Abstract
The torn rotator cuff remains a persistent orthopedic challenge, with poor outcomes disproportionately associated with chronic, massive tears. Degenerative changes in the tissues that comprise the rotator cuff organ, including muscle, tendon, and bone, contribute to the poor healing capacity of chronic tears, resulting in poor function and an increased risk for repair failure. Tissue engineering strategies to augment rotator cuff repair have been developed in an effort to improve rotator cuff healing and have focused on three principal aims: (1) immediate mechanical augmentation of the surgical repair, (2) restoration of muscle quality and contractility, and (3) regeneration of native enthesis structure. Work in these areas will be reviewed in sequence, highlighting the relevant pathophysiology, developmental biology, and biomechanics, which must be considered when designing therapeutic applications. While the independent use of these strategies has shown promise, synergistic benefits may emerge from their combined application given the interdependence of the tissues that constitute the rotator cuff organ. Furthermore, controlled mobilization of augmented rotator cuff repairs during postoperative rehabilitation may provide mechanotransductive cues capable of guiding tissue regeneration and restoration of rotator cuff function. Present challenges and future possibilities will be identified, which if realized, may provide solutions to the vexing condition of chronic massive rotator cuff tears.
Keywords: : developmental engineering, enthesis, mechanobiology, rotator cuff
Introduction
The torn rotator cuff remains a persistent orthopedic challenge, affecting up to 50% of patients over age 60.1 While acute tears of the rotator cuff can be caused by trauma, the majority of rotator cuff disease entails chronic, degenerative changes of the tendon, with initially small, partial thickness tears propagating in size and producing sequential degenerative changes in the adjacent bone and muscle. While conservative treatment of rotator cuff tears can temporarily improve glenohumeral joint kinematics and patient-reported outcomes,2 there is little evidence to suggest that restoration of normal tendon structure and function occurs. Rather, a substantial proportion of small asymptomatic tears increase in size, resulting in increased pain and decreased function.3,4 Consequently, early surgical repair is often advocated to prevent tear propagation and further tissue degeneration.1 Early intervention has proven effective in restoring tendon integrity, while late intervention, such as when performing surgical repairs of chronic massive tears (>5 cm), results in re-tear rates as high as 94%.5 However, given the insidious nature of rotator cuff disease, many patients do not seek treatment until considerable degeneration has occurred, presenting a formidable challenge to the surgeon. Current practice guidelines for treating rotator cuff tears, as recommended by the American Academy of Orthopedic Surgeons, are inconclusive in part due to the paucity of prospective randomized control studies.6
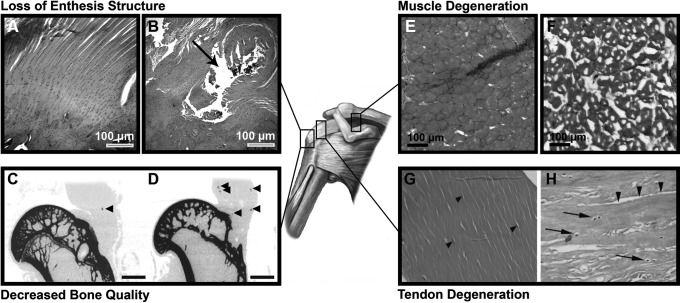
The risk of repair failure (i.e., re-tear) is correlated with muscle degeneration, tear size, and patient age, all of which increase with tear chronicity.7–9 With propagation of a chronic rotator cuff tear, the unloaded muscles undergo degenerative changes, including increased fibrosis and fatty infiltration, which causes further medial retraction of the tendon edge.1,10 Concurrently, bone density of the humeral head decreases in the absence of mechanical stress.11,12 As a result, increased repair tension is required to surgically appose the tendon to the anatomical footprint of the now osteoporotic humeral head, both of which compromise repair integrity.10,13,14 Furthermore, the complex structure of the tendon–bone interface (i.e., enthesis) is not restored following surgical repair,15,16 causing a focal stress concentration as the compliant tendon abruptly adjoins stiff bone. Taken together, these factors predispose the surgical repair to fail, which occurs most frequently at the suture–tendon interface.17 Moreover, the repair strength is further reduced due to the compromised tendon quality found in chronic tears (Fig. 1).18
FIG. 1.
The effect of chronic, massive tears on the elements of the rotator cuff organ. The native enthesis (A) contains a complex structure that is not restored following surgical repair (B); instead of a gradient in mineral content and interpositional fibrocartilage, the repaired tendon abruptly adjoins bone. The black arrow indicates the suture hole. As shown with micro-computed tomography, the humeral head of uninjured or acutely repaired rotator cuff tears (C) exhibit superior bone quality (e.g., bone mineral density) to the humeral heads of chronically torn rotator cuff tendons (D). Adapted with permission from Killian et al.75 Native muscle fibers are polygonal with peripheral nuclei (E) whereas degenerated muscle in the context of chronic tears undergoes atrophy, fibrosis, and fatty infiltration (F). Lastly, uninjured tendon consists of fibroblasts (arrow heads) elongated in the direction of aligned collagen fibrils (G). With chronic degeneration, collagen fibrils become disorganized and delaminated (arrow heads), with concurrent heterotopic cartilage formation (H). Black arrows indicate chondrocyte-like cells. Adapted with permission from Buck et al.244
Novel surgical techniques have been developed in an effort to improve footprint coverage and reduce shear stresses at the suture–tendon interface, theoretically enhancing healing at the interface and reducing repair failure.19,20 While these techniques have shown promise in studies with cadaveric specimens, clinical studies have not consistently found improvements in structural healing or functional outcomes.21,22 The discrepancies between in vitro and in vivo findings suggest that increases in initial repair strength are insufficient to restore the integrated structure and function of the tissues that comprise the rotator cuff organ, including muscle, tendon, enthesis, and bone. Indeed, the degenerative muscle changes seen in chronic rotator cuff tears, including fatty infiltration and atrophy, do not improve despite successful surgical repair (i.e., no evidence of re-tear) and correlate with poor functional outcomes.23 If progress is to be made in reducing the re-tear rate and improving clinical outcomes following repair of chronic massive rotator cuff tears, therapeutic strategies must address not only the mechanical integrity of the surgical repair but also the restoration of tissue structure, anatomy, and function.
This review highlights the recent progress made in the application of tissue engineering strategies, including the independent or combined use of cells, scaffolds, and biomolecules (e.g., growth factors, gene therapy), to enhance healing of the tissues that comprise the rotator cuff organ—muscle, tendon, enthesis, and bone. While preclinical results are promising, an emerging understanding of both rotator cuff development and healing suggests that therapeutic strategies for treating individual tissues of the rotator cuff organ might provide synergistic benefit if combined. Moreover, the timing and delivery of these strategies must be considered in the context of current surgical techniques and rehabilitation protocols.
Tissue engineering strategies to augment rotator cuff repair have focused on three principal aims: (1) immediate mechanical augmentation of the surgical repair, (2) restoration of muscle quality and contractility, and (3) regeneration of native enthesis structure. Work in these areas will be reviewed in sequence, highlighting the relevant pathophysiology, developmental biology, and biomechanics, which must be considered when designing therapeutic applications. Thereafter, the role of mechanical loading, as might be controlled through postoperative physical therapy, will be discussed as a rehabilitative stimulus to promote restoration of the integrated tissues that comprise the rotator cuff organ. Present challenges and future possibilities will be identified, which if realized, may provide solutions to the vexing condition of the treatment of chronic massive rotator cuff tears.
Mechanical Augmentation of the Surgical Repair
Rotator cuff repairs fail most commonly in the early postoperative period (within the first 6 months)24–26 when the suture shears through the tendon.17 Consequently, there has been considerable effort to develop novel strategies to increase the early strength of surgical repairs in hopes of reducing failure rates. Improved suture techniques, most notably the double row and its derivatives, have been shown to increase repair strength when loading cadaveric specimens to failure, but when applied clinically, neither reductions in re-tear rates nor enhanced shoulder function have been reported, as shown in meta-analyses.19–22
An alternative strategy to improve the mechanical strength of rotator cuff repairs is to augment the surgical repair with a scaffold,27,28 which could also serve as a delivery vehicle for cells and/or biomolecules intended to restore tendon quality.29 To offload the repair, thereby improving failure mechanics, the scaffold should possess (1) material properties approaching those of the native tendon, and (2) suture retention strength equal to, or exceeding, that of the nonaugmented repair.30,31 When incorporated into surgical repairs of cadaveric human shoulders, both tissue-derived32–34 and synthetic35 scaffolds have been reported to reduce gap formation upon cyclic loading while also enhancing ultimate failure load. In doing so, the mode of failure shifted from suture pull-through to suture breakage.34,35
Application of these scaffolds in large animal models demonstrated benefit in vivo as well. For instance, augmentation of an acutely transected and repaired infraspinatus tendon with a reinforced fascia patch (derived from human fascia lata) in a canine model increased the ultimate load at time 0 by 46%, as compared to nonaugmented repairs.36 However, this advantage in mechanical strength provided by scaffold augmentation dissipated by 12 weeks, at which time there were no differences between groups.36 Conversely, repair augmentation with a poly-l-lactide scaffold (i.e., X-Repair; Synthasome) in the same canine model not only enhanced the ultimate load at time 0, but also increased cross-sectional area, stiffness, and ultimate load at 12 weeks, with a corresponding reduction in tendon retraction.37 In a related ovine model of acute infraspinatus tendon transection and repair, augmentation with a porcine small intestine submucosa (SIS) patch (Biomet) significantly enhanced repair stiffness at 12 weeks.38 Nevertheless, not all studies have reported positive results. As investigated in an ovine model, no benefit was found when augmenting repairs of acutely transected infraspinatus tendons with either a cross-linked acellular porcine dermal (PD) patch (Zimmer Collagen Repair Patch) or a porcine SIS patch (Restore Orthobiologic Soft Tissue Implant; DePuy Orthopaedics), as evaluated by histology and biomechanical testing at weeks 9 and 24.39
A similar pattern is seen in clinical studies. While several case series have documented improved shoulder function (both objective and patient-reported) following scaffold augmentation of rotator cuff repairs,40,41 only two prospective randomized trials have been performed (Table 1). Augmentation of surgical repairs of large and massive chronic rotator cuff tears with a porcine SIS patch (Restore; DePuy Orthopaedics) did not improve the rate of tendon healing or clinical outcomes scores, with a trend toward impaired healing in the augmentation group.42 In a similar case–control study, no benefit was found when employing the same porcine SIS patch, but a pronounced inflammatory reaction to the xenograft was observed.43 On the other hand, when repairs of large, but not massive, rotator cuff tears (i.e., 3–5 cm) were augmented with acellular human dermal matrix (GraftJacket; Wright Medical Technology), the healing rate and outcome scores were significantly greater than nonaugmented repairs.44 Repair augmentation with synthetic scaffolds, such as those composed of biodegradable poly-l-lactide (X-Repair; Synthasome)45 or nondegradable polypropylene (Repol Angimesh; Angiologica BM SRL),46 have also shown promise, as investigated in retrospective case–control studies.
Table 1.
Clinical (Human) Studies with a Minimum of Level 3 Evidence (Cohort Studies) Examining the Benefit of Scaffolds, Augmentation at the Healing Tendon–Bone Interface, or a Combination of the Two
| Augmentation strategy | Study | Level of evidence | Tear size | Exclusion of tears with fatty infiltration? | Sample size | Follow-up period (range) | Failure rate on US/MRI | Functional outcome | Adverse events |
|---|---|---|---|---|---|---|---|---|---|
| Scaffold (porcine SIS) | Iannotti et al.42 | 2 (RCT) | Large and massive (>4 cm) | No | CG: 15 | 12 months (12–26.5) | CG: 6/15 | No difference between groups using PENN | AG: 3/15 postoperative inflammatory reaction |
| AG: 15 | AG: 11/15 | ||||||||
| Scaffold (porcine SIS) | Walton et al.43 | 3 (case–control) | — | No | CG: 16 | 24 months | CG: 7/12 | AG had significantly less strength in internal rotation and adduction than CG | AG: 4/10 postoperative inflammatory reaction |
| AG: 15 | AG: 6/10 | ||||||||
| Scaffold (human dermal) | Barber et al.44 | 2 (RCT) | Large two-tendon tears (>3 cm) | No | CG: 20 | 24 months (12–38) | CG: 9/15 | AG had significantly better ASES and constant score | None |
| AG: 22 | AG: 3/20 | ||||||||
| Scaffold (polypropylene or bovine collagen) | Ciampi et al.46 | 3 (Cohort) | Full thickness, two-tendon tear with <2 cm postoperative residual retraction | Yes, advanced fatty infiltration | CG: 51 | 36 months | CG: 21/51 | UCLA scores were significantly higher for the polypropylene group; Elevation and strength of the polypropylene group were significantly higher than other groups | None |
| Collagen: 49 | Collagen: 25/49 | ||||||||
| Polypropylene: 52 | Polypropylene: 9/52 | ||||||||
| Scaffold (fascia lata autograft) | Mori et al.77 | 3 (Cohort) | Massive rotator cuff tears | No | LG: 26 | 24 months | LG: 7/26 | Constant score and ASES were significantly higher in low-grade group compared to high-grade group | None |
| HG: 19 | HG: 17/19 | ||||||||
| Enthesis (microfracture) | Osti etal.245 | 2 (RCT) | — | Yes, severe fatty infiltration | CG: 29 | 24 months (24–53) | CG: 3/29 | At 3 months, UCLA, VAS, and constant scores better in the microfracture group. No difference at 2 years | None |
| MG: 28 | MG: 2/26 | ||||||||
| Enthesis (microfracture) | Milano et al.246 | 2 (RCT) | Full thickness | No | CG: 38 | 24 months (25–31) | CG: 18/38 | No significant difference in DASH score. Large tear had significantly greater healing with microfracture | None |
| MG: 35 | MG: 12/35 | ||||||||
| Enthesis (MSCs) | Hernigou et al.170 | 3 (Case–control) | Tear <3 cm | No | CG: 45 | 10 years | CG: 35/45 | Number of MSCs correlated with grade of healing. Total MSCs >2500/mL had more healing and less failure than when MSCs <1500/mL. | None |
| MSCG: 45 | MSCG: 6/45 | ||||||||
| Scaffold (human dermis) and enthesis (microfracture) | Yoon et al.225 | 3 (Cohort) | Massive rotator cuff tear | No | CG: 54 | 24 months (14–53) | CG: 25/54 | No difference in VAS, constant, and ASES score | None |
| MPG: 21 | MPG: 4/21 |
No clinical studies investigating cell or pharmaceutical strategies for reversing muscle degeneration were identified. Reviews of preclinical studies are not included here, but have focused on scaffold augmentation,31,57 tendon-bone healing,109,235 and muscle regeneration.198
AG, augmented group; CG, control group; HG, high grade degeneration of both infraspinatus and supraspinatus; LG, low grade fatty degeneration of infraspinatus but high grade degeneration supraspinatus; MG, microfracture group; MPG, marrow stimulation and patch group; MRI, magnetic imaging resonance; MSC, mesenchymal stem cell; MSCG, mesenchymal stem cell group; PENN, PENN shoulder score; RCT, randomized controlled trial; SIS, small intestine submucosa; UCLA, University of California at Los Angeles score; US, ultrasound; VAS, visual analog scale.
For large to massive rotator cuff tears deemed irreparable, reconstruction with an interpositional auto/allograft has been employed to restore continuity between the humeral head and the torn rotator cuff. As reported in several cases series, reconstruction of massive tears with dermal tissue allografts improved pain and function in patients with minimal preexisting glenohumeral arthritis.47,48 Similarly, reconstruction with a fascia lata autograft reduced re-tear rates and improved clinical scores and strength as compared to partial repair in a cohort study of patients with large to massive irreparable tears and low-grade fatty degeneration of the infraspinatus muscle.49
Given the anatomical proximity of the superior glenohumeral capsule and rotator cuff tendons, the former is often damaged concomitant with rotator cuff tears.50 Reconstruction of the superior capsule has been shown in cadaveric models to restore stability, reducing the superior migration of the humeral head that often results from massive rotator cuff tears.51,52 In a limited number of case series, superior capsule reconstruction grossly restored superior glenohumeral stability and improved shoulder function, as reported by patients.53,54 Similar to augmented repairs, long-term outcomes following rotator cuff or superior capsule reconstruction are lacking.
As suggested by the equivocal results found across preclinical and clinical studies, consistent enhancement in surgical outcomes mediated by scaffold augmentation of rotator cuff repairs or reconstruction will require more than mere optimization of a scaffold's mechanical properties. In particular, the immune response to an implanted scaffold, particularly one derived from allo- or xeno-geneic tissue sources, appears to have a great influence in mediating any beneficial (or adverse) effect. The presence of cells55 and nonhomologous protein epitopes56,57 in certain xenograft scaffolds may explain the null effects of scaffold augmentation noted above, as both have been associated with an M1 (proinflammatory) macrophage response, which can lead to poor healing and excessive scar tissue formation.58,59 At the same time, the ultrastructure and biochemical composition of scaffolds differentially affect the phenotype of tendon fibroblasts, with subsequent influence on extracellular matrix (ECM) deposition and scaffold remodeling.60 Novel scaffolds designed to match the mechanical, topographical, and biochemical properties of native tendon, with resulting promotion of a tenogenic cell phenotype, have been recently reported but their in vivo effects remain unexplored.61,62
Additional consideration should also be given to the quality of the tendon tissue itself. Retracted tendons, as seen in chronic rotator cuff tears, exhibit frank deterioration characterized by increased collagen fibril crimp and collagen fibril atrophy and disorganization.63 Similarly, cell apoptosis is seen throughout degenerated tendon,64,65 with concurrent elevations in inflammatory and catabolic mediators.66,67 These degenerative changes not only weaken the suture retention strength, thereby predisposing to surgical failure,18 but also impair healing. Namely, it was recently reported that the severity of tendinosis, rather than tear size or fatty infiltration, was the greatest predictor of repair integrity at least 6 months after surgery.68 Compromised tendon quality likely contributes as well to “failure with continuity,” in which there is no overt defect in the repaired rotator cuff tendon, yet the musculotendinous junction is medially retracted with associated disruption of muscle mechanics.69
Chemical cross-linking of the degenerated tendon may enhance failure properties70 but likely interferes with tissue remodeling and the restoration of native tendon structure and function.58 Conversely, cell- and pharmaceutic-based therapies have shown benefit in reversing tendinopathic changes.71,72 However, animal models with chronic degenerative changes in the tendon have only recently been developed,73–75 with few studies exploring the effect of scaffold augmentation. An investigation in a chronic ovine model found that augmentation of a rotator cuff repair with polyurethane scaffold mesh (Biomerix RCR Mesh; Biomeric Corp.) increased the force at failure compared to nonaugmented controls at 12 weeks, but further analysis of tendon structure was not performed.76 In a cohort study, the effect of augmenting arthroscopic repairs of large to massive rotator cuff tears with a fascia lata autograft patch was evaluated, distinguishing between shoulders with low-grade and high-grade fatty infiltration.77 Interestingly, patch augmentation was not beneficial for massive tears with high-grade fatty infiltration,77 highlighting the importance of considering the muscle quality when applying tissue engineering strategies intended to support the healing tendon–bone interface. To that end, restoring the structure and function of the degenerated muscle in the context of a chronic rotator cuff tear may further enhance the benefit of a scaffold applied to augment the surgical repair.
Restoration of Muscle Quality and Contractility
Unfortunately, progressive deterioration in muscle quality is seen with increasing severity (and chronicity) of rotator cuff tears.78 These degenerative changes, including muscle atrophy, fibrosis, and fatty infiltration, increase muscle stiffness79,80 and necessitate higher repair tension, which increases the risk for re-tear.81 Stress deprivation of the humeral head in the context of chronic tears compromises bone density and architecture,11,82 which further contributes to poor healing.83 Yet even with successful surgical repair, as defined by the continuity of tendon to bone on imaging, the degenerative changes in muscle are considered irreversible.23,84–88 Beyond increasing the risk for repair failure, muscle degeneration is directly correlated with clinical outcomes,7,89 likely due to adverse effects on musculotendinous mechanics, including disruption of myofibril architecture and reductions in contractility.90 As a result, early rotator cuff repair is advocated in an effort to halt further muscle degeneration.87,91–93
The etiology and pathogenesis of muscle degeneration are multifactorial.94–96 It was hypothesized that increasing medial tendon retraction in the context of a chronic tear places undue tension on the suprascapular nerve as it passes through the suprascapular notch, resulting in a stretch-induced neuropathy.97 However, the association of suprascapular neuropathy (SSN) with rotator cuff pathology remains unclear; SSN is correlated with tear size, but not fatty degeneration.98–100 Furthermore, the morphological patterns of fatty infiltration differ when comparing muscle changes following massive rotator cuff tears against those seen in isolated SSN.101 Nevertheless, animal studies have consistently found worse muscle degeneration with combined tendon tear and neuromuscular compromise, induced by injecting Botulinum toxin A74,79,80 or transecting the suprascapular nerve.102,103 The necessity of chemical or surgical denervation to induce pronounced fatty infiltration and fibrosis following tendon transection in small animal models may be due to the robust scar formation that prevents muscle unloading to the same degree seen in human shoulders in which there is minimal scar formation.80,104 This may contribute to the noted differences between animals as passive muscle biomechanical properties influence motor endplate properties105 and satellite cell behavior.106 Additionally, the rotator cuff muscles may be particularly sensitive to denervation, as compared to other muscle groups,107 although the mechanisms underlying such discrepancies remain uncertain.
Other studies have sought to elucidate the alterations in gene expression,104,108–110 proteostasis,111,112 and inflammation,111,113,114 underlying the noted structural and functional deficits. The Akt/mammalian target of rapamycin (mTOR) pathway has been implicated in both muscle atrophy and fatty infiltration following tendon rupture and/or denervation.115 Regarding the latter, Akt/mTOR has been shown to regulate the transcription factor sterol regulatory element binding protein 1 (SREBP-1) and its downstream mediators of adipogenesis, peroxisome proliferator activated receptor gamma (PPARγ) and CCAAT/enhancer-binding protein alpha (C/EBPα).108–110 While PPARγ and C/EBPα are likely instrumental in promoting adipogenic differentiation in putative progenitor cells, the resulting ectopic fat accumulation may occur through noncanonical intramyocellular lipid storage and synthesis pathways.116
At the same time, upregulation of transforming growth factor-beta (TGF-β) signaling is likely causative in promoting increased fibrosis following injury104,117 while also mediating some effect on fatty infiltration.118 There is emerging data to suggest that the p38 mitogen-activated protein kinase (MAPK) molecule orchestrates many of these changes, as inhibition of p38 MAPK in a rodent model of a degenerative rotator cuff tear significantly reduced fibrosis and the accumulation of intramuscular lipid.119 However, muscle is comprised of numerous cell types beyond the contractile myofibers, with each differentially contributing to the restoration or compromise of muscle function following cuff injury.
The satellite cell is central to muscle homeostasis, providing a reserve of renewable progenitor cells that can differentiate into contractile myocytes. Recent work has shown that the age-associated reduction in regenerative capacity of the muscle is at least partially attributable to cell-autonomous defects in satellite cell function driven by increased p38 MAPK signaling.120–122 Given the role of increased p38 MAPK in promoting fibrosis and fatty infiltration of muscles following rotator cuff tear,119 it is reasonable to speculate that aberrant satellite cell function contributes to tear-induced degenerative changes, but definitive studies have not yet been performed. Furthermore, the sources of cells that ultimately become the adipocytes and fibroblasts of the degenerated muscle are unclear. Recent work found that Tie2+ muscle mesenchymal progenitors were the major source of fibroblasts while PDGFRα+ fibro/adipogenic progenitors (FAPs) principally became adipocytes following rotator cuff tear,123 yet related work showed that FAPs can become fibroblasts as well.124 Interestingly, FAPs appear to facilitate myogenic differentiation of satellite cells in healthy muscles but contribute to ectopic fat formation when the muscle is injured, suggesting the importance of environmental factors in mediating FAP function.124,125
Paracrine signaling from macrophages, as opposed to lymphocytes,114 also contributes to degenerative changes, with prolonged macrophage infiltration worsening fibrosis and fatty infiltration following rotator cuff tears.111,113 A detailed understanding of how signaling networks and cellular composition evolve over location and time during rotator cuff disease pathogenesis will be essential for developing targeted therapeutic interventions. Differences in gene expression were found when comparing distal to proximal regions of the rotator cuff muscles following injury,104 while upregulated expression of many genes in full thickness tears were noted to be largely blunted in massive tears,110 perhaps contributing to the diminished healing capacity found with increasing tear size.1,102
Although much remains to be understood about the mechanisms of muscle degeneration, a growing body of research argues against an immutable progression of increasing fibrosis and fatty infiltration following a rotator cuff tear. Inhibition of TGF-β signaling by the small molecule inhibitor SB431542 reduced fibrosis and fatty infiltration in a mouse model when administered daily as an intraperitoneal injection following tendon and nerve transection.118 Statins, likely through their effects on inflammation,126 reduced collagen accumulation and preserved muscle fiber contractility when provided orally in a rat model of a massive rotator cuff tear.127 Similarly, intramuscular injection of an anabolic steroid (nandrolone decanoate) immediately following tendon release in a rabbit128 and sheep129 model mitigated fatty infiltration over 6 or 16 weeks, respectively. However, treatment with steroids for 6 weeks subsequent to surgical repair in the sheep model, after 16 weeks of degeneration without steroids, could not reverse the fatty infiltration that previously accumulated.129 These findings are consistent with clinical reports that surgical repair of rotator cuff tears may halt, but not reverse, degenerative changes in the muscles.1,86 As such, there may be limited clinical utility of any approach that requires administration at the time of initial injury given the insidious nature of most rotator cuff tears, where slow degenerative changes progress in the context of an asymptomatic, but propagating, tendon tear.
On the other hand, continuous traction by a transcutaneous device in a sheep model was shown to gradually return a chronically torn tendon to its anatomical footprint, thereby restoring normal muscle architecture, partially reversing muscle atrophy, and arresting the progression of fatty infiltration.130,131 While promising, a transcutaneous device would present numerous clinical challenges, most notably a risk for infection. However, more recent studies employing pharmaceutical and cell-based therapies suggest reversal of muscle degeneration may be possible. Both inhibition of cyclooxygenase enzymes132 and intramuscular injection of mesenchymal stem cells (MSCs)133,134 were found to reduce muscle fibrosis and lipid accumulation when administered following surgical repair of chronic rotator cuff tears. Additional benefit may also be possible by combining these strategies with interventions to prevent acute myofibril damage during repair. In particular, repair tension disrupts the myofibril membrane,135 with resulting disruption in Ca+2 handling and deficits in specific force.136 Damage may be mitigated by controlling repair tension137 or inclusion of membrane-stabilizing compounds.136,138 The extent to which these various strategies can be combined synergistically is unknown. For an expanded discussion on muscle biology and therapeutic interventions to reverse muscle degeneration associated with chronic rotator cuff tears, see the excellent recent review.139 Although efforts to reverse muscle degeneration are relatively new, their success will likely be required to optimize strategies intended to regenerate the structure and function of the tendon–bone interface at which most rotator cuff tears occur, given the known importance of in utero muscle contractions in enthesis development.
Regeneration of Native Enthesis Structure
The tendon–bone interface (i.e., enthesis) of the rotator cuff is a complex structure traditionally conceptualized as four distinct zones: (1) tendon, (2) uncalcified fibrocartilage, (3) calcified fibrocartilage, and (4) bone.140,141 Recent studies have demonstrated a more gradual transition across the interface, rather than discrete regions. It is this graded transition in mineral content,142,143 collagen fiber orientation,142,144 and biochemical composition,145,146 which minimizes the stress concentrations inherent in bi-material interfaces.147 The complex structure of the native enthesis is not restored following injury, even if surgical repair is performed.15,16 The resulting stress concentration following innate healing is thought to contribute to the high re-tear rate of rotator cuff repair. Furthermore, augmentation of surgical repairs with overlying scaffolds has not been shown to recapitulate the native enthesis structure.148 Composite scaffolds have been engineered with gradual or discrete transitions in fiber architecture, mineral content, biochemical composition, and cell phenotype.149–152 In vitro characterization of the composite scaffolds has shown region-specific differences in the target parameters, yet no study has explored the utility of these scaffolds when applied in vivo. Future studies must also consider how these composite scaffolds will be applied surgically153 and scaled anatomically, given the technical constraints of arthroscopic approaches and the small size of the native enthesis (i.e., ≤1 mm from tendon to bone).
An alternative strategy to restore the structure and function of the healing enthesis is to identify, and subsequently manipulate, the biological mediators that inhibit regeneration. The intra-articular environment, in which tissues are surrounded by synovial fluid, is particularly inhospitable to tendon-bone healing.154–156 Although in the nascent stages, the spatiotemporal patterns of the molecular mediators involved in intrinsic enthesis healing have begun to be identified, at least in rodent models.157,158 Following surgical repair of an acutely transected supraspinatus tendon in a rat model, a wound healing response resulted in a histologically unorganized and mechanically inferior insertion site, even at the longest time point (56 days).159 As scarless healing in embryonic tendons has been at least partially attributed to differences in TGF-β isoforms across the age of an organism—TGF-β3 predominates in in utero healing while TGF-β1 is principally expressed in the mature animal—it was hypothesized that an artificial predominance of TGF-β3 in the healing enthesis of a mature animal would promote better regeneration.160 While neutralization of TGF-β signaling compromised healing, neither isoform preferential enhanced the mechanical properties of the repair when applied exogenously through an osmotic pump.160 Overall, augmentation of the healing insertion site with growth factors,161,162 bone adhesives,163,164 or anti-inflammatory agents,165–167 has yielded equivocal results; structural changes, based upon histology, do not consistently correspond to changes in mechanical properties. These findings are reviewed in greater detail elsewhere.168
Concurrent studies have explored the utility of augmenting rotator cuff repairs with cells, most commonly employing adult tissue-derived MSCs. A reduced number of MSCs at the enthesis were found in patients with symptomatic rotator cuff tears,169 suggesting potential benefit of exogenous MSC supplementation. Indeed, a recent cohort study found improved healing rates and tissue quality up to 10 years following repair of small- to medium-sized tears (i.e., 1.5–3.5 cm) augmented with autologous MSCs (Table 1).170 On the other hand, the more extensive body of preclinical studies examining the efficacy of MSC supplementation of rotator cuff repairs contains mixed results.171 Several investigations have reported benefit172–174 while others found no effect of cell augmentation, whether exogenously delivered175,176 or endogenously recruited.177,178 At present, it is unknown what factors contribute to the inconsistent effects of MSCs, but likely include differences in animal models, experimental design, delivery vehicle, MSC concentration, and MSC phenotype, which may be further modulated by concomitant inclusion of growth factors or viral gene transduction constructs.171,179,180 For instance, augmentation with MSCs overexpressing tenogenic growth factor bone morphogenetic protein-13 (BMP-13) did not improve rotator cuff healing over MSCs alone,176 while MSCs overexpressing Scleraxis (Scx), a transcription factor associated with tenogenesis, did enhance histological and mechanical properties as compared to untransduced MSCs.181 Of interest, Scx is required for the formation of a functional enthesis in development, whereas the role of BMP-13 remains uncertain.182–184
As regeneration recapitulates development, elucidating the mechanisms of enthesis formation will likely prove essential in guiding interventions to restore rotator cuff structure and function following injury.185,186 Recent work has begun to unravel the complex spatiotemporal expression patterns of numerous molecular mediators governing enthesis formation.145,187–190 Instrumental to the maturation of the nascent enthesis is the presence of postnatal mechanical loading provided through spontaneous muscle activity.191–193 Botulinum toxin-induced paralysis of the supraspinatus muscle immediately after birth in a mouse model delayed fibrocartilage formation and decreased bone mineralization,193 resulting in sustained deficits in strength, modulus, and toughness.192 In parallel studies, it was shown that ablation of hedgehog (Hh) signaling in progenitor cells that constitute the primordial enthesis results in loss of mineralized fibrocartilage with corresponding reductions in mechanical properties (Fig. 2A–D).194–196 Given the known roles of Hh signaling in endochondral ossification197,198 and mechanotransduction,199,200 it was hypothesized that mechanical loading in the early postnatal period drives enthesis formation by modulating Hh activity in the enthesis fibrocartilage cells.201,202 Indeed, botox-mediated muscle paralysis in the first week of postnatal development actually increased the number of Hh-responsive cells due to compensatory feedback.202 On the other hand, ablation of these Hh-responsive cells resulted in a loss of mineralized fibrocartilage, with little remodeling up to 5 weeks later.202 Taken together, these studies demonstrate that mechanical loading, through active muscle contractions, drive enthesis maturation by modulating Hh signaling in fibrocartilage cells.
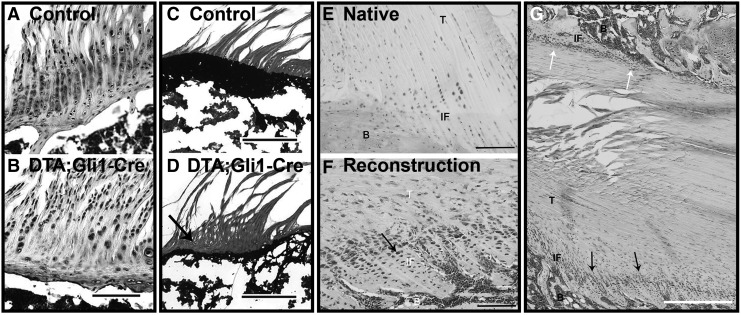
FIG. 2.
The role of hedgehog signaling in enthesis formation and healing. Parallel columns of fibrochondrocytes are embedded in a proteoglycan-rich extracellular matrix (A) that has undergone robust mineralization (C) by postnatal day 42 in the developing rotator cuff enthesis of a mouse, as shown through Safranin O (A, B) and Von Kossa (C, D) staining, respectively. Conditional ablation of Hh-responsive cells disrupts enthesis formation with reductions in proteoglycan (B) and mineral content (D, black arrow). Adapted with permission from Schwartz et al.202 Cells located at the native ACL–bone interface in a mature rat do not strongly express Hh mediators (E). Conversely, Hh signaling is strongly upregulated in cells at the tendon graft–bone tunnel interface following ACL reconstruction (F). Focal stresses appear to differentially affect Hh signaling and tissue organization (G), as interfaces presumably under greater tension (bottom, black arrows) exhibit greater organization and fibrochondrocyte alignment than unloaded interfaces (G, white arrows). ACL, anterior cruciate ligament; B, bone; Hh, hedgehog; IF, interface; T, tendon. Adapted with permission from Carbone et al.203
The extent to which failed regeneration of enthesis following injury is attributable to aberrant mechanical loading or dysregulated Hh signaling in reparative cells remains unknown. However, positive Hh immunohistochemical staining was recently observed at the graft–bone tunnel interface at 3 and 6 weeks following anterior cruciate ligament (ACL) reconstruction in a rat model (Fig. 2E, F).203 Interestingly, animals that received pretensioned tendon grafts showed increased staining area and intensity, suggesting an influence of mechanical stress on Hh signaling during tendon-bone healing.203 As Indian Hedgehog (Ihh) and parathyroid hormone (PTH)-related peptide (PTHrP) are reciprocal mediators of a feedback loop controlling chondrogenesis and mineralization at the growth plate204,205 and enthesis,189,201 it was hypothesized that the subcutaneous supplementation of the related PTH might improve enthesis healing in rat model of acute supraspinatus transection and repair.206 Although treatment with PTH increased bone and mineralized fibrocartilage formation and improved collagen fiber organization, this did not correspond to improved mechanical properties,206 further demonstrating the complex interaction of soluble factors and mechanical stimuli in directing the emergence of enthesis structure and function.
Mechanical Loading in the Context of Tissue Engineering
Given the fundamental role of mechanical loading in rotator cuff development and homeostasis, investigation of its influence on healing naturally follows. To that end, it has been shown, principally through animal models, that the complete removal of load postoperatively is detrimental to rotator cuff healing207 but premature loading risks surgical failure or tendon lengthening. As a result, controlled mobilization is advocated but its operationalization remains a challenge.208 The early wound callus is mechanically weak with increased cellular content, including both progenitor cells and a time-specific predominance of a particular phenotype of inflammatory cell.209,210 As most rotator cuff repairs fail within the first 3–6 months,24–26 it had been hypothesized that botox-mediated unloading could transiently protect the repair site from damaging muscular forces, ultimately resulting in enhanced tissue structure and strength.211
When applied in a rat model, botox-treated specimens showed accelerated formation of a normal tidemark and increased collagen fiber organization, but negligible or inferior mechanical properties and bone morphometry at weeks 4, 8, and 24.212 Similar detriments were found in a rabbit model of chronic rotator cuff tears.213 In a related study, an external fixator was applied to the tibiae and femurs of rats that underwent ACL reconstruction, eliminating tissue strain with the exception of 50 cycles of 2% strain applied daily and commenced (1) immediately postoperatively, (2) on postoperative day 4, (3) postoperative day 10, or (4) never (i.e., complete immobilization).214 Specimens from the delayed immobilization group (i.e., postoperative day 10) demonstrated superior mechanical and histological properties along with greater bone formation when compared against the other loading conditions.214 Similarly, immediate postoperative passive motion was detrimental to shoulder mechanics in a rat model of rotator cuff repair.215 Lastly, scapular dyskinesis, as induced through transection of the accessory and long thoracic nerves, decreased mechanical properties, altered histology, and diminished tendon organization at the enthesis following rotator cuff repair.216 Taken together, these studies validate the importance of controlled mobilization postoperatively as a means to optimize healing. In the clinical setting, where the practical postoperative loading protocols have been far more constrained than those investigated in animal studies, there is no long-term difference between early mobilization compared to delayed rehabilitation.217–219 However, there is growing evidence that in vivo mechanical loads can and should be considered if the promise of tissue engineering strategies for improved rotator cuff healing is to be realized.
In a seminal study, the influence of in vivo loading on a braided porcine SIS graft used to replace the rabbit Achilles tendon was examined.220 After a 2-week immobilization period, unrestricted motion and weight bearing during the early remodeling phase accelerated tendon remodeling, as compared to totally immobilized joints.220 In related work, acellular dermal matrix patches were used to reconstruct the rotator cuff in a rat model, with variable periods of immobilization to follow. Two weeks of immobilization yielded superior collagen organization and mechanical strength compared to no immobilization or 6 weeks of immobilization.221,222 Beyond scaffolds, the importance of in vivo loading also extends to the application of growth factors and cells. Injection of cartilage-derived morphogenetic protein 2 (CDMP-2) into unloaded rat tendons produced heterotopic bone formation, which was greatly reduced in loaded tendons.223 Likewise, muscle-derived stem cells (MDSCs) formed more myofibrils and reduced fibrosis when injected into injured muscle of mice exposed to daily treadmill running, as compared to littermates that only engaged in normal cage activity.224
Though limited, these studies suggest that the utility of many tissue engineering strategies will be influenced by the mechanical microenvironment of the injured tissue to which they are applied. With particular regard to efforts to enhance rotator cuff healing, it is important to consider all the elements that comprise the rotator cuff organ and the possibility of synergistic interactions of biological therapies heretofore applied to an individual element. For instance, a bioactive scaffold sheet that can offload the surgical repair may serve to reverse the degenerative changes in the tendinopathic tendon while also allowing earlier joint mobilization with the resulting strains at the healing enthesis providing mechanotransductive cues to exogenous Hh-responsive MSCs, thereby recapitulating the developmental signals essential for the formation of a mineralized fibrocartilage interface. These combinatorial approaches have yet to be systematically investigated, but may offer novel approaches to addressing the otherwise recalcitrant challenge that is the chronic rotator cuff tear. In a recent clinical study, arthroscopic repairs of massive rotator cuff tears that were augmented with both bone marrow stimulation and patch augmentation resulted in a significantly lower re-tear rate than conventional repairs.225 Although the study design limits conclusions about any additive benefit of bone marrow stimulation and scaffold augmentation, the results were encouraging.
Present Challenges and Future Perspectives
Despite a dramatic increase in research on rotator cuff repair over the past two decades, there is little evidence that clinical outcomes of rotator cuff repair have improved.226 However, poor outcomes are disproportionately attributable to chronic massive tears, which remain a persistent challenge.227 Increasingly sophisticated tissue engineering strategies may provide orthopedic surgeons with the capacity to restore the structure and function of the rotator cuff, which has traditionally been considered to possess a limited intrinsic healing capacity. In this review, we have highlighted the concept of the rotator cuff as an organ, for the various tissues that comprise it each exhibit degenerative changes in the context of a chronic tear. It is therefore posited that tissue engineering strategies aimed at regenerating one element might provide synergistic benefits when combined. Investigation of this hypothesis in animal studies is necessary, as are studies examining how mechanical loading of augmented repairs might provide mechanotransductive cues capable of promoting tissue-specific cell differentiation and favorable tissue remodeling.
The judicious application of loading protocols could be enhanced by further characterization of in vivo forces in both healthy and healing tissues228,229 and the use of these results as design parameters for novel biomaterials and computational models of rotator cuff mechanics.230–232 For instance, recent work has begun to elucidate how tear size and location affect tear propagation when tissues are cyclically loaded with forces likely experienced in postoperative rehabilitation.233,234 Parallel in vivo investigations have explored how conservative therapy (i.e., physical therapy)2 and surgical stabilization235 affect glenohumeral kinematics in patients. In combining these results, models may be developed that are predictive of the in vivo stresses experienced by a healing tissue and possibly prescriptive for the exercise protocols that constitute a rehabilitation program at a given time after surgery.236 In the future, it may be possible to combine these emerging regenerative rehabilitation approaches with tissue engineering strategies to more consistently restore tissue structure and function at an accelerated pace without sacrificing safety.
The successful translation of tissue engineering strategies into clinical practice will also require continued refinement of animal models, such that the pathogenesis in the model organism more closely recapitulates that of human patients, notwithstanding insurmountable differences in anatomy and biology across species. With regards to rotator cuff pathology, increased consideration has been given to the effect of tear chronicity and aging on the healing potential of the injured shoulder.179 By their very nature, chronic rotator cuff tears are most commonly seen in the context of increased age, which itself is correlated with poor postoperative rotator cuff integrity.237 Until recently, most investigations of the potential benefit of tissue engineering strategies in enhancing rotator cuff healing were performed in young, healthy animals in which the tendon was acutely transected and immediately repaired with or without augmentation. Novel animal models of chronic degeneration have now been reported in mice,238,239 rats,75,103 and sheep,130,240 with associated impairment in healing potential.74,75 These animal models may be more predictive of the utility of a particular tissue engineering strategy when applied to patients. For instance, no benefit to augmenting repairs with a fascia lata autograft patch was found in patients with high-grade fatty infiltration of their muscles.77 Preclinical models employing aged animals may also be of value, as older animals also experience age-associated exacerbation in muscle stiffness241 and fatty infiltration116 in addition to reduced healing capacity.242
Conclusions
This review highlights the three principal aims toward which tissue engineering strategies have been applied in an effort to improve rotator cuff healing: (1) immediate mechanical augmentation of the surgical repair, (2) restoration of muscle quality and contractility, and (3) regeneration of native enthesis structure. Given the seamless integration of multiple tissues comprising the rotator cuff, and their concurrent degeneration in the context of chronic massive tears, it is instructive to consider the concept of the rotator cuff as an organ. In doing so, it is posited that combining the individual approaches to address the three principal aims may offer synergistic benefit. At the same time, it must be emphasized that the application of a tissue engineering strategy to any given tissue of the rotator cuff organ must likely be temporally discrete and spatially confined, as signaling pathways of putative benefit in one tissue may be detrimental in adjacent tissues. For instance, TGF-β-mediated upregulation of the tenogenic transcription factor Scx may reverse degenerative changes in the tendon midsubstance or enhance enthesis healing but could also exacerbate muscle atrophy and fibrosis if TGF-β delivery is inadequately localized.243 Much work remains to validate this approach and will invariably require strong collaboration among engineers, biologists, and clinicians to bring the promise of tissue engineering and regenerative medicine to come to fruition in treating massive chronic rotator cuff tears.
Acknowledgments
This work was supported in part by the University of Pittsburgh Physicians Academic Foundation, the NIH (NIH R01 AR062947, and CATER Training Grant, 5T32 EB001026), and the U.S. Department of Defense (W81XWH-15-1-0104).
Disclosure Statement
No competing financial interests exist.
References
- 1.Tashjian R.Z. Epidemiology, natural history, and indications for treatment of rotator cuff tears. Clin Sports Med 31, 589, 2012 [DOI] [PubMed] [Google Scholar]
- 2.Miller R.M., Popchak A., Vyas D., Tashman S., Irrgang J.J., Musahl V., and Debski R.E. Effects of exercise therapy for the treatment of symptomatic full-thickness supraspinatus tears on in vivo glenohumeral kinematics. J Shoulder Elbow Surg 25, 641, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Moosmayer S., Tariq R., Stiris M., and Smith H.J. The natural history of asymptomatic rotator cuff tears a three-year follow-up of fifty cases. J Bone Joint Surg Am 95A, 1249, 2013 [DOI] [PubMed] [Google Scholar]
- 4.Keener J.D., Galatz L.M., Teefey S.A., Middleton W.D., Steger-May K., Stobbs-Cucchi G., Patton R., and Yamaguchi K. A prospective evaluation of survivorship of asymptomatic degenerative rotator cuff tears. J Bone Joint Surg Am 97A, 89, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galatz L.M., Ball C.M., Teefey S.A., Middleton W.D., and Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am 86A, 219, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Tashjian R.Z. Aaos clinical practice guideline: optimizing the management of rotator cuff problems. J Am Acad Orthop Surg 19, 380, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Oh J.H., Kim S.H., Ji H.M., Jo K.H., Bin S.W., and Gong H.S. Prognostic factors affecting anatomic outcome of rotator cuff repair and correlation with functional outcome. Arthroscopy 25, 30, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Le B.T.N., Wu X.L., Lam P.H., and Murrell G.A.C. Factors predicting rotator cuff retears an analysis of 1000 consecutive rotator cuff repairs. Am J Sports Med 42, 1134, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Barry J.J., Lansdown D.A., Cheung S., Feeley B.T., and Benjamin C. The relationship between tear severity, fatty infiltration, and muscle atrophy in the supraspinatus. J Shoulder Elbow Surg 22, 18, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Tashjian R.Z., Hung M., Burks R.T., and Greis P.E. Influence of preoperative musculotendinous junction position on rotator cuff healing using single-row technique. Arthroscopy 29, 1748, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Oh J.H., Song B.W., and Lee Y.S. Measurement of volumetric bone mineral density in proximal humerus using quantitative computed tomography in patients with unilateral rotator cuff tear. J Shoulder Elbow Surg 23, 993, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Waldorff E.I., Lindner J., Kijek T.G., Downie B.K., Hughes R.E., Carpenter J.E., and Miller B.S. Bone density of the greater tuberosity is decreased in rotator cuff disease with and without full-thickness tears. J Shoulder Elbow Surg 20, 904, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Braunstein V., Ockert B., Windolf M., Sprecher C.M., Mutschler W., Imhoff A., Postl L.K.L., Biberthaler P., and Kirchhoff C. Increasing pullout strength of suture anchors in osteoporotic bone using augmentation—a cadaver study. Clin Biomech (Bristol, Avon) 30, 243, 2015 [DOI] [PubMed] [Google Scholar]
- 14.Chung S.W., Oh J.H., Gong H.S., Kim J.Y., and Kim S.H. Factors affecting rotator cuff healing after arthroscopic repair osteoporosis as one of the independent risk factors. Am J Sports Med 39, 2099, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Newsham-West R., Nicholson H., Walton M., and Milburn P. Long-term morphology of a healing bone-tendon interface: a histological observation in the sheep model. J Anat 210, 318, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodeo S.A., Arnoczky S.P., Torzilli P.A., Hidaka C., and Warren R.F. Tendon healing in a bone tunnel – a biomechanical and histological study in the dog. J Bone Joint Surg Am 75A, 1795, 1993 [DOI] [PubMed] [Google Scholar]
- 17.Cummins C.A., and Murrell G.A.C. Mode of failure for rotator cuff repair with suture anchors identified at revision surgery. J Shoulder Elbow Surg 12, 128, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Wlk M.V., Abdelkafy A., Hexel M., Krasny C., Aigner N., Meizer R., and Landsiedl F. Biomechanical evaluation of suture-tendon interface and tissue holding of three suture configurations in torn and degenerated versus intact human rotator cuffs. Knee Surg Sports Traumatol Arthrosc 23, 386, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Nassos J.T., ElAttrache N.S., Angel M.J., Tibone J.E., Limpisvasti O., and Lee T.Q. A watertight construct in arthroscopic rotator cuff repair. J Shoulder Elbow Surg 21, 589, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Brown M.J., Pula D.A., Kluczynski M.A., Mashtare T., and Bisson L.J. Does suture technique affect re-rupture in arthroscopic rotator cuff repair? A meta-analysis. Arthroscopy 31, 1576, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Dines J.S., Bedi A., ElAttrache N.S., and Dines D.M. Single-row versus double-row rotator cuff repair: techniques and outcomes. J Am Acad Orthop Surg 18, 83, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Mall N.A., Tanaka M.J., Choi L.S., and Paletta G.A., Jr. Factors affecting rotator cuff healing. J Bone Joint Surg Am 96A, 778, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Gladstone J.N., Bishop J.Y., Lo I.K.Y., and Flatow E.L. Fatty infiltration and atrophy of the rotator cuff do not improve after rotator cuff repair and correlate with poor functional outcome. Am J Sports Med 35, 719, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Iannotti J.P., Deutsch A., Green A., Rudicel S., Christensen J., Marraffino S., and Rodeo S. Time to failure after rotator cuff repair: a prospective imaging study. J Bone Joint Surg Am 95A, 965, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Kim J.H., Hong I.T., Ryu K.J., Bong S.T., Lee Y.S., and Kim J.H. Retear rate in the late postoperative period after arthroscopic rotator cuff repair. Am J Sports Med 42, 2606, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Miller B.S., Downie B.K., Kohen R.B., Kijek T., Lesniak B., Jacobson J.A., Hughes R.E., and Carpenter J.E. When do rotator cuff repairs fail? Serial ultrasound examination after arthroscopic repair of large and massive rotator cuff tears. Am J Sports Med 39, 2064, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Derwin K.A., Baker A.R., Spragg R.K., Leigh D.R., and Iannotti J.P. Commercial extracellular matrix scaffolds for rotator cuff tendon repair – biomechanical, biochemical, and cellular properties. J Bone Joint Surg Am 88A, 2665, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Chainani A., and Little D. Current status of tissue-engineered scaffolds for rotator cuff repair. Tech Orthop 31, 91, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hakimi O., Mouthuy P.-A., and Carr A. Synthetic and degradable patches: an emerging solution for rotator cuff repair. Int J Exp Pathol 94, 287, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aurora A., McCarron J.A., van den Bogert A.J., Gatica J.E., Iannotti J.P., and Derwin K.A. The biomechanical role of scaffolds in augmented rotator cuff tendon repairs. J Shoulder Elbow Surg 21, 1064, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Ratcliffe A., Butler D.L., Dyment N.A., Cagle P.J., Jr., Proctor C.S., Ratcliffe S.S., and Flatow E.L. Scaffolds for tendon and ligament repair and regeneration. Ann Biomed Eng 43, 819, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shea K.P., Obopilwe E., Sperling J.W., and Iannotti J.P. A biomechanical analysis of gap formation and failure mechanics of a xenograft-reinforced rotator cuff repair in a cadaveric model. J Shoulder Elbow Surg 21, 1072, 2012 [DOI] [PubMed] [Google Scholar]
- 33.McCarron J.A., Milks R.A., Mesiha M., Aurora A., Walker E., Iannotti J.P., and Derwin K.A. Reinforced fascia patch limits cyclic gapping of rotator cuff repairs in a human cadaveric model. J Shoulder Elbow Surg 21, 1680, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Barber F.A., Herbert M.A., and Boothby M.H. Ultimate tensile failure loads of a human dermal allograft rotator cuff augmentation. Arthroscopy 24, 20, 2008 [DOI] [PubMed] [Google Scholar]
- 35.McCarron J.A., Milks R.A., Chen X., Iannotti J.P., and Derwin K.A. Improved time-zero biomechanical properties using poly-l-lactic acid graft augmentation in a cadaveric rotator cuff repair model. J Shoulder Elbow Surg 19, 688, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Baker A.R., McCarron J.A., Tan C.D., Iannotti J.P., and Derwin K.A. Does augmentation with a reinforced fascia patch improve rotator cuff repair outcomes? Clin Orthop Relat Res 470, 2513, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derwin K.A., Codsi M.J., Milks R.A., Baker A.R., McCarron J.A., and Iannotti J.P. Rotator cuff repair augmentation in a canine model with use of a woven poly-l-lactide device. J Bone Joint Surg Am 91A, 1159, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schlegel T.F., Hawkins R.J., Lewis C.W., Motta T., and Turner A.S. The effects of augmentation with swine small intestine submucosa on tendon healing under tension - histologic and mechanical evaluations in sheep. Am J Sports Med 34, 275, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Nicholson G.P., Breur G.J., Van Sickle D., Yao J.Q., Kim J., and Blanchard C.R. Evaluation of a cross-linked acellular porcine dermal patch for rotator cuff repair augmentation in an ovine model. J Shoulder Elbow Surg 16, 184S, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Thangarajah T., Pendegrass C.J., Shahbazi S., Lambert S., Alexander S., and Blunn G.W. Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med 3, 2325967115587495, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferguson D.P., Lewington M.R., Smith T.D., and Wong I.H. Graft utilization in the augmentation of large-to-massive rotator cuff repairs. Am J Sports Med 44, 2984, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Iannotti J.P., Codsi M.J., Kwon Y.W., Derwin K., Ciccone J., and Brems J.J. Porcine small intestine submucosa augmentation of surgical repair of chronic two-tendon rotator cuff tears – a randomized, controlled trial. J Bone Joint Surg Am 88A, 1238, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Walton J.R., Bowman N.K., Khatib Y., Linklater J., and Murrell G.A.C. Restore orthobiologic implant: not recommended for augmentation of rotator cuff repairs. J Bone Joint Surg Am 89A, 786, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Barber F.A., Burns J.P., Deutsch A., Labbe M.R., and Litchfield R.B. A prospective, randomized evaluation of acellular human dermal matrix augmentation for arthroscopic rotator cuff repair. Arthroscopy 28, 8, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Lenart B.A., Martens K.A., Kearns K.A., Gillespie R.J., Zoga A.C., and Williams G.R. Treatment of massive and recurrent rotator cuff tears augmented with a poly-l-lactide graft, a preliminary study. J Shoulder Elbow Surg 24, 915, 2015 [DOI] [PubMed] [Google Scholar]
- 46.Ciampi P., Scotti C., Nonis A., Vitali M., Di Serio C., Peretti G.M., and Fraschini G. The benefit of synthetic versus biological patch augmentation in the repair of posterosuperior massive rotator cuff tears a 3-year follow-up study. Am J Sports Med 42, 1169, 2014 [DOI] [PubMed] [Google Scholar]
- 47.Gupta A.K., Hug K., Boggess B., Gavigan M., and Toth A.P. Massive or 2-tendon rotator cuff tears in active patients with minimal glenohumeral arthritis clinical and radiographic outcomes of reconstruction using dermal tissue matrix xenograft. Am J Sports Med 41, 872, 2013 [DOI] [PubMed] [Google Scholar]
- 48.Gupta A.K., Hug K., Berkoff D.J., Boggess B.R., Gavigan M., Malley P.C., and Toth A.P. Dermal tissue allograft for the repair of massive irreparable rotator cuff tears. Am J Sports Med 40, 141, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Mori D., Funakoshi N., and Yamashita F. Arthroscopic surgery of irreparable large or massive rotator cuff tears with low-grade fatty degeneration of the infraspinatus: patch autograft procedure versus partial repair procedure. Arthroscopy 29, 1911, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Nimura A., Kato A., Yamaguchi K., Mochizuki T., Okawa A., Sugaya H., and Akita K. The superior capsule of the shoulder joint complements the insertion of the rotator cuff. J Shoulder Elbow Surg 21, 867, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Mihata T., McGarry M.H., Kahn T., Goldberg I., Neo M., and Lee T.Q. Biomechanical role of capsular continuity in superior capsule reconstruction for irreparable tears of the supraspinatus tendon. Am J Sports Med 44, 1423, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Mihata T., McGarry M.H., Kahn T., Goldberg I., Neo M., and Lee T.Q. Biomechanical effects of acromioplasty on superior capsule reconstruction for irreparable supraspinatus tendon tears. Am J Sports Med 44, 191, 2016 [DOI] [PubMed] [Google Scholar]
- 53.Mihata T., Lee T.Q., Watanabe C., Fukunishi K., Ohue M., Tsujimura T., and Kinoshita M. Clinical results of arthroscopic superior capsule reconstruction for irreparable rotator cuff tears. Arthroscopy 29, 459, 2013 [DOI] [PubMed] [Google Scholar]
- 54.Tokish J.M., and Beicker C. Superior capsule reconstruction technique using an acellular dermal allograft. Arthrosc Tech 4, E833, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keane T.J., Londono R., Turner N.J., and Badylak S.F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials 33, 1771, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Sandor M., Xu H., Connor J., Lombardi J., Harper J.R., Silverman R.P., and McQuillan D.J. Host response to implanted porcine-derived biologic materials in a primate model of abdominal wall repair. Tissue Eng Part A 14, 2021, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Ricchetti E.T., Aurora A., Iannotti J.P., and Derwin K.A. Scaffold devices for rotator cuff repair. J Shoulder Elbow Surg 21, 251, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Brown B.N., Londono R., Tottey S., Zhang L., Kukla K.A., Wolf M.T., Daly K.A., Reing J.E., and Badylak S.F. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater 8, 978, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown B.N., Ratner B.D., Goodman S.B., Amar S., and Badylak S.F. Macrophage polarization: an opportunity for improved outcomes in and regenerative medicine. Biomaterials 33, 3792, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith R.D.J., Carr A., Dakin S.G., Snelling S.J.B., Yapp C., and Hakimi O. The response of tenocytes to commercial scaffolds used for rotator cuff repair. Eur Cell Mater 31, 107, 2016 [DOI] [PubMed] [Google Scholar]
- 61.Hakimi O., Mouthuy P.A., Zargar N., Lostis E., Morrey M., and Carr A. A layered electrospun and woven surgical scaffold to enhance endogenous tendon repair. Acta Biomater 26, 124, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Yang G., Lin H., Rothrauff B.B., Yu S., and Tuan R.S. Multilayered polycaprolactone/gelatin fiber-hydrogel composite for tendon tissue engineering. Acta Biomater 35, 68, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Farshad M., Gerber C., Snedeker J.G., Frauenfelder T., and Meyer D.C. Structure of retracted tendons after staged repair following continuous traction. Knee Surg Sports Traumatol Arthrosc 19, 2131, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Lee H.-J., Kim Y.-S., Ok J.-H., and Song H.-J. Apoptosis occurs throughout the diseased rotator cuff. Am J Sports Med 41, 2249, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Yuan J., Murrell G.A.C., Wei A.Q., and Wang M.X. Apoptosis in rotator cuff tendonopathy. J Orthop Res 20, 1372, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Fabis J., Szemraj J., Strek M., Fabis A., Dutkiewicz Z., and Zwierzchowski T.J. Is resection of the tendon edge necessary to enhance the healing process? An evaluation of the homeostasis of apoptotic and inflammatory processes in the distal 1 cm of a torn supraspinatus tendon: part I. J Shoulder Elbow Surg 23, 1772, 2014 [DOI] [PubMed] [Google Scholar]
- 67.Fabis J., Szemraj J., Strek M., Fabis A., Dutkiewicz Z., and Zwierzchowski T.J. Is resection of the tendon edge necessary to enhance the healing process? An evaluation of the expression of collagen type I, Il-1 beta, IFN-gamma, Il-4, and Il-13 in the distal 1 cm of a torn supraspinatus tendon: part II. J Shoulder Elbow Surg 23, 1779, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Chung S.W., Kim J.Y., Yoon J.P., Lyu S.H., Rhee S.M., and Oh S.B. Arthroscopic repair of partial-thickness and small full-thickness rotator cuff tears tendon quality as a prognostic factor for repair integrity. Am J Sports Med 43, 588, 2015 [DOI] [PubMed] [Google Scholar]
- 69.McCarron J.A., Derwin K.A., Bey M.J., Polster J.M., Schils J.P., Ricchetti E.T., and Iannotti J.P. Failure with continuity in rotator cuff repair “healing.” Am J Sports Med 41, 134, 2013 [DOI] [PubMed] [Google Scholar]
- 70.Fessel G., Gerber C., and Snedeker J.G. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. J Shoulder Elbow Surg 21, 209, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Chen J.M., Yu Q., Wu B., Lin Z., Pavlos N.J., Xu J.K., Ouyang H.W., Wang A., and Zheng M.H. Autologous tenocyte therapy for experimental Achilles tendinopathy in a rabbit model. Tissue Eng Part A 17, 2037, 2011 [DOI] [PubMed] [Google Scholar]
- 72.Obaid H., and Connell D. Cell therapy in tendon disorders. Am J Sports Med 38, 2123, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Wieser K., Farshad M., Meyer D.C., Conze P., von Rechenberg B., and Gerber C. Tendon response to pharmaco-mechanical stimulation of the chronically retracted rotator cuff in sheep. Knee Surg Sports Traumatol Arthrosc 23, 577, 2015 [DOI] [PubMed] [Google Scholar]
- 74.Killian M.L., Cavinatto L., Shah S.A., Sato E.J., Ward S.R., Havlioglu N., Galatz L.M., and Thomopoulos S. The effects of chronic unloading and gap formation on tendon-to-bone healing in a rat model of massive rotator cuff tears. J Orthop Res 32, 439, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Killian M.L., Cavinatto L.M., Ward S.R., Havlioglu N., Thomopoulos S., and Galatz L.M. Chronic degeneration leads to poor healing of repaired massive rotator cuff tears in rats. Am J Sports Med 43, 2401, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Santoni B.G., McGilvray K.C., Lyons A.S., Bansal M., Turner A.S., MacGillivray J.D., Coleman S.H., and Puttlitz C.M. Biomechanical analysis of an ovine rotator cuff repair via porous patch augmentation in a chronic rupture model. Am J Sports Med 38, 679, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Mori D., Funakoshi N., Yamashita F., and Wakabayashi T. Effect of fatty degeneration of the infraspinatus on the efficacy of arthroscopic patch autograft procedure for large to massive rotator cuff tears. Am J Sports Med 43, 1108, 2015 [DOI] [PubMed] [Google Scholar]
- 78.Melis B., DeFranco M.J., Chuinard C., and Walch G. Natural history of fatty infiltration and atrophy of the supraspinatus muscle in rotator cuff tears. Clin Orthop Relat Res 468, 1498, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sato E.J., Killian M.L., Choi A.J., Lin E., Esparza M.C., Galatz L.M., Thomopoulos S., and Ward S.R. Skeletal muscle fibrosis and stiffness increase after rotator cuff tendon injury and neuromuscular compromise in a rat model. J Orthop Res 32, 1111, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato E.J., Killian M.L., Choi A.J., Lin E., Choo A.D., Rodriguez-Soto A.E., Lim C.T., Thomopoulos S., Galatz L.M., and Ward S.R. Architectural and biochemical adaptations in skeletal muscle and bone following rotator cuff injury in a rat model. J Bone Joint Surg Am 97A, 565, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gimbel J.A., Van Kleunen J.P., Lake S.P., Williams G.R., and Soslowsky L.J. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech 40, 561, 2007 [DOI] [PubMed] [Google Scholar]
- 82.Cadet E.R., Hsu J.W., Levine W.N., Bigliani L.U., and Ahmad C.S. The relationship between greater tuberosity osteopenia and the chronicity of rotator cuff tears. J Shoulder Elbow Surg 17, 73, 2008 [DOI] [PubMed] [Google Scholar]
- 83.Galatz L.M., Rothermich S.Y., Zaegel M., Silva M.J., Havlioglu N., and Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res 23, 1441, 2005 [DOI] [PubMed] [Google Scholar]
- 84.Osti L., Buda M., and Buono A.D. Fatty infiltration of the shoulder: diagnosis and reversibility. Muscles Ligaments Tendons J 3, 351, 2013 [PMC free article] [PubMed] [Google Scholar]
- 85.Chaudhury S., Dines J.S., Delos D., Warren R.F., Voigt C., and Rodeo S.A. Role of fatty infiltration in the pathophysiology and outcomes of rotator cuff tears. Arthritis Care Res 64, 76, 2012 [DOI] [PubMed] [Google Scholar]
- 86.Deniz G., Kose O., Tugay A., Guler F., and Turan A. Fatty degeneration and atrophy of the rotator cuff muscles after arthroscopic repair: does it improve, halt or deteriorate? Arch Orthop Trauma Surg 134, 985, 2014 [DOI] [PubMed] [Google Scholar]
- 87.Kuzel B.R., Grindel S., Papandrea R., and Ziegler D. Fatty infiltration and rotator cuff atrophy. J Am Acad Orthop Surg 21, 613, 2013 [DOI] [PubMed] [Google Scholar]
- 88.Park Y.B., Ryu H.Y., Hong J.H., Ko Y.H., and Yoo J.C. Reversibility of supraspinatus muscle atrophy in tendon-bone healing after arthroscopic rotator cuff repair. Am J Sports Med 44, 981, 2016 [DOI] [PubMed] [Google Scholar]
- 89.Jost B., Pfirrmann C.W.A., and Gerber C. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am 82A, 304, 2000 [DOI] [PubMed] [Google Scholar]
- 90.Mendias C.L., Roche S.M., Harning J.A., Davis M.E., Lynch E.B., Enselman E.R.S., Jacobson J.A., Claflin D.R., Calve S., and Bedi A. Reduced muscle fiber force production and disrupted myofibril architecture in patients with chronic rotator cuff tears. J Shoulder Elbow Surg 24, 111, 2015 [DOI] [PubMed] [Google Scholar]
- 91.Trudel G., Ramachandran N., Ryan S.E., Rakhra K., and Uhthoff H.K. Improved strength of early versus late supraspinatus tendon repair: a study in the rabbit. J Shoulder Elbow Surg 21, 828, 2012 [DOI] [PubMed] [Google Scholar]
- 92.Uhthoff H.K., Coletta E., and Trudel G. Effect of timing of surgical ssp tendon repair on muscle alterations. J Orthop Res 32, 1430, 2014 [DOI] [PubMed] [Google Scholar]
- 93.Goutallier D., Postel J.M., Bernageau J., Lavau L., and Voisin M.C. Fatty muscle degeneration in cuff ruptures – pre- and postoperative evaluation by CT scans. Clin Orthop Relat Res 304, 78, 1994 [PubMed] [Google Scholar]
- 94.Nho S.J., Yadav H., Shindle M.K., and MacGillivray J.D. Rotator cuff degeneration – etiology and pathogenesis. Am J Sports Med 36, 987, 2008 [DOI] [PubMed] [Google Scholar]
- 95.Kang J.R., and Gupta R. Mechanisms of fatty degeneration in massive rotator cuff tears. J Shoulder Elbow Surg 21, 175, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Laron D., Samagh S.P., Liu X.H., Kim H.T., and Feeley B.T. Muscle degeneration in rotator cuff tears. J Shoulder Elbow Surg 21, 164, 2012 [DOI] [PubMed] [Google Scholar]
- 97.Albritton M.J., Graham R.D., Richards R.S., and Basamania C.J. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg 12, 497, 2003 [DOI] [PubMed] [Google Scholar]
- 98.Shi L.L., Boykin R.E., Lin A., and Warner J.J.P. Association of suprascapular neuropathy with rotator cuff tendon tears and fatty degeneration. J Shoulder Elbow Surg 23, 339, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Collin P., Treseder T., Ladermann A., Benkalfate T., Mourtada R., Courage O., and Favard L. Neuropathy of the suprascapular nerve and massive rotator cuff tears: a prospective electromyographic study. J Shoulder Elbow Surg 23, 28, 2014 [DOI] [PubMed] [Google Scholar]
- 100.Denard P.J., Ladermann A., Brady P.C., Narbona P., Adams C.R., Arrigoni P., Huberty D., Zlatkin M.B., Sanders T.G., and Burkhart S.S. Pseudoparalysis from a massive rotator cuff tear is reliably reversed with an arthroscopic rotator cuff repair in patients without preoperative glenohumeral arthritis. Am J Sports Med 43, 2373, 2015 [DOI] [PubMed] [Google Scholar]
- 101.Beeler S., Ek E.T.H., and Gerber C. A comparative analysis of fatty infiltration and muscle atrophy in patients with chronic rotator cuff tears and suprascapular neuropathy. J Shoulder Elbow Surg 22, 1537, 2013 [DOI] [PubMed] [Google Scholar]
- 102.Kim H.M., Galatz L.M., Lim C., Havlioglu N., and Thomopoulos S. The effect of tear size and nerve injury on rotator cuff muscle fatty degeneration in a rodent animal model. J Shoulder Elbow Surg 21, 847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu X.H., Manzano G., Kim H.T., and Feeley B.T. A rat model of massive rotator cuff tears. J Orthop Res 29, 588, 2011 [DOI] [PubMed] [Google Scholar]
- 104.Killian M.L., Lim C.T., Thomopoulos S., Charlton N., Kim H.M., and Galatz L.M. The effect of unloading on gene expression of healthy and injured rotator cuffs. J Orthop Res 31, 1240, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gigliotti D., Leiter J.R.S., Macek B., Davidson M.J., MacDonald P.B., and Anderson J.E. Atrophy, inducible satellite cell activation, and possible denervation of supraspinatus muscle in injured human rotator-cuff muscle. Am J Physiol Cell Physiol 309, C383, 2015 [DOI] [PubMed] [Google Scholar]
- 106.Gigliotti D., Leiter J.R.S., MacDonald P.B., Peelerm J., and Anderson J.E. Altered satellite cell responsiveness and denervation implicated in progression of rotator-cuff injury. Plos One 11, e0162494, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davies M.R., Ravishankar B., Laron D., Kim H.T., Liu X.H., and Feeley B.T. Rat rotator cuff muscle responds differently from hindlimb muscle to a combined tendon-nerve injury. J Orthop Res 33, 1046, 2015 [DOI] [PubMed] [Google Scholar]
- 108.Frey E., Regenfelder F., Sussmann P., Zumstein M., Gerber C., Born W., and Fuchs B. Adipogenic and myogenic gene expression in rotator cuff muscle of the sheep after tendon tear. J Orthop Res 27, 504, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Joshi S.K., Liu X.H., Samagh S.P., Lovett D.H., Bodine S.C., Kim H.T., and Feeley B.T. mTOR regulates fatty infiltration through SREBP-1 and PPAR gamma after a combined massive rotator cuff tear and suprascapular nerve injury in rats. J Orthop Res 31, 724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choo A., McCarthy M., Pichika R., Sato E.J., Lieber R.L., Schenk S., Lane J.G., and Ward S.R. Muscle gene expression patterns in human rotator cuff pathology. J Bone Joint Surg Am 96A, 1558, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gumucio J.P., Davis M.E., Bradley J.R., Stafford P.L., Schiffman C.J., Lynch E.B., Claflin D.R., Bedi A., and Mendias C.L. Rotator cuff tear reduces muscle fiber specific force production and induces macrophage accumulation and autophagy. J Orthop Res 30, 1963, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Joshi S.K., Kim H.T., Feeley B.T., and Liu X.H. Differential ubiquitin-proteasome and autophagy signaling following rotator cuff tears and suprascapular nerve injury. J Orthop Res 32, 138, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Davies M.R., Lee L., Feeley B.T., Kim H.T., and Liu X. Lysophosphatidic acid-induced RHOA signaling and prolonged macrophage infiltration worsens fibrosis and fatty infiltration following rotator cuff tears. J Orthop Res 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gumucio J., Flood M., Harning J.A., Phan A., Roche S.M., Lynch E.B., Bedi A., and Mendias C. T lymphocytes are not required for the development of fatty degeneration after rotator cuff tear. Bone Joint Res 3, 262, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liu X.H., Joshi S.K., Samagh S.P., Dang Y.X., Laron D., Lovett D.H., Bodine S.C., Kim H.T., and Feeley B.T. Evaluation of Akt/mTOR activity in muscle atrophy after rotator cuff tears in a rat model. J Orthop Res 30, 1440, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Gumucio J.P., Korn M.A., Saripalli A.L., Flood M.D., Phan A.C., Roche S.M., Lynch E.B., Claflin D.R., Bedi A., and Mendias C.L. Aging-associated exacerbation in fatty degeneration and infiltration after rotator cuff tear. J Shoulder Elbow Surg 23, 99, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liu X., Joshi S.K., Ravishankar B., Laron D., Kim H.T., and Feeley B.T. Upregulation of transforming growth factor-beta signaling in a rat model of rotator cuff tears. J Shoulder Elbow Surg 23, 1709, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davies M.R., Liu X.H., Lee L., Laron D., Ning A.Y., Kim H.T., and Feeley B.T. Tgf-beta small molecule inhibitor SB431542 reduces rotator cuff muscle fibrosis and fatty infiltration by promoting fibro/adipogenic progenitor apoptosis. Plos One 11, e0155486, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wilde J.M., Gumucio J.P., Grekin J.A., Sarver D.C., Noah A.C., Ruehlmann D.G., Davis M.E., Bedi A., and Mendias C.L. Inhibition of p38 mitogen-activated protein kinase signaling reduces fibrosis and lipid accumulation after rotator cuff repair. J Shoulder Elbow Surg 25, 1501, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cosgrove B.D., Gilbert P.M., Porpiglia E., Mourkioti F., Lee S.P., Corbel S.Y., Llewellyn M.E., Delp S.L., and Blau H.M. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med 20, 255, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bernet J.D., Doles J.D., Hall J.K., Tanaka K.K., Carter T.A., and Olwin B.B. P38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med 20, 265, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fry C.S., Lee J.D., Mula J., Kirby T.J., Jackson J.R., Liu F., Yang L., Mendias C.L., Dupont-Versteegden E.E., McCarthy J.J., and Peterson C.A. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med 21, 76, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu X., Ning A.Y., Chang N.C., Kim H., Nissenson R., Wang L., and Feeley B.T. Investigating the cellular origin of rotator cuff muscle fatty infiltration and fibrosis after injury. Muscles Ligaments Tendons J 6, 6, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Joe A.W.B., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M.A., and Rossi F.M.V. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natarajan A., Lemos D.R., and Rossi F.M.V. Fibro/adipogenic progenitors a double-edged sword in skeletal muscle regeneration. Cell Cycle 9, 2045, 2010 [DOI] [PubMed] [Google Scholar]
- 126.Dolkart O., Liron T., Chechik O., Somjen D., Brosh T., Maman E., and Gabet Y. Statins enhance rotator cuff healing by stimulating the COX2/PGE2/EP4 pathway an in vivo and in vitro study. Am J Sports Med 42, 2869, 2014 [DOI] [PubMed] [Google Scholar]
- 127.Davis M.E., Korn M.A., Gumucio J.P., Harning J.A., Saripalli A.L., Bedi A., and Mendias C.L. Simvastatin reduces fibrosis and protects against muscle weakness after massive rotator cuff tear. J Shoulder Elbow Surg 24, 280, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gerber C., Meyer D.C., Nuss K.M., and Farshad M. Anabolic steroids reduce muscle damage caused by rotator cuff tendon release in an experimental study in rabbits. J Bone Joint Surg Am 93A, 2189, 2011 [DOI] [PubMed] [Google Scholar]
- 129.Gerber C., Meyer D.C., Flück M., Benn M.C., von Rechenberg B., and Wieser K. Anabolic steroids reduce muscle degeneration associated with rotator cuff tendon release in sheep. Am J Sports Med 43, 2393, 2015 [DOI] [PubMed] [Google Scholar]
- 130.Gerber C., Meyer D.C., Frey E., von Rechenberg B., Hoppeler H., Frigg R., Jost B., and Zumstein M.A. Neer award 2007: reversion of structural muscle changes caused by chronic rotator cuff tears using continuous musculotendinous traction. An experimental study in sheep. J Shoulder Elbow Surg 18, 163, 2009 [DOI] [PubMed] [Google Scholar]
- 131.Zumstein M.A., Frey E., von Rechenberg B., Frigg R., Gerber C., and Meyer D.C. Device for lengthening of a musculotendinous unit by direct continuous traction in the sheep. BMC Vet Res 8, 50, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Oak N.R., Gumucio J.P., Flood M.D., Saripalli A.L., Davis M.E., Harning J.A., Lynch E.B., Roche S.M., Bedi A., and Mendias C.L. Inhibition of 5-LOX, COX-1, and COX-2 increases tendon healing and reduces muscle fibrosis and lipid accumulation after rotator cuff repair. Am J Sports Med 42, 2860, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gumucio J.P., Flood M.D., Roche S.M., Sugg K.B., Momoh A.O., Kosnik P.E., Bedi A., and Mendias C.L. Stromal vascular stem cell treatment decreases muscle fibrosis following chronic rotator cuff tear. Int Orthop 40, 759, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oh J.H., Chung S.W., Kim S.H., Chung J.Y., and Kim J.Y. 2013 Neer award: effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg 23, 445, 2014 [DOI] [PubMed] [Google Scholar]
- 135.Davis M.E., Stafford P.L., Jergenson M.J., Bedi A., and Mendias C.L. Muscle fibers are injured at the time of acute and chronic rotator cuff repair. Clin Orthop Relat Res 473, 226, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ng R., Metzger J.M., Claflin D.R., and Faulkner J.A. Poloxamer 188 reduces the contraction-induced force decline in lumbrical muscles from mdx mice. Am J Physiol Cell Physiol 295, C146, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mannava S., Plate J.F., Whitlock P.W., Callahan M.F., Seyler T.M., Koman L.A., Smith T.L., and Tuohy C.J. Evaluation of in vivo rotator cuff muscle function after acute and chronic detachment of the supraspinatus tendon. J Bone Joint Surg Am 93A, 1702, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Han R., and Campbell K.P. Dysferlin and muscle membrane repair. Curr Opin Cell Biol 19, 409, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Meyer G.A., and Ward S.R. Developmental biology and regenerative medicine: addressing the vexing problem of persistent muscle atrophy in the chronically torn human rotator cuff. Phys Ther 96, 722, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Benjamin M., Toumi H., Ralphs J.R., Bydder G., Best T.M., and Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat 208, 471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Benjamin M., Kumai T., Milz S., Boszczyk B.M., Boszczyk A.A., and Ralphs J.R. The skeletal attachment of tendons – tendon ‘entheses.’ Comp Biochem Physiol A Mol Integr Physiol 133, 931, 2002 [DOI] [PubMed] [Google Scholar]
- 142.Genin G.M., Kent A., Birman V., Wopenka B., Pasteris J.D., Marquez P.J., and Thomopoulos S. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J 97, 976, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wopenka B., Kent A., Pasteris J.D., Yoon Y., and Thomopoulos S. The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl Spectrosc 62, 1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Thomopoulos S., Marquez J.P., Weinberger B., Birman V., and Genin G.M. Collagen fiber orientation at the tendon to bone insertion and its influence on stress concentrations. J Biomech 39, 1842, 2006 [DOI] [PubMed] [Google Scholar]
- 145.Galatz L., Rothermich S., VanderPloeg K., Petersen B., Sandell L., and Thomopoulos S. Development of the supraspinatus tendon-to-bone insertion: localized expression of extracellular matrix and growth factor genes. J Orthop Res 25, 1621, 2007 [DOI] [PubMed] [Google Scholar]
- 146.Thomopoulos S., Williams G.R., Gimbel J.A., Favata M., and Soslowsky L.J. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res 21, 413, 2003 [DOI] [PubMed] [Google Scholar]
- 147.Liu Y.X., Thomopoulos S., Birman V., Li J.S., and Genin G.M. Bi-material attachment through a compliant interfacial system at the tendon-to-bone insertion site. Mech Mater 44, 83, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Dejardin L.M., Arnoczky S.P., Ewers B.J., Haut R.C., and Clarke R.B. Tissue-engineered rotator cuff tendon using porcine small intestine submucosa – histologic and mechanical evaluation in dogs. Am J Sports Med 29, 175, 2001 [DOI] [PubMed] [Google Scholar]
- 149.Ji X.X., Chen Q.S., Thoreson A.R., Qu J., An K.N., Amadio P.C., Steinmann S.P., and Zhao C.F. Rotator cuff repair with a tendon-fibrocartilage-bone composite bridging patch. Clin Biomech (Bristol, Avon) 30, 976, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Spalazzi J.P., Doty S.B., Moffat K.L., Levine W.N., and Lu H.H. Development of controlled matrix heterogeneity on a triphasic scaffold for orthopedic interface tissue engineering. Tissue Eng 12, 3497, 2006 [DOI] [PubMed] [Google Scholar]
- 151.Kolluru P.V., Lipner J., Liu W., Xia Y., Thomopoulos S., Genin G.M., and Chasiotis I. Strong and tough mineralized plga nanofibers for tendon-to-bone scaffolds. Acta Biomater 9, 9442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu W.Y., Lipner J., Xie J.W., Manning C.N., Thomopoulos S., and Xia Y.N. Nanofiber scaffolds with gradients in mineral content for spatial control of osteogenesis. ACS Appl Mater Interfaces 6, 2842, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam A., Bohl M.S., Tsai A.G., Younesi M., Gillespie R., and Akkus O. Biomechanical evaluation of a novel suturing scheme for grafting load-bearing collagen scaffolds for rotator cuff repair. Clin Biomech (Bristol, Avon) 30, 669, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bedi A., Kawamura S., Ying L., and Rodeo S.A. Differences in tendon graft healing between the intra-articular and extra-articular ends of a bone tunnel. HSS J 5, 51, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sun L., Zhou X.H., Wu B., and Tian M. Inhibitory effect of synovial fluid on tendon-to-bone healing: an experimental study in rabbits. Arthroscopy 28, 1297, 2012 [DOI] [PubMed] [Google Scholar]
- 156.Funakoshi T., Martin S.D., Schmid T.M., and Spector M. Distribution of lubricin in the ruptured human rotator cuff and biceps tendon: a pilot study. Clin Orthop Relat Res 468, 1588, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wurgler-Hauri C.C., Dourte L.M., Baradet T.C., Williams G.R., and Soslowsky L.J. Temporal expression of 8 growth factors in tendon-to-bone healing in a rat supraspinatus model. J Shoulder Elbow Surg 16, 198S, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thomopoulos S., Hattersley G., Rosen V., Mertens M., Galatz L., Williams G.R., and Soslowsky L.J. The localized expression of extracellular matrix components in healing tendon insertion sites: an in situ hybridization study. J Orthop Res 20, 454, 2002 [DOI] [PubMed] [Google Scholar]
- 159.Galatz L.M., Sandell L.J., Rothermich S.Y., Das R., Mastny A., Havlioglu N., Silva M.J., and Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res 24, 541, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Kim H.M., Galatz L.M., Das R., Havlioglu N., Rothermich S.Y., and Thomopoulos S. The role of transforming growth factor beta isoforms in tendon-to-bone healing. Connect Tissue Res 52, 87, 2011 [DOI] [PubMed] [Google Scholar]
- 161.Kovacevic D., Gulotta L.V., Ying L., Ehteshami J.R., Deng X.H., and Rodeo S.A. rhPDGF-BB promotes early healing in a rat rotator cuff repair model. Clin Orthop Relat Res 473, 1644, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Seeherman H.J., Archambault J.M., Rodeo S.A., Turner A.S., D'Augusta D., Li X.J., Smith E., and Wozney J.M. rhBMP-12 accelerates healing of rotator cuff repairs in a sheep model. J Bone Joint Surg Am 90A, 2206, 2008 [DOI] [PubMed] [Google Scholar]
- 163.Itoigawa Y., Suzuki O., Sano H., Anada T., Handa T., Hatta T., Kuwahara Y., Takahashi A., Ezoe Y., Kaneko K., and Itoi E. The role of an octacalcium phosphate in the re-formation of infraspinatus tendon insertion. J Shoulder Elbow Surg 24, E175, 2015 [DOI] [PubMed] [Google Scholar]
- 164.Thomopoulos S., Zampiakis E., Das R., Kim M., Silva M.J., Havlioglu N., and Gelberman R.H. Use of a magnesium-based bone adhesive for flexor tendon-to-bone healing. J Hand Surg Am 34A, 1066, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Maman E., Yehuda C., Pritsch T., Morag G., Brosh T., Sharfman Z., and Dolkart O. Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: a biomechanical and imaging study in rats. Am J Sports Med 44, 177, 2016 [DOI] [PubMed] [Google Scholar]
- 166.Bedi A., Fox A.J.S., Kovacevic D., Deng X.H., Warren R.F., and Rodeo S.A. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med 38, 308, 2010 [DOI] [PubMed] [Google Scholar]
- 167.Gulotta L.V., Kovacevic D., Cordasco F., and Rodeo S.A. Evaluation of tumor necrosis factor alpha blockade on early tendon-to-bone healing in a rat rotator cuff repair model. Arthroscopy 27, 1351, 2011 [DOI] [PubMed] [Google Scholar]
- 168.Paxton J.Z., Bannerman A., Wang A., and Grover L.M. Engineering the enthesis – formation of a multiphase tissue transition in vitro. Int J Exp Pathol 94, A6, 2013 [Google Scholar]
- 169.Hernigou P., Merouse G., Duffiet P., Chevalier N., and Rouard H. Reduced levels of mesenchymal stem cells at the tendon-bone interface tuberosity in patients with symptomatic rotator cuff tear. Int Orthop 39, 1219, 2015 [DOI] [PubMed] [Google Scholar]
- 170.Hernigou P., Lachaniette C.H.F., Delambre J., Zilber S., Duffiet P., Chevallier N., and Rouard H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int Orthop 38, 1811, 2014 [DOI] [PubMed] [Google Scholar]
- 171.Rothrauff B.B., and Tuan R.S. Cellular therapy in bone-tendon interface regeneration. Organogenesis 10, 13, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Kida Y., Morihara T., Matsuda K.I., Kajikawa Y., Tachiiri H., Iwata Y., Sawamura K., Yoshida A., Oshima Y., Ikeda T., Fujiwara H., Kawata M., and Kubo T. Bone marrow-derived cells from the footprint infiltrate into the repaired rotator cuff. J Shoulder Elbow Surg 22, 197, 2013 [DOI] [PubMed] [Google Scholar]
- 173.Loeffler B.J., Scannell B.P., Peindl R.D., Connor P., Davis D.E., Hoelscher G.L., Norton H.J., Hanley E.N., and Gruber H.E. Cell-based tissue engineering augments tendon-to-bone healing in a rat supraspinatus model. J Orthop Res 31, 407, 2013 [DOI] [PubMed] [Google Scholar]
- 174.Chen C.H., Chang C.H., Wang K.C., Su C.I., Liu H.T., Yu C.M., Wong C.B., Wang I.C., Whu S.W., and Liu H.W. Enhancement of rotator cuff tendon-bone healing with injectable periosteum progenitor cells-BMP-2 hydrogel in vivo. Knee Surg Sports Traumatol Arthrosc 19, 1597, 2011 [DOI] [PubMed] [Google Scholar]
- 175.Gulotta L.V., Kovacevic D., Ehteshami J.R., Dagher E., Packer J.D., and Rodeo S.A. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med 37, 2126, 2009 [DOI] [PubMed] [Google Scholar]
- 176.Gulotta L.V., Kovacevic D., Packer J.D., Ehteshami J.R., and Rodeo S.A. Adenoviral-mediated gene transfer of human bone morphogenetic protein-13 does not improve rotator cuff healing in a rat model. Am J Sports Med 39, 180, 2011 [DOI] [PubMed] [Google Scholar]
- 177.Ficklscherer A., Loitsch T., Serr M., Guelecyuez M.F., Niethammer T.R., Mueller H.-H., Milz S., Pietschmann M.F., and Muller P.E. Does footprint preparation influence tendon-to-bone healing after rotator cuff repair in an animal model? Arthroscopy 30, 188, 2014 [DOI] [PubMed] [Google Scholar]
- 178.Ross D., Maerz T., Kurdziel M., Hein J., Doshi S., Bedi A., Anderson K., and Baker K. The effect of granulocyte-colony stimulating factor on rotator cuff healing after injury and repair. Clin Orthop Relat Res 473, 1655, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Depres-tremblay G., Chevrier A., Snow M., Hurtig M.B., Rodeo S., and Buschmann M.D. Rotator cuff repair: a review of surgical techniques, animal models, and new technologies under development. J Shoulder Elbow Surg 25, 2078, 2016 [DOI] [PubMed] [Google Scholar]
- 180.Derwin K.A., Baker A.R., Iannotti J.P., and McCarron J.A. Preclinical models for translating regenerative medicine therapies for rotator cuff repair. Tissue Eng Part B 16, 21, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gulotta L.V., Kovacevic D., Packer J.D., Deng X.H., and Rodeo S.A. Bone marrow-derived mesenchymal stem cells transduced with scleraxis improve rotator cuff healing in a rat model. Am J Sports Med 39, 1282, 2011 [DOI] [PubMed] [Google Scholar]
- 182.Killian M.L., and Thomopoulos S. Scleraxis is required for the development of a functional tendon enthesis. FASEB J 30, 301, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sugimoto Y., Takimoto A., Akiyama H., Kist R., Scherer G., Nakamura T., Hiraki Y., and Shukunami C. Scx(+)/Sox9(+) progenitors contribute to the establishment of the junction between cartilage and tendon/ligament. Development 140, 2280, 2013 [DOI] [PubMed] [Google Scholar]
- 184.Blitz E., Sharir A., Akiyama H., and Zelzer E. Tendon-bone attachment unit is formed modularly by a distinct pool of Scx- and Sox9-positive progenitors. Development 140, 2680, 2013 [DOI] [PubMed] [Google Scholar]
- 185.Lenas P., Moos M., Jr., and Luyten F.P. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part I: from three-dimensional cell growth to biomimetics of in vivo development. Tissue Eng Part B 15, 381, 2009 [DOI] [PubMed] [Google Scholar]
- 186.Liu C.F., Aschbacher-Smith L., Barthelery N.J., Dyment N., Butler D., and Wylie C. What we should know before using tissue engineering techniques to repair injured tendons: a developmental biology perspective. Tissue Eng Part B 17, 165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu C.F., Aschbacher-Smith L., Barthelery N.J., Dyment N., Butler D., and Wylie C. Spatial and temporal expression of molecular markers and cell signals during normal development of the mouse patellar tendon. Tissue Eng Part A 18, 598, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Schweitzer R., Zelzer E., and Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development 137, 2807, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Zelzer E., Blitz E., Killian M.L., and Thomopoulos S. Tendon-to-bone attachment: from development to maturity. Birth Defects Res C Embryo Today 102, 101, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Liu C.-F., Breidenbach A., Aschbacher-Smith L., Butler D., and Wylie C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. Plos One 8, e65411, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Tatara A.M., Lipner J.H., Das R., Kim M., Patel N., Ntouvali E., Silva M.J., and Thomopoulos S. The role of muscle loading on bone (re)modeling at the developing enthesis. Plos One 9, e97375, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Schwartz A.G., Lipner J.H., Pasteris J.D., Genin G.M., and Thomopoulos S. Muscle loading is necessary for the formation of a functional tendon enthesis. Bone 55, 44, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thomopoulos S., Kim H.M., Rothermich S.Y., Biederstadt C., Das R., and Galatz L.A. Decreased muscle loading delays maturation of the tendon enthesis during postnatal development. J Orthop Res 25, 1154, 2007 [DOI] [PubMed] [Google Scholar]
- 194.Dyment N.A., Breidenbach A.P., Schwartz A.G., Russell R.P., Aschbacher-Smith L., Liu H., Hagiwara Y., Jiang R., Thomopoulos S., Butler D.L., and Rowe D.W. Gdf5 progenitors give rise to fibrocartilage cells that mineralize via hedgehog signaling to form the zonal enthesis. Dev Biol 405, 96, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Breidenbach A.P., Aschbacher-Smith L., Lu Y., Dyment N.A., Liu C.-F., Liu H., Wylie C., Rao M., Shearn J.T., Rowe D.W., Kadler K.E., Jiang R., and Butler D.L. Ablating hedgehog signaling in tenocytes during development impairs biomechanics and matrix organization of the adult murine patellar tendon enthesis. J Orthop Res 33, 1142, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang M.N., VanHouten J.N., Nasiri A.R., Johnson R.L., and Broadus A.E. PTHrP regulates the modeling of cortical bone surfaces at fibrous insertion sites during growth. J Bone Miner Res 28, 598, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chung U.I., Lanske B., Lee K.C., Li E., and Kronenberg H. The parathyroid hormone parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci U S A 95, 13030, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Amano K., Densmore M., Nishimura R., and Lanske B. Indian hedgehog signaling regulates transcription and expression of collagen type X via Runx2/Smads interactions. J Biol Chem 289, 24898, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rais Y., Reich A., Simsa-Maziel S., Moshe M., Idelevich A., Kfir T., Miosge N., and Monsonego-Ornan E. The growth plate's response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell Mol Life Sci 72, 597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wu Q.Q., Zhang Y., and Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. J Biol Chem 276, 35290, 2001 [DOI] [PubMed] [Google Scholar]
- 201.Chen X., Macica C., Nasiri A., Judex S., and Broadus A.E. Mechanical regulation of PTHrP expression in entheses. Bone 41, 752, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Schwartz A.G., Long F.X., and Thomopoulos S. Enthesis fibrocartilage cells originate from a population of hedgehog-responsive cells modulated by the loading environment. Development 142, 196, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Carbone A., Carballo C., Ma R., Wang H.S., Deng X.H., Dahia C., and Rodeo S. Indian hedgehog signaling and the role of graft tension in tendon-to-bone healing: evaluation in a rat ACL reconstruction model. J Orthop Res 34, 641, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kobayashi T., Chung U.I., Schipani E., Starbuck M., Karsenty G., Katagiri T., Goad D.L., Lanske B., and Kronenberg H.M. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development 129, 2977, 2002 [DOI] [PubMed] [Google Scholar]
- 205.Kronenberg H.M. Developmental regulation of the growth plate. Nature 423, 332, 2003 [DOI] [PubMed] [Google Scholar]
- 206.Hettrich C.M., Beamer B.S., Bedi A., Deland K., Deng X.H., Ying L., Lane J., and Rodeo S.A. The effect of rhPTH on the healing of tendon to bone in a rat model. J Orthop Res 30, 769, 2012 [DOI] [PubMed] [Google Scholar]
- 207.Galatz L.M., Charlton N., Das R., Kim H.M., Havlioglu N., and Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg 18, 669, 2009 [DOI] [PubMed] [Google Scholar]
- 208.Killian M.L., Cavinatto L., Galatz L.M., and Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg 21, 228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hogan M.V., Bagayoko N., James R., Starnes T., Katz A., and Chhabra A.B. Tissue engineering solutions for tendon repair. J Am Acad Orthop Surg 19, 134, 2011 [DOI] [PubMed] [Google Scholar]
- 210.Hjorthaug G.A., Madsen J.E., Nordsletten L., Reinholt F.P., Steen H., and Dimmen S. Tendon to bone tunnel healing-a study on the time-dependent changes in biomechanics, bone remodeling, and histology in a rat model. J Orthop Res 33, 216, 2015 [DOI] [PubMed] [Google Scholar]
- 211.Ma J., Shen J., Smith B.P., Ritting A., Smith T.L., and Koman L.A. Bioprotection of tendon repair: adjunctive use of botulinum toxin A in Achilles tendon repair in the rat. J Bone Joint Surg Am 89A, 2241, 2007 [DOI] [PubMed] [Google Scholar]
- 212.Hettrich C.M., Rodeo S.A., Hannafin J.A., Ehteshami J., and Stein B.E.S. The effect of muscle paralysis using Botox on the healing of tendon to bone in a rat model. J Shoulder Elbow Surg 20, 688, 2011 [DOI] [PubMed] [Google Scholar]
- 213.Gilotra M., Thao N., Christian M., Davis D., Henn R.F., III, and Hasan S.A. Botulinum toxin is detrimental to repair of a chronic rotator cuff tear in a rabbit model. J Orthop Res 33, 1152, 2015 [DOI] [PubMed] [Google Scholar]
- 214.Bedi A., Kovacevic D., Fox A.J.S., Imhauser C.W., Stasiak M., Packer J., Brophy R.H., Deng X.H., and Rodeo S.A. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am 92A, 2387, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Peltz C.D., Dourte L.M., Kuntz A.F., Sarver J.J., Kim S.Y., Williams G.R., and Soslowsky L.J. The effect of postoperative passive motion on rotator cuff healing in a rat model. J Bone Joint Surg Am 91A, 2421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Reuther K.E., Tucker J.J., Thomas S.J., Vafa R.P., Liu S.S., Gordon J.A., Caro A.C., Yannascoli S.M., Kuntz A.F., and Soslowsky L.J. Effect of scapular dyskinesis on supraspinatus repair healing in a rat model. J Shoulder Elbow Surg 24, 1235, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hsu J.E., Horneff J.G., and Gee A.O. Immobilization after rotator cuff repair what evidence do we have now? Orthop Clin North Am 47, 169, 2016 [DOI] [PubMed] [Google Scholar]
- 218.Ross D., Maerz T., Lynch J., Norris S., Baker K., and Anderson K. Rehabilitation following arthroscopic rotator cuff repair: a review of current literature. J Am Acad Orthop Surg 22, 1, 2014 [DOI] [PubMed] [Google Scholar]
- 219.Keener J.D., Galatz L.M., Stobbs-Cucchi G., Patton R., and Yamaguchi K. Rehabilitation following arthroscopic rotator cuff repair a prospective randomized trial of immobilization compared with early motion. J Bone Joint Surg Am 96A, 11, 2014 [DOI] [PubMed] [Google Scholar]
- 220.Hodde J.P., Badylak S.F., and Shelbourne K.D. The effect of range of motion on remodeling of small intestinal submucosa (SIS) when used as an Achilles tendon repair material in the rabbit. Tissue Eng 3, 27, 1997 [Google Scholar]
- 221.Uezono K., Ide J., Tokunaga T., Sakamoto H., Okamoto N., and Mizuta H. Effect of immobilization on rotator cuff reconstruction with acellular dermal matrix grafts in an animal model. J Shoulder Elbow Surg 22, 1290, 2013 [DOI] [PubMed] [Google Scholar]
- 222.Uezono K., Ide J., Tokunaga T., Arimura H., Sakamoto H., Nakanishi Y., and Mizuta H. Effect of postoperative passive motion on rotator cuff reconstruction with acellular dermal matrix grafts in a rat model. Am J Sports Med 42, 1930, 2014 [DOI] [PubMed] [Google Scholar]
- 223.Forslund C., and Aspenberg P. CDMP-2 induces bone or tendon-like tissue depending on mechanical stimulation. J Orthop Res 20, 1170, 2002 [DOI] [PubMed] [Google Scholar]
- 224.Ambrosio F., Ferrari R.J., Distefano G., Plassmeyer J.M., Carvell G.E., Deasy B.M., Boninger M.L., Fitzgerald G.K., and Huard J. The synergistic effect of treadmill running on stem-cell transplantation to heal injured skeletal muscle. Tissue Eng Part A 16, 839, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Yoon J.P., Chung S.W., Kim J.Y., Lee B.J., Kim H.S., Kim J.E., and Cho J.H. Outcomes of combined bone marrow stimulation and patch augmentation for massive rotator cuff tears. Am J Sports Med 44, 963, 2016 [DOI] [PubMed] [Google Scholar]
- 226.McElvany M.D., McGoldrick E., Gee A.O., Neradilek M.B., and Matsen F.A. Rotator cuff repair published evidence on factors associated with repair integrity and clinical outcome. Am J Sports Med 43, 491, 2015 [DOI] [PubMed] [Google Scholar]
- 227.Henry P., Wasserstein D., Park S., Dwyer T., Chahal J., Slobogean G., and Schemitsch E. Arthroscopic repair for chronic massive rotator cuff tears: a systematic review. Arthroscopy 31, 2472, 2015 [DOI] [PubMed] [Google Scholar]
- 228.Juncosa N., West J.R., Galloway M.T., Bolvin G.P., and Butler D.L. In vivo forces used to develop design parameters for tissue engineered implants for rabbit patellar tendon repair. J Biomech 36, 483, 2003 [DOI] [PubMed] [Google Scholar]
- 229.Butler D.L., Juncosa-Melvin N., Boivin G.P., Galloway M.T., Shearn J.T., Gooch C., and Awad H. Functional tissue engineering for tendon repair: a multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res 26, 1, 2008 [DOI] [PubMed] [Google Scholar]
- 230.Jackson M., Sylvestre E., Bleau J., Allard P., and Begon M. Estimating optimal shoulder immobilization postures following surgical repair of massive rotator cuff tears. J Biomech 46, 179, 2013 [DOI] [PubMed] [Google Scholar]
- 231.Jackson M., Tetreault P., Allard P., and Begon M. Optimal shoulder immobilization postures following surgical repair of rotator cuff tears: a simulation analysis. J Shoulder Elbow Surg 22, 1011, 2013 [DOI] [PubMed] [Google Scholar]
- 232.Mannava S., Plate J.F., Tuohy C.J., Seyler T.M., Whitlock P.W., Curl W.W., Smith T.L., and Saul K.R. The science of rotator cuff tears: translating animal models to clinical recommendations using simulation analysis. Knee Surg Sports Traumatol Arthrosc 21, 1610, 2013 [DOI] [PubMed] [Google Scholar]
- 233.Araki D., Miller M., Fujimaki Y., Hoshino Y., Musahl V., and Debski R.E. Effect of tear location on propagation of isolated supraspinatus tendon tears during increasing levels of cyclic loading. J Bone Joint Surg Am 97A, 273, 2015 [DOI] [PubMed] [Google Scholar]
- 234.Thunes J., Miller R.M., Pal S., Damle S., Debski R.E., and Maiti S. The effect of size and location of tears in the supraspinatus tendon on potential tear propagation. J Biomech Eng 137, 081012, 2015 [DOI] [PubMed] [Google Scholar]
- 235.Bakshi N.K., Jameel O.F., Merrill Z.F., Debski R.E., and Sekiya J.K. The influence of surgical stabilization on glenohumeral abduction using 3-dimensional computed tomography in patients with shoulder instability. Arthroscopy 32, 1495, 2016 [DOI] [PubMed] [Google Scholar]
- 236.Haering D., Blache Y., Raison M., and Begon M. Mechanical risk of rotator cuff repair failure during passive movements: a simulation-based study. Clin Biomech (Bristol, Avon) 30, 1181, 2015 [DOI] [PubMed] [Google Scholar]
- 237.Oh J.H., Kim S.H., Kang J.Y., Oh C.H., and Gong H.S. Effect of age on functional and structural outcome after rotator cuff repair. Am J Sports Med 38, 672, 2010 [DOI] [PubMed] [Google Scholar]
- 238.Samagh S.P., Kramer E.J., Melkus G., Laron D., Bodendorfer B.M., Natsuhara K., Kim H.T., Liu X., and Feeley B.T. MRI quantification of fatty infiltration and muscle atrophy in a mouse model of rotator cuff tears. J Orthop Res 31, 421, 2013 [DOI] [PubMed] [Google Scholar]
- 239.Bell R., Taub P., Cagle P., Flatow E.L., and Andarawis-Puri N. Development of a mouse model of supraspinatus tendon insertion site healing. J Orthop Res 33, 25, 2015 [DOI] [PubMed] [Google Scholar]
- 240.Coleman S.H., Fealy S., Ehteshami J.R., MacGillivray J.D., Altchek D.W., Warren R.F., and Turner A.S. Chronic rotator cuff injury and repair model in sheep. J Bone Joint Surg Am 85A, 2391, 2003 [DOI] [PubMed] [Google Scholar]
- 241.Wood L.K., Kayupov E., Gumucio J.P., Mendias C.L., Claflin D.R., and Brooks S.V. Intrinsic stiffness of extracellular matrix increases with age in skeletal muscles of mice. J Appl Physiol 117, 363, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Plate J.F., Brown P.J., Walters J., Clark J.A., Smith T.L., Freehill M.T., Tuohy C.J., Stitzel J.D., and Mannava S. Advanced age diminishes tendon-to-bone healing in a rat model of rotator cuff repair. Am J Sports Med 42, 859, 2014 [DOI] [PubMed] [Google Scholar]
- 243.Mendias C.L., Gumucio J.P., Davis M.E., Bromley C.W., Davis C.S., and Brooks S.V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 45, 55, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Buck F.M., Grehn H., Hilbe M., Pfirrmann C.W.A., Manzanell S., and Hodler J. Magnetic resonance histologic correlation in rotator cuff tendons. J Magn Reson Imaging 32, 165, 2010 [DOI] [PubMed] [Google Scholar]
- 245.Osti L., Del Buono A., and Maffulli N. Microfractures at the rotator cuff footprint: A randomised controlled study. Int Orthop 37, 2165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Milano G., Saccomanno M.F., Careri S., Taccardo G., De Vitis R., and Fabbriciani C. Efficacy of marrow-stimulating technique in arthroscopic rotator cuff repair: A prospective randomized study. Arthroscopy 29, 802, 2013 [DOI] [PubMed] [Google Scholar]