due to the rate-limiting step of PGH2 synthesis in the absence of Cox1. It is not surprising that 6KP levels decrease as vector dosage exceeds the saturating dose of ∼1E9, as higher doses induce massive inflammation and organ dysfunction. We confirmed that the Cox1-PGIS fusion protein was physically present in the liver and that it was of the predicted molecular weight by Western blot analysis (Fig. 1B). No band of the corresponding size was observed in the absence of the vector. On the basis of these in vivo studies, we proceeded to testing the central hypothesis of our study.
Expression of the Cox1-PGIS transgene in the submandibular glands of rats
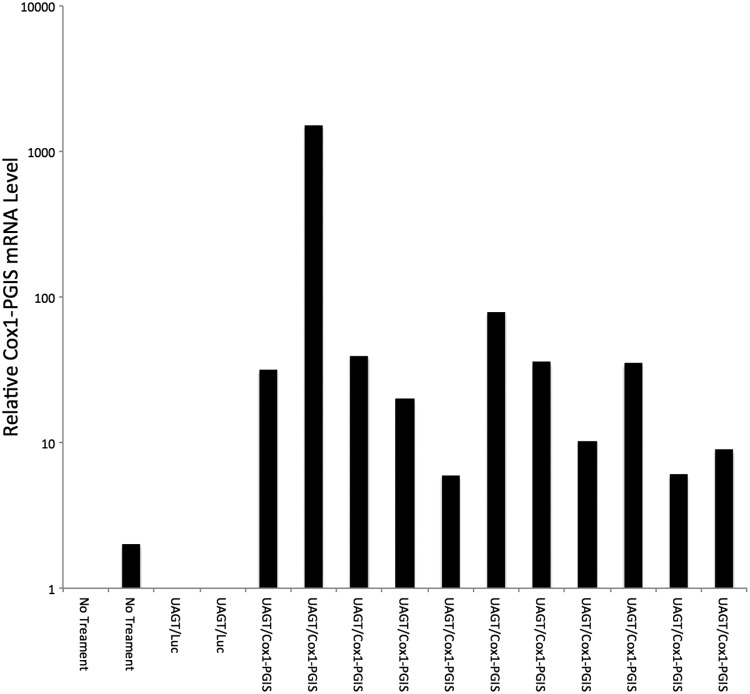
UAGT with the pCMV-Cox1-PGIS vector was carried out in the submandibular glands of rats and 48 h later, glands were dissected and cDNA was synthesized. Quantitative PCR analysis was performed to establish relative expression levels of the Cox1-PGIS transgene, based upon probes designed to detect human Cox1 but not rat or mouse Cox1. Relative mRNA levels are shown in Fig 4. The Cox1-PGIS transgene was detected in all animals treated with UAGT/Cox1-PGIS, although there was animal-to-animal variability that is typical of UAGT in the salivary glands of rodents.12
Figure 4.
Quantitative PCR relative quantification of human Cox1 transcripts in pooled homogenates of left and right submandibular glands from rats treated as indicated. Cox1 signal was normalized to rat GAPDH in each sample.
Expression of Cox1-PGIS in the submandibular glands elevates serum 6KP levels dramatically
Forty-eight hours after UAGT of the pCMV-Cox1-PGIS vector to the submandibular glands of rats, we measured serum 6KP and found it massively upregulated relative to control rats, who received UAGT of pCMV-GL3. To further query the clinical translational potential of this gene therapy strategy, we measured 6KP levels in human PAH patients undergoing stable and continuous intravenous prostacyclin (epoprostenol) treatment, and we discovered that our gene therapy raised serum 6KP to ∼30% of clinically therapeutic levels in humans (Fig. 3A compared with 3C).
Finally, we undertook a study wherein rats were injured with monocrotaline (MCT) to induce a PAH-like disease state, then treated with UAGT/Cox1-PGIS salivary gland gene transfer or UAGT/GL3 salivary gland gene transfer 7 days and 14 days post MCT, before being subjected to right ventricular puncture (a fatal procedure) 21 days post MCT. We did not observe a clear relationship between Cox1-PGIS gene therapy and RV pressure relative to animals treated with irrelevant (Luc) gene therapy. However, we did make the unexpected observation that animals receiving Luc gene transfer to the salivary gland had substantially elevated serum levels of 6KP relative to normal rats (Fig. 3B). We also found that the two treatments with UAGT/Cox1-PGIS were partially additive, increasing circulating 6KP levels equivalent to almost 50% of therapeutic levels in humans (Fig. 3B compared with 3C).
Discussion
This study supports the idea that the salivary glands may have the capability to function as endogenous bioreactors to produce prostacyclin as an alternative to exogenous administration for the treatment of PAH. UAGT offers a minimally-invasive and theoretically safe means of carrying out this gene therapy strategy. By virtue of avoiding extracellular immune response, this technique is assumed to be repeatable. One previous study and our own unpublished data indicates that while UAGT transduces only a portion of the cells in the salivary gland,16 this is a stochastic process that recruits additional cells with each successive treatment. This suggests that it may be possible to “titrate” the level of prostacyclin produced endogenously by closely spaced UAGT/Cox1-PGIS treatments in order to reach the desired serum level of 6KP. This study did utilize two serial treatments in PAH rats, but was not designed to address this titration question in a conclusive way.
Due to the inherent imprecision of the relationship of vector dose-to-transgene produced in gene therapy applications, our clinical translational strategy is not to use gene therapy to entirely replace exogenous prostacyclin therapy in PAH. Rather, we propose a synergy between exogenous prostacyclin therapy and gene therapy to overcome the requirement for continuous intravenous infusion, in favor of oral or inhaled prostacyclin formulations. At present, PAH specialists face a challenging dilemma in deciding when to initiate intravenous prostacyclin therapy. While intravenous prostacyclins deliver therapeutic efficacy that is substantially superior to oral or inhaled treatments, the logistical complexity of continuous intravenous infusion makes these drugs a “last resort” in all but the most compliant and sophisticated patients. Our reasoning is that if effective prostacyclin therapy is a consequence of achieving a target serum concentration (and our 6KP measurements in PAH patients undergoing intravenous prostacyclin therapy certainly suggest that this is the case), then the therapeutic shortfall of oral or inhaled formulations is presumably their inability to reach these high serum levels (∼2000 pg/mL). If gene therapy is capable of achieving ∼50% of these levels, effectively setting a higher serum baseline, oral or inhaled formulations might then be effectively utilized for finer titration up to the therapeutic levels as an “add on” to gene therapy, but without the logistical challenges of intravenous infusion. Our translational development of this gene therapy strategy going forward will be predicated upon this schema.
Our finding that the Cox1-PGIS fusion protein produces substantially more PGI2 than PGIS alone support those of Ruan and coworkers, who originally conceived of this fusion protein design.11 Given that the Cox1-PGIS fusion protein can catalyze the production of PGI2 directly from arachidonic acid, and since arachidonic acid can be easily supplemented by diet, the capacity of this PGI2 endogenous synthesis strategy is likely to be very deep. Further, the two catalytic domains of the fusion protein are human proteins, and would not be expected to elicit host response when delivered with our nonviral UAGT method. These advantages make our gene therapy appear practical from the standpoint of patient safety.
The observation that MCT treatment alone (MCT animals treated with UAGT/Luc) is associated with very substantial increases in serum 6KP is unexpected and, to our knowledge, novel. Since Luc gene transfer could not be the cause of this phenomenon, and since human PAH patients naïve to prostacyclin therapy do not exhibit this phenomenon, our explanation is that this is simply an artifact of the rat MCT model of PAH, which has been often criticized as imperfectly modeling the human PAH condition. Although our efforts to observe an effect of Cox1-PGIS gene therapy upon right ventricular pressure in the MCT rats was disappointing, this is rendered of lesser importance by our observation that there is little variation in the circulating 6KP levels in PAH patients on optimized and stable prostacyclin therapy. The MCT rat, and indeed therapeutic response in any animal model, is unlikely to be an essential element of further efforts to move this gene therapy strategy into larger animals and ultimately humans based upon the idea of achieving circulating 6KP targets as proof of therapeutic potential.
Our studies demonstrate that our gene therapy strategy can induce the salivary gland of the rat to produce endogenous PGI2 at levels that correspond to roughly 35% of the circulating PGI2 levels that are therapeutic in humans, and that a second treatment boosts this effect further. Great caution should be exercised in extrapolating these observations to what might be possible in larger animals or humans. It is clear that the salivary gland's capacity for endocrine secretion of a transgene is conserved across the three mammalian species thus far studied,17–20 but species-specific differences in capacity and trafficking have also been reported, indicating that nothing observed in one species can be assumed to translate with perfect precision to another species. Nothing has yet been empirically determined regarding the use of salivary glands as endogenous biotherapeutic synthesis sites in humans. Based upon this initial study, interpreted in the context of previous literature, we can conclude that this gene therapy strategy has potential to reach our therapeutic goals, but a number of pitfalls remain further down the clinical translational pathway.
Acknowledgments
This work was supported by National Institutes of Health grant HL106114 (to R.L.B.) and the Entelligence Young Investigator Award (to M.J.P.). Z.W. performed molecular analyses as well as edited the manuscript. K.J.S. performed the statistical analysis of the data edited the manuscript. M.J.P., R.L.B. designed the study, interpreted the results, and wrote the manuscript. K.B.H. edited the manuscript. The authors would like to thank Paul Kennedy, PhD, from Cayman Chemical for developing and carrying out the 6KP analytical chemistry.
Author Disclosure
The authors state that no conflicts of interest exist relevant to this manuscript.
References
- 1.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012;142:448–456 [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343–349 [DOI] [PubMed] [Google Scholar]
- 3.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J 2008;31:891–901 [DOI] [PubMed] [Google Scholar]
- 4.Christman BW, McPherson CD, Newman JH, et al. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327:70–75 [DOI] [PubMed] [Google Scholar]
- 5.O'Connell C, Amar D, Boucly A, et al. Comparative Safety and Tolerability of Prostacyclins in Pulmonary Hypertension. Drug Saf 2016;39:287–294 [DOI] [PubMed] [Google Scholar]
- 6.Passineau MJ, Fahrenholz T, Machen L, et al. α-Galactosidase A expressed in the salivary glands partially corrects organ biochemical deficits in the fabry mouse through endocrine trafficking. Hum Gene Ther 2011;22:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Samuni Y, Baum BJ. Gene delivery in salivary glands: from the bench to the clinic. Biochim Biophys Acta 2011;1812:1515–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baum BJ, Alevizos I, Chiorini JA, et al. Advances in salivary gland gene therapy — oral and systemic implications. Expert Opin Biol Ther 2015;15:1443–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruan CH, So SP, Ruan KH. Inducible COX-2 dominates over COX-1 in prostacyclin biosynthesis: mechanisms of COX-2 inhibitor risk to heart disease. Life Sci 2011;88:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lalich JJ, Ehrhart LA. Monocrotaline-induced pulmonary arteritis in rats. J Atheroscler Res 1962;2:482–492 [DOI] [PubMed] [Google Scholar]
- 11.Ruan KH, So SP, Cervantes V, et al. An active triple-catalytic hybrid enzyme engineered by linking cyclo-oxygenase isoform-1 to prostacyclin synthase that can constantly biosynthesize prostacyclin, the vascular protector. FEBS J 2008;275:5820–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passineau MJ, Zourelias L, Machen L, et al. Ultrasound-assisted non-viral gene transfer to the salivary glands. Gene Ther 2010;17:1318–1324 [DOI] [PubMed] [Google Scholar]
- 13.Cawello W, Schweer H, Muller R, et al. Metabolism and pharmacokinetics of prostaglandin E1 administered by intravenous infusion in human subjects. Eur J Clin Pharmacol 1994;46:275–277 [DOI] [PubMed] [Google Scholar]
- 14.Brash AR, Jackson EK, Saggese CA, et al. Metabolic disposition of prostacyclin in humans. J Pharmacol Exp Ther 1983;226:78–87 [PubMed] [Google Scholar]
- 15.Miller DK, Sadowski S, DeSousa D, et al. Development of enzyme-linked immunosorbent assays for measurement of leukotrienes and prostaglandins. J Immunol Methods 1985;81:169–185 [DOI] [PubMed] [Google Scholar]
- 16.Geguchadze R, Wang Z, Zourelias L, et al. Proteomic profiling of salivary gland after nonviral gene transfer mediated by conventional plasmids and minicircles. Mol Ther Methods Clin Dev 2014;1:14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baum BJ, Berkman ME, Marmary Y, et al. Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther 1999;10:2789–2797 [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Cawley NX, Voutetakis A, et al. Partial redirection of transgenic human growth hormone secretion from rat salivary glands. Hum Gene Ther 2005;16:571–583 [DOI] [PubMed] [Google Scholar]
- 19.Samuni Y, Zheng C, Cawley NX, et al. Sorting of growth hormone-erythropoietin fusion proteins in rat salivary glands. Biochem Biophys Res Commun 2008;373:136–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Voutetakis A, Zheng C, et al. Sorting of transgenic secretory proteins in miniature pig parotid glands following adenoviral-mediated gene transfer. J Gene Med 2007;9:779–787 [DOI] [PubMed] [Google Scholar]