Abstract
Omecamtiv mecarbil (OM) is a pharmacological agent that augments cardiac contractile function by enhancing myofilament Ca2+ sensitivity. Given that interventions that increase myofilament Ca2+ sensitivity have the potential to alter length-dependent activation (LDA) of cardiac myofilaments, we tested the influence of OM on this fundamental property of the heart. This is significant not only because LDA is prominent in cardiac muscle but also because it contributes to the Frank-Starling law, a mechanism by which the heart increases stroke volume in response to an increase in venous return. We measured steady-state and dynamic contractile indices in detergent-skinned guinea pig (Cavia porcellus) cardiac muscle fibers in the absence and presence of 0.3 and 3.0 μM OM at two different sarcomere lengths (SLs), short SL (1.9 μm) and long SL (2.3 μm). Myofilament Ca2+ sensitivity, as measured by pCa50 (−log of [Ca2+]free concentration required for half-maximal activation), increased significantly at both short and long SLs in OM-treated fibers when compared to untreated fibers; however, the magnitude of increase in pCa50 was twofold greater at short SL than at long SL. A consequence of this greater increase in pCa50 at short SL was that pCa50 did not increase any further at long SL, suggesting that OM abolished the SL dependency of pCa50. Furthermore, the SL dependency of rate constants of cross-bridge distortion dynamics (c) and force redevelopment (ktr) was abolished in 0.3-μM-OM-treated fibers. The negative impact of OM on the SL dependency of pCa50, c, and ktr was also observed in 3.0-μM-OM-treated fibers, indicating that cooperative mechanisms linked to LDA were altered by the OM-mediated effects on cardiac myofilaments.
Introduction
Omecamtiv mecarbil (OM) is a cardiac-specific myosin activator that has been shown to improve cardiac function in both animal models and humans. At the whole-heart level, OM increases systolic function by increasing stroke volume and ejection fraction (1, 2, 3, 4). The basis for OM-mediated enhancement of cardiac contractile function is highlighted by recent studies (5, 6, 7, 8) demonstrating that OM increases cardiac myofilament Ca2+ sensitivity and tension. A recent study (5) also suggests that OM-mediated effects augment cross-bridge (XB)-based cooperative mechanisms such that cardiac thin filaments are activated, leading to an increase in myofilament Ca2+ sensitivity.
XB-mediated cooperative activation of thin filaments is also implicated in other fundamental aspects of cardiac muscle regulation. One such aspect is length-dependent activation (LDA), whereby a given increase in sarcomere length (SL) increases myofilament Ca2+ sensitivity to a greater extent in cardiac muscle compared to skeletal muscle (9, 10, 11). This enhanced dependency of myofilament Ca2+ sensitivity on SL in cardiac muscle relies on regulatory components of the cooperative system that respond steeply to feedback effects of strong XBs on thin filaments (9, 12, 13, 14, 15). At the whole-heart level, LDA may contribute to the Frank-Starling (FS) law (16, 17, 18, 19), a mechanism by which the heart increases its stroke volume in response to an increase in venous return. With regard to cooperative activation of cardiac thin filaments, a consistent observation made in the last two decades of research is that interventions that alter XB-based cooperative mechanisms or modifications that enhance thin-filament Ca2+ sensitivity affect LDA (12, 17, 20). For example, NEM-S1 (a strong XB analog) significantly increases myofilament Ca2+ sensitivity at short SL by elevating cooperative recruitment of strong XBs such that an increase in SL leads to no further increase in myofilament Ca2+ sensitivity (12); in other words, NEM-S1 abolishes LDA. Likewise, expression of slow skeletal troponin I in cardiac muscle enhances myofilament Ca2+ sensitivity but attenuates LDA (17) and R92L mutation in cardiac troponin T enhances myofilament Ca2+ sensitivity but abolishes LDA (20) in cardiac muscle fibers. Because OM also augments strong XB-based cooperative activation of thin filaments and myofilament Ca2+ sensitivity, the observations made above highlight the need to assess the OM-mediated effect on LDA. Whether or not the OM-mediated effects on cardiac myofilament alter LDA in cardiac muscle fibers remains unknown.
The physiological significance of understanding the effect of OM on LDA in cardiac muscle is highlighted by observations that LDA is impaired in patients with heart failure (21, 22, 23). There is also experimental evidence to suggest that attenuation of LDA at the myofilament level may translate as an impaired FS mechanism in the intact heart (16, 17, 18, 19). To test the hypothesis that OM alters cardiac myofilament length-sensing mechanisms to modulate LDA, we characterized steady-state and dynamic contractile features in cardiac muscle fibers from Cavia porcellus (guinea pigs) in the absence and presence of 0.3 and 3.0 μM OM at short (1.9 μm) and long SLs (2.3 μm). Regardless of the OM concentration, steady-state contractile measurements demonstrated that myofilament Ca2+ sensitivity increased to a greater extent at short SL than at long SL; consequently, OM abolished the SL-dependent increase in myofilament Ca2+ sensitivity. Moreover, the SL dependency of XB turnover rate and XB detachment kinetics was abolished, suggesting that cooperative mechanisms associated with LDA were altered by the OM-mediated effects on cardiac myofilaments.
Materials and Methods
Animal protocols
Six- to eight-month-old male Dunkin-Hartley guinea pigs (Charles River, Burlington, MA) were used in this study. All animals were housed in environmentally controlled rooms (accredited by the American Association for Laboratory Animal Care) under 12 h light and dark cycles and received proper care and treatment in accordance with the pre-approved protocols of the Washington State University Institutional Animal Care and Use Committee. The procedures for euthanizing guinea pigs conform to the recommendations of the American Veterinary Medical Association as outlined in the Guidelines for the Euthanasia of Animals.
Measurements of pCa-tension relationship
Left ventricular papillary muscle fibers from guinea pigs were prepared and detergent-skinned, as described in the Supporting Material. Muscle fibers were attached between a motor arm (322C; Aurora Scientific, Ontario, Canada) and a force transducer (AE 801; Sensor One Technologies, Sausalito, CA) using T-shaped aluminum clips. The resting SL of muscle fibers was adjusted to either 1.9 or 2.3 μm and steady-state tension measurements were made in various test solutions with pCa (−log of [Ca2+]free) ranging from 9.0 to 4.3. The compositions of pCa solutions are listed in the Supporting Material. The pH and ionic strength of pCa solutions were adjusted to 7.0 and 180 mM, respectively. All measurements were made at 20°C.
Preparation of OM solution
OM (CK-1827452) was acquired from Selleckchem (Houston, TX) and an initial 15 mM stock was prepared in dimethylsulfoxide (DMSO) as per the manufacturer’s instructions. Appropriate volumes of concentrated OM stock solutions were added to various pCa solutions to achieve final concentrations ranging from 0.1 to 10 μM; the final concentration of DMSO was 0.5%. To make comparisons relevant, we also adjusted the final concentration of DMSO in pCa solutions used for control (untreated) fibers to 0.5%. During the experiment, each fiber was incubated in DMSO- or OM-containing solution for 10 min before making contractile measurements.
Dynamic muscle fiber stiffness
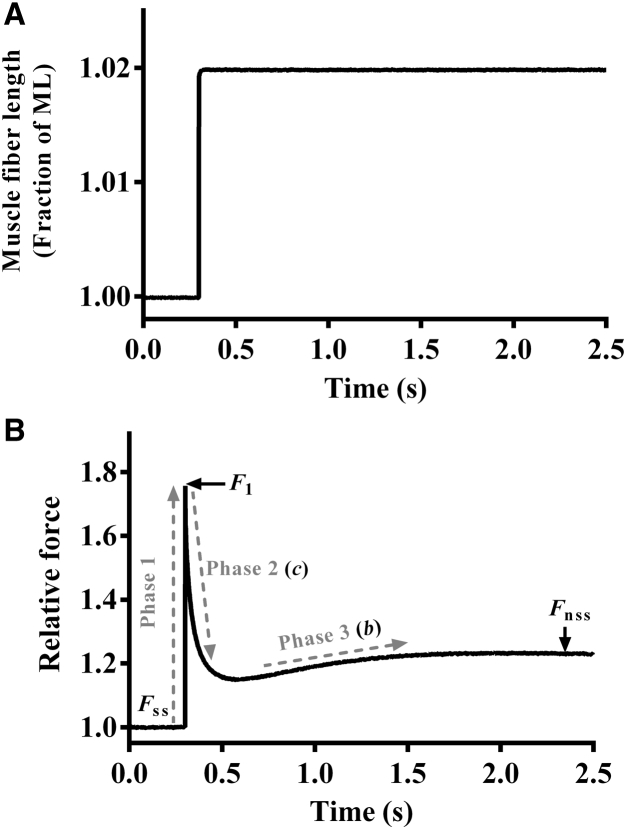
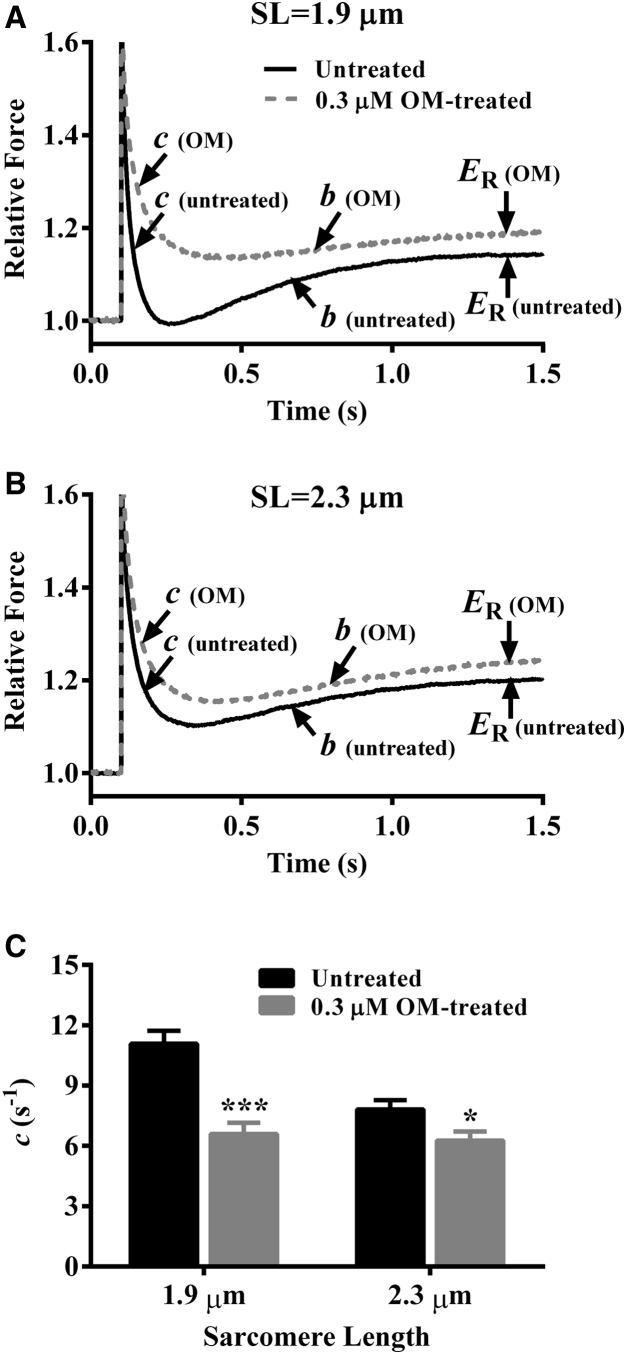
A series of various amplitude stretch/release perturbations (±0.5, ±1.0, ±1.5, and ±2.0% of the initial muscle length (ML)) was applied on muscle fibers and the corresponding force responses were recorded (24). Representative traces of a 2% step-like increase in ML and the corresponding force response elicited by an untreated muscle fiber are shown in Fig. 1, A and B, respectively. A nonlinear recruitment-distortion (NLRD) model was fit to force responses (24) to estimate the following four model parameters: the magnitude of the instantaneous muscle fiber stiffness caused by a sudden change in ML (ED); the rate at which the strain within bound XBs dissipates to a steady-state level (c); the rate at which new XBs are recruited into the force-bearing state due to a change in ML (b); and the magnitude of increase in the muscle fiber stiffness due to ML-mediated recruitment of additional force-bearing XBs (ER). Details on the characteristic features of force responses to step-like length perturbations and the physiological significance of model parameters are included in the Supporting Material.
Figure 1.
Force response to a sudden 2% stretch. (A) A representative 2% increase in ML imposed on a control (untreated) guinea pig cardiac muscle fiber at maximal Ca2+ activation (pCa 4.3). (B) The corresponding force responses normalized to the isometric steady-state force (Fss) before ML change. Different phases (dashed lines) from which respective parameters were estimated are highlighted. F1, the instantaneous increase in force due to sudden increase in ML (phase 1); c, the rate at which the sudden ML-induced strain within force-bearing XBs dissipates to a minimal force point (phase 2); b, the rate of delayed force rise after an increase in ML (phase 3); Fnss, the new steady-state force corresponding to an increase in ML.
Rate constant of tension redevelopment, ktr
ktr was estimated using force response in maximally activated muscle fibers in response to a large slack/restretch perturbation, as described in the Supporting Material.
Statistical analysis
Our experimental model involved two factors, treatment (untreated and OM-treated) and SL (1.9 and 2.3 μm). Therefore, we used two-way analysis of variance (ANOVA) to analyze the data. A significant treatment-SL interaction effect suggested that the effect of OM on a given contractile parameter varied significantly with SL. When the interaction effect was not significant, we assessed the main effects of both OM and SL. To probe the underlying cause for a significant interaction effect or a main effect, we performed multiple post hoc t-tests using Fisher’s least-squares difference method. The criterion for statistical significance was set to p < 0.05. Data were presented as the mean ± SE.
Results
Phosphorylation status of sarcomeric proteins in untreated and OM-treated fibers
To determine the phosphorylation status of sarcomeric proteins in untreated and OM-treated fibers, solubilized protein samples from untreated and OM-treated fibers were subjected to Pro-Q phospho-analysis, as described in the Supporting Material. The representative gel shown in Fig. S1 demonstrates that the phosphorylation levels of various sarcomeric proteins are similar in untreated and OM-treated fibers; this finding is in agreement with another recent study of OM (5).
Dose response of OM
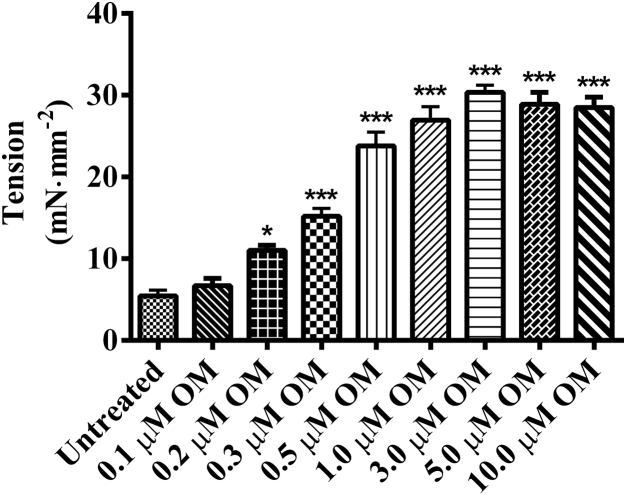
We generated a dose-response curve for OM by measuring tension of muscle fibers exposed to various OM concentrations ranging from 0.1 to 10 μM at pCa 6.0. As illustrated in Fig. 2, tension increased progressively with increasing OM concentration, reaching a maximum value at 3 μM. The OM concentration required to attain a half-maximal effect (EC50) in guinea pig was 0.36 μM, which is comparable to the EC50 of 0.31 μM reported in a previous study in mice (8). Our choice of low (0.3 μM) and high (3.0 μM) OM concentrations in this study spans the range of doses used in clinical trials (3).
Figure 2.
Dose response of OM. Steady-state tensions in muscle fibers were measured in pCa 6.0 solution, with OM concentration ranging from 0.1 to 10 μM. Tension increased with increasing OM concentration up to 3.0 μM and saturated thereafter. Asterisks indicate a significant difference from untreated fibers (∗p < 0.05, ∗∗∗p < 0.001; NS, not significant). A separate set of four fibers was measured at each concentration. Data are expressed as the mean ± SE.
Effect of OM on maximal tension and ED
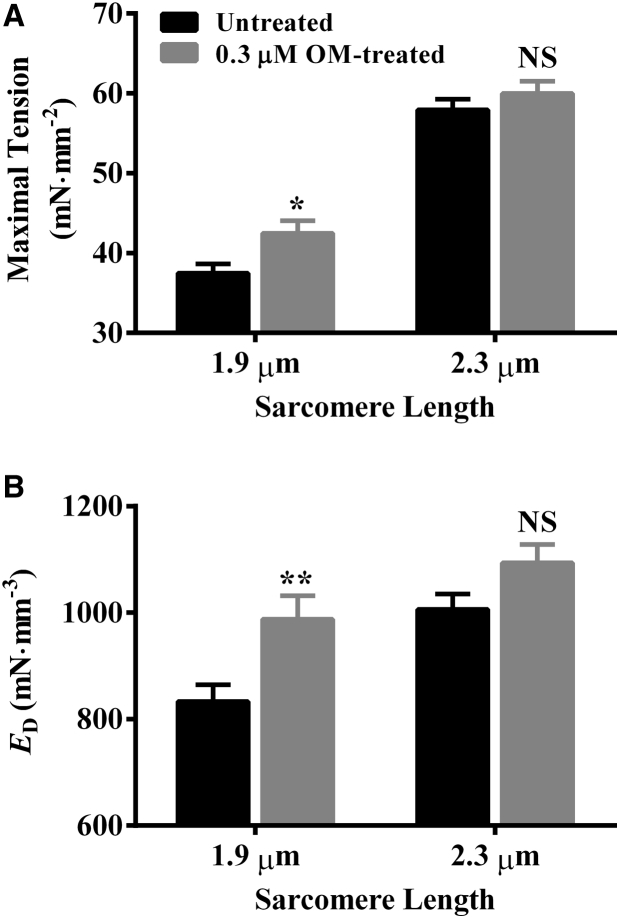
Two-way ANOVA of maximal tension (pCa 4.3) did not show a significant interaction effect (p = 0.30), but it showed significant main effects of both OM (p < 0.05) and SL (p < 0.001). To probe the underlying cause of significant effects of OM and SL on maximal tension, we performed post hoc t-tests. OM (0.3 μM) significantly increased maximal tension by 13% (p < 0.05; Fig. 3 A) at short SL, but it showed no significant effect (p = 0.85; Fig. 3 A) at long SL. Our observation at long SL (2.3 μm) is consistent with another study conducted at an SL of 2.24 μm (7). As for the main effect of SL on Ca2+-activated maximal tension, increasing the SL from 1.9 to 2.3 μm significantly increased maximal tension by 55% (p < 0.001; Fig. 3 A) in untreated fibers and by 41% (p < 0.001; Fig. 3 A) in 0.3-μM-OM-treated fibers. Fibers treated with 3.0 μM OM (Table S1) also substantiated our observations on maximal tension in 0.3-μM-OM-treated fibers.
Figure 3.
Effect of 0.3 μM OM on maximal tension and ED. Steady-state tension was measured in muscle fibers at pCa 4.3, as described in the Supporting Material. ED was estimated as the slope of the linear relationship between F1 and Fss (see Fig. 1) and the corresponding ML changes (ΔL). Bar graphs show the effect of 0.3 μM OM on (A) maximal tension and (B) ED at short and long SL. Statistical differences were analyzed by two-way ANOVA and subsequent post hoc multiple pairwise comparisons using Fisher’s least-squares difference method. Asterisks indicate a significant difference from untreated fibers (∗p < 0.05, ∗∗p < 0.01; NS, not significant). A separate set of fibers from three hearts was used for each group. The numbers of fibers measured for untreated and 0.3-μM-OM-treated groups at short SL were 12 and 11, whereas those at long SL were 12 and 12, respectively. Data are expressed as the mean ± SE.
We compared estimates of ED (pCa 4.3) among groups to validate our observations on maximal tension. ED was estimated from the instantaneous increase in force (F1; Fig. 1 B) in response to a sudden increase in ML (Fig. 1 A), as described in the Supporting Material. We have previously shown that ED is correlated with maximal tension and that it is an approximation of the number of force-bearing XBs (24, 25, 26, 27). Two-way ANOVA of ED did not show a significant interaction effect (p = 0.62), but it confirmed significant main effects of both OM (p < 0.05) and SL (p < 0.01). The 0.3 μM OM significantly increased ED by 19% (p < 0.01; Fig. 3 B) at short SL but it did not show a significant effect (p = 0.17; Fig. 3 B) at long SL. An increase in SL significantly increased ED by 20% (p < 0.01; Fig. 3 B) in untreated fibers and by 14% (p < 0.05; Fig. 3 B) in 0.3-μM-OM-treated fibers. Our observations on ED were similar in both 0.3- and 3.0-μM-OM-treated fibers (Table S1).
Effect of OM on the pCa-tension relationship
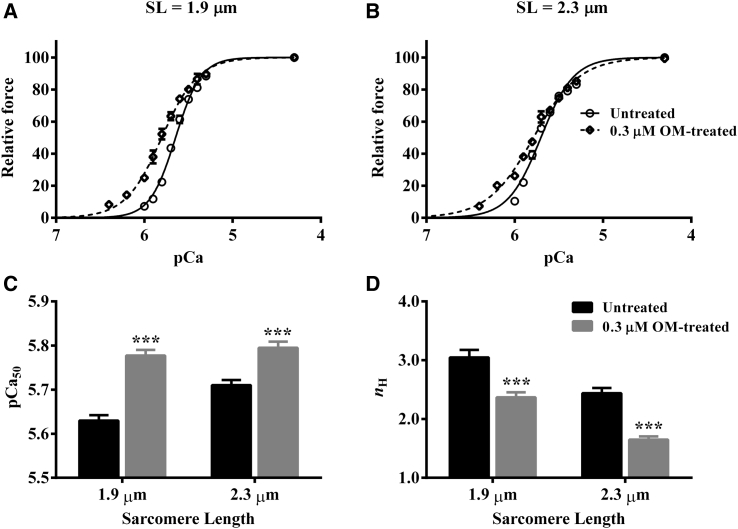
pCa-tension plots showed a greater leftward shift in the pCa-tension relationship of 0.3-μM-OM-treated fibers at short (Fig. 4 A) than at long SL (Fig. 4 B). This suggested that OM induced a greater increase in myofilament Ca2+ sensitivity at short than at long SL. Fig. 4, A and B, also indicated a decrease in the steepness of the pCa-tension relationship in 0.3-μM-OM-treated fibers, suggesting that OM attenuated myofilament cooperativity. To quantify the magnitude of the OM-mediated effect on the pCa-tension relationship, we used Hill model parameters, pCa50 (myofilament Ca2+ sensitivity), and nH (myofilament cooperativity).
Figure 4.
Effect of 0.3 μM OM on the pCa-tension relationship. pCa-tension relationships were measured using methods described in the Supporting Material. The Hill model was fitted to the pCa-tension relationships to derive pCa50 and nH. (A and B) Effect of 0.3 μM OM on the pCa-tension relationship at (A) short SL and (B) long SL. (C and D) Bar graphs show the effect of 0.3 μM OM on (C) pCa50 and (D) nH at short and long SL. Statistical differences were analyzed by two-way ANOVA and subsequent post hoc multiple pairwise comparisons using Fisher’s least-squares difference method. Asterisks indicate a significant difference from untreated fibers (∗∗∗p < 0.001). A separate set of fibers from three hearts was used for each group. The numbers of fibers measured for untreated and 0.3 μM OM groups at short SL were 12 and 11, whereas those at long SL were 12 and 12, respectively. Data are expressed as the mean ± SE.
Two-way ANOVA of pCa50 showed a significant interaction effect (p < 0.05), suggesting that the effect of 0.3 μM OM on pCa50 varied in an SL-dependent manner. Post hoc analysis showed that the magnitude of increase in pCa50 was nearly twofold greater at short than at long SL in OM-treated fibers. When compared to the untreated group, 0.3 μM OM significantly increased pCa50 by 0.15 pCa units (p < 0.001; Fig. 4 C) at short SL, whereas it increased pCa50 by only 0.08 pCa units (p < 0.001; Fig. 4 C) at long SL; this suggested that LDA was altered. LDA was normal in untreated fibers because Ca2+ sensitivity increased significantly by 0.08 pCa units (p < 0.001; Fig. 4 C) when the SL was increased from 1.9 to 2.3 μm. In contrast, increasing the SL did not alter Ca2+ sensitivity significantly (p = 0.33; Fig. 4 C) in 0.3 μM OM-treated fibers, demonstrating that OM blunted LDA. A similar effect was also observed in 3.0-μM-OM-treated fibers (Table S1), demonstrating that LDA was blunted regardless of the OM concentration. As for nH, two-way ANOVA did not show a significant interaction effect (p = 0.56), but it confirmed significant main effects of both OM (p < 0.001) and SL (p < 0.001). Treatment with 0.3 μM OM significantly decreased nH in cardiac fibers by 22% (p < 0.001; Fig. 4 D) at short SL and by 32% (p < 0.001; Fig. 4 D) at long SL. An increase in SL significantly decreased nH by 20% (p < 0.001; Fig. 4 D) in untreated fibers and by 30% (p < 0.001; Fig. 4 D) in 0.3-μM-OM-treated fibers. Although the SL dependency of nH was not different between untreated and 0.3-μM-OM-treated fibers, OM attenuated nH at both SLs, a finding that was also substantiated by our observations in the presence of 3.0 μM OM (Table S1).
Effect of OM on c
To determine whether OM altered XB detachment kinetics in an SL-dependent manner, we measured c at pCa 4.3. As demonstrated previously, c is an approximate measure of the XB detachment rate, g (24, 25). A comparison of force responses to a sudden 2% stretch showed that OM induced a rightward shift in the force decay phase at both short (Fig. 5 A) and long SLs (Fig. 5 B), suggesting slower c; however, such OM-induced rightward shift in the force decay phase was more pronounced at short SL. This differential attenuation of c in 0.3-μM-OM-treated fibers at short and long SL gave rise to a significant interaction effect (p < 0.01). Treatment with 0.3 μM OM significantly decreased c by 40% (p < 0.001; Fig. 5 C) at short SL, whereas it decreased c by only 19% (p < 0.05; Fig. 5 C) at long SL. An increase in SL from 1.9 to 2.3 μm significantly attenuated c by 29% (p < 0.001; Fig. 5 C) in untreated fibers, whereas it did not show a significant effect on c (p = 0.66; Fig. 5 C) in 0.3-μM-OM-treated fibers. This observation demonstrated that OM abolished the SL dependency of c, which was supported by our data from 3.0-μM-OM-treated fibers (Table S1).
Figure 5.
Effect of 0.3 μM OM on c. Effect of 0.3 μM OM on force responses to a 2% sudden stretch at (A) short SL and (B) long SL. Force data were normalized by the isometric steady-state value before ML perturbation. c describes the rate of force decay to a minimal force point, b represents the rate constant of delayed force rise, and ER represents the ML-mediated increase in the steady-state force (24). Parameters b, c, and ER are estimated by fitting the nonlinear recruitment-distortion model to a family of force responses to various-amplitude ML perturbations (24). (C) Bar graph showing the effect of 0.3 μM OM on c at short and long SL. Statistical differences were analyzed by two-way ANOVA and subsequent post hoc multiple pairwise comparisons using Fisher’s least-squares difference method. Asterisks indicate a significant difference from untreated fibers (∗p < 0.05, ∗∗∗p < 0.001). A separate set of fibers from three hearts was used for each group. The numbers of fibers measured for untreated and 0.3 μM OM groups at short SL were 12 and 11, whereas those at long SL were 12 and 12, respectively. Data are expressed as mean ± SE.
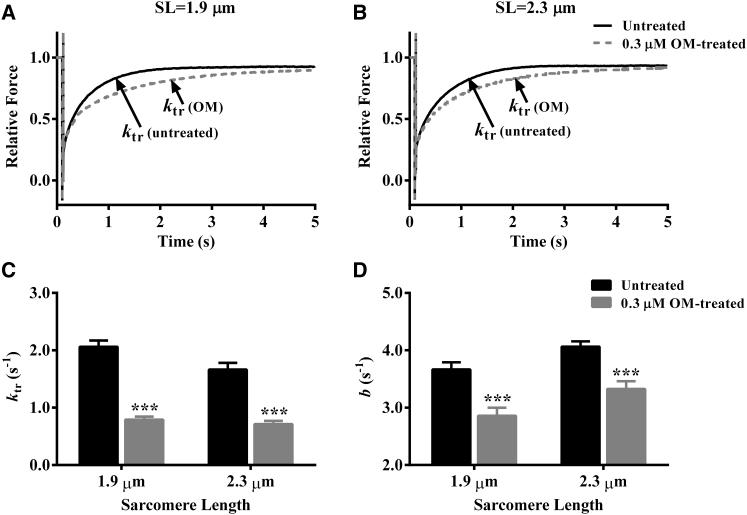
Effect of OM on ktr and b
To determine whether OM altered the XB turnover rate in an SL-dependent manner, we measured ktr and b at pCa 4.3. A comparison of force responses to the slack-restretch maneuver showed a rightward shift in the rising phase of 0.3-μM-OM-treated fibers at both SLs (Fig. 6, A and B), suggesting a slower ktr. Our analysis of ktr did not show a significant interaction effect (p = 0.10), but it showed significant main effects of both OM (p < 0.001) and SL (p < 0.05). Treatment with 0.3 μM OM significantly decreased ktr by 61% (p < 0.001; Fig. 6 C) at short SL and by 57% (p < 0.001; Fig. 6 C) at long SL. An increase in SL from 1.9 to 2.3 μm significantly decreased ktr by 0.40 s−1 (p < 0.001; Fig. 6 C) in untreated fibers, wheras it did not decrease ktr significantly (0.08 s−1; p = 0.54; Fig. 6 C) in 0.3 μM OM-treated fibers. This observation demonstrated that OM abolished the SL dependency of ktr, a finding that was substantiated by our data from 3.0-μM-OM-treated fibers (Table S1).
Figure 6.
Effect of 0.3 μM OM on ktr and b. ktr describes the rate of tension redevelopment and is estimated by fitting a mono-exponential function to the rising phase of the force response to a large release-restretch length maneuver (38). b represents the rate constant of delayed force rise after a sudden stretch and is estimated by fitting the nonlinear recruitment-distortion model (24) to a family of force responses to various-amplitude length perturbations (see Figs. 1 and 5, A and B). (A and B) Effect of 0.3 μM OM on the force response to a large release-restretch maneuver at (A) short SL and (B) long SL. Force data were normalized by the isometric steady-state value before length perturbation. (C and D) Bar graph showing the effect of 0.3 μM OM on (C) ktr and (D) b at short and long SL. Statistical differences were analyzed by two-way ANOVA and subsequent post hoc multiple pairwise comparisons using Fisher’s least-squares difference method. Asterisks indicate a significant difference from untreated fibers (∗∗∗p < 0.001). A separate set of fibers from three hearts was used for each group. The numbers of fibers measured for untreated and 0.3-μM-OM-treated groups at short SL were 12 and 11, whereas those at long SL were 12 and 12, respectively. Data are expressed as the mean ± SE.
The OM-mediated effect on XB recruitment dynamics was determined by estimating b. A comparison of force responses to a sudden 2% stretch indicated a slower rate of delayed force rise at both SLs (Fig. 5, A and B), suggesting slower b. Two-way ANOVA of b did not show a significant interaction effect (p = 0.78), but it confirmed significant main effects of both OM (p < 0.001) and SL (p < 0.01). Treatment with 0.3 μM OM significantly decreased b at both SLs by 22% (p < 0.001; Fig. 6 D) at short SL and 17% (p < 0.001; Fig. 6 D) at long SL, suggesting that the attenuating effect of OM on b was similar at both SLs. Consequently, the SL dependency of b was unaltered by OM, because an increase in SL significantly increased b by 10% (p < 0.05; Fig. 6 D) in untreated fibers and by 16% (p < 0.05; Fig. 6 D) in 0.3-μM-OM-treated fibers. Our observations on the attenuating effect of OM on b are corroborated by our observations in the presence of 3.0 μM OM (Table S1).
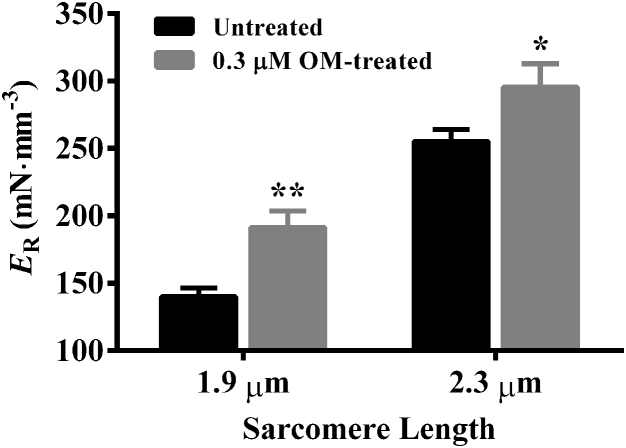
Effect of OM on ER
To investigate whether OM altered the magnitude of stretch activation in an SL-dependent manner, we measured ER at pCa 4.3. ER is estimated from the new steady-state force (Fnss; Fig. 1 B) that results from a sudden increase in ML (Fig. 1 A), as described in the Supporting Material. Two-way ANOVA of ER did not show a significant interaction effect (p = 0.65), but it confirmed significant main effects of both OM (p < 0.001) and SL (p < 0.001). Treatment with 0.3 μM OM significantly increased ER by 36% (p < 0.01; Fig. 7) at short SL and 16% (p < 0.05; Fig. 7) at long SL, demonstrating that the OM-mediated increase in ER was lower at long SL. Consequently, our data showed that the increase in ER associated with an increase in SL from 1.9 to 2.3 μm (ΔER) is attenuated by OM. For example, ΔER was 82% (p < 0.001; Fig. 7) in untreated fibers, but this was only 54% (p < 0.001; Fig. 7) in 0.3-μM-OM-treated fibers. The attenuating effect of 0.3 μM OM on ΔER was substantiated by our data from 3.0-μM-OM-treated fibers (Table S1).
Figure 7.
Effect of 0.3 μM OM on ER. ER is the magnitude of stretch activation and is estimated as the slope of the linear relationship between (Fnss-Fss) and imposed ML changes, ΔL (also see Figs. 1 and 5, A and B). Statistical differences were analyzed by two-way ANOVA and subsequent post-hoc multiple pairwise comparisons using Fisher’s least-squares difference method. Asterisks indicate significantly different from untreated fibers (∗p < 0.05, ∗∗p < 0.01). A separate set of fibers from three hearts was used for each group. The number of fibers measured for untreated and 0.3 μM OM groups at short SL were 12 and 11, while those at long SL were 12 and 12, respectively. Data are expressed as mean ± SE.
Discussion
The impact of OM on the SL dependency of myofilament Ca2+ sensitivity has not been studied before. A major finding in our study is that the SL-dependent increase in myofilament Ca2+ sensitivity, ΔpCa50, is abolished by OM. Our data also demonstrate that OM abolishes the SL dependency of c and ktr at both concentrations (0.3 and 3.0 μM) of OM. We discuss the mechanisms by which OM-mediated effects on cardiac myofilaments are differently affected by changes in SL.
As others have observed before (5, 6, 7, 8), pCa50 increases at long SL in OM-treated fibers (Fig. 4 C). A novel finding of our study, to our knowledge, is that OM increases pCa50 to a greater extent at short SL than at long SL (Fig. 4 C); a consequence of this effect at short SL is that an increase in SL does not increase pCa50 any further in OM-treated fibers. This observation suggests that mechanisms that underlie SL-mediated increase in pCa50 are saturated at short SL. Therefore, OM abolishes the ML-mediated increase in pCa50 (Fig. 4 C), which is known to be prominent in normal cardiac muscle fibers (9, 10, 11). To decompose mechanisms that are saturated at short SL, we must consider some fundamental aspects of LDA that are associated with the length-mediated increase in pCa50. The basic prevailing view of LDA is that the steeper dependency of pCa50 on SL in cardiac muscle results from the SL-mediated effects on thin-filament cooperativity. SL-mediated effects increase pCa50, leading to the recruitment of new force-bearing XBs (9, 12, 13, 14, 15). Because OM-treated fibers deviate significantly from normal behavior, it suggests that mechanisms associated with LDA are altered in OM-treated fibers, especially at short SL.
Our contractile studies at short and long SL show several changes in contractile parameters that may help explain altered LDA in OM-treated fibers. Although there is a small but significant increase (13%) in tension at maximal activation at short SL, what is striking is the augmenting effect of OM on tension at submaximal activation. For instance, relative to untreated fibers, there is an ∼160% increase in tension at half-maximal activation (pCa 5.8) in 0.3-μM-OM-treated fibers (Table S2), indicating that OM significantly increases the number of force-bearing XBs at submaximal activation. A consequence of such an increase in force-bearing XBs at submaximal activation is that it enhances XB-based activation of thin filaments via XB-XB and XB-regulatory unit (RU; troponin-tropomyosin) cooperative mechanisms; with respect to these two cooperative mechanisms, the consequences of augmented XB-RU cooperativity are of particular importance to our focus on pCa50. It is generally known that XB-RU cooperativity is one of the important mechanisms that underpins the molecular basis for the length-mediated increase in pCa50 (14, 28). An indirect effect of such an increase in XB-based effects is that it engages more RUs in XB-RU cooperativity, leaving behind fewer RUs in the recruitable pool. Therefore, RU-RU cooperativity is expected to decrease in OM-treated fibers; our data confirm this because a decrease in RU-RU cooperativity increases pCa50 (Fig. 4 C) and decreases nH (Fig. 4 D; (14)). The combined effects of increased XB-based cooperativity and decreased RU-RU cooperativity (an indirect effect of OM on thin-filament activation) increases pCa50 substantially at short SL.
At long SL, pCa50 does not increase above that of short SL in OM-treated fibers. The saturation of increased XB-RU and decreased RU-RU cooperativity at short SL may provide the molecular basis for the lack of increase in pCa50 as SL increases. Due to the OM-mediated increase in the engagement of RUs in XB-RU cooperativity at short SL, there is limited capacity for the XB-RU population to rise as SL increases. The observation made at 0.3 μM OM was also valid for 3.0 μM, because pCa50 increased at short SL to such an extent that pCa50 did not increase any further at long SL (Table S1). We also observe a differential effect on the magnitude of ER at short and long SL in OM-treated fibers; for example, OM increases ER to a greater extent at short SL (36% at 1.9 μm and 16% at 2.3 μm). This effect is even more pronounced at submaximal activation (pCa 5.8); OM increases ER by 58% at short SL, without any change at long SL (Table S2). Previous studies have shown that an increase in XB-based cooperativity augments ER, whereas a decrease in XB-based cooperativity attenuates ER (25, 29, 30). Collectively, these observations substantiate the idea that XB-based cooperativity is greater at short SL in OM-treated fibers.
OM-mediated increase in XB-based cooperativity may also affect other contractile parameters. Previous studies have shown that an increase in XB-based cooperativity slows ktr (14, 31) and OM attenuates ktr at long SL (6, 7). A new finding of our study, to our knowledge, shows that ktr is also attenuated at short SL (Fig. 6 C). Therefore, significant attenuation of ktr in OM-treated fibers at both short and long SL (Fig. 6 C) may be attributed to enhanced XB-based cooperativity. This is further substantiated by the observation that b is attenuated significantly at both short and long SL in OM-treated fibers (Fig. 6 D). Such observations regarding b are also valid at submaximal activation in 0.3-μM-OM-treated fibers (Table S2). Attenuation of ktr in muscle fibers (5, 6, 7), including the finding from the study presented here, are in disagreement with previous in vitro studies (4, 32, 33), which have suggested that OM accelerates both the phosphate release and the transition of XBs from a weakly to a strongly bound state. Such discrepancy between muscle fiber studies and in vitro assays may result from the absence of lattice structure and poorly preserved cooperative mechanisms in in vitro assays. Despite some differences in observations, what is consistently observed is that OM increases the number of XBs at low levels of activation (5, 6, 7, 8). The basis for the OM-mediated increase in the number of force-bearing XBs and the subsequent increase in XB-based cooperativity may be linked to the enhanced duty ratio of XBs, as suggested by previous studies (5, 6, 7), which is consistent with a significant decrease in g observed in our study. The increased duty ratio of XBs may promote recruitment of additional XBs via XB-based cooperative mechanisms (5, 7).
The differential effects of OM on XB-based cooperativity at short and long SL has another interesting consequence for SL-mediated effects on XB cycling kinetics. Length-mediated effects on cardiac myofilaments have been shown to attenuate ktr and g at long SL (34, 35, 36). Such changes are also evident in g (Fig. 5 C) and ktr (Fig. 6 C) of untreated fibers in this study. What is interesting is that such an SL-mediated attenuating effect on ktr is abolished in OM-treated fibers. Attenuation of ktr at short SL may be attributed to enhanced XB-based cooperativity (31). This attenuating effect on ktr appears to be saturated at short SL, because there is no further attenuation at long SL (Fig. 6 C). Attenuation of ktr in OM-treated fibers may be explained by a decrease in g (34, 37, 38, 39).
Two plausible mechanisms have been suggested to explain how OM decreases g (7): 1) OM may decrease g by slowing the rate of ADP release, although direct evidence is lacking; and 2) OM may decrease g by slowing the isomerization step between strongly bound actomyosin-ADP states that precedes the XB detachment step. The new finding from this study, to our knowledge, shows that g is also attenuated at short SL, but the magnitude of attenuation is greater at short SL; for example, g is attenuated by 40% at short SL and by 20% at long SL in 0.3-μM-OM-treated fibers (Fig. 5 C). Such observations regarding g are also valid at submaximal activations in 0.3-μM-OM-treated fibers (Table S2). A consequence of this greater attenuation of g at short SL is that the SL dependency of g is ablated (Fig. 5 C) in a manner similar to that observed for ktr (Fig. 6 C). These observations are also supported by data from fibers treated with 3.0 μM OM (Table S1). Collectively, the observations made above substantiate the notion that OM alters mechanisms linked to LDA.
Relevance of our findings to whole heart function
Previous studies in both animal models (2, 4) and humans (1, 3) suggest that OM enhances systolic function by prolonging the systolic ejection time. Changes in b and ER shed some light on how OM may tune the late phase of ejection. The slower b in OM-treated fibers suggests a slower rise in XB recruitment, which prolongs the effect of stretch activation on ventricular force output (39, 40, 41, 42). Augmentation of ER suggests an increase in ventricular force output during the late phase of ejection. Such enhanced force output during the late stage of systole—paired with the increased Ca2+ sensitivity and lengthened duty ratio of XBs (due to slower g)—may significantly increase stroke volume by prolonging the duration of systole. Although these changes suggest improved systolic function, they may adversely affect diastole not only by delaying the onset of ventricular relaxation but also by slowing isovolumic relaxation. Such OM-mediated effects on relaxation may reduce the amount of venous filling in the next beat, a possibility that requires further investigation.
New observations from our study demonstrate that OM has a major effect on Ca2+ sensitivity and LDA, leading us to speculate that it may modify the FS mechanism to some extent in the intact heart. Indeed, changes in myofilament Ca2+ sensitivity have been shown to impair FS mechanism in intact hearts (16, 17, 18, 19). OM-mediated blunting of LDA may attenuate the ability of the heart to increase stroke volume in response to an increase in venous return during exertion. This may have some relevance to a recent clinical study in which OM-treated patients with systolic dysfunction exhibited a decreasing trend in exercise time by 30%, although authors noted that this decrease did not reach statistical significance due to the smaller sample size (43). Thus, new findings from our contractile study, along with the observations from the aforementioned study, warrant further investigation regarding the effect of OM on the FS mechanism at the whole-heart level.
Author Contributions
Study conception and design, data acquisition, data analysis and interpretation, and drafting of the manuscript, S.K.G.; Data acquisition, data analysis and interpretation, and drafting of the manuscript, S.M.R.; Study conception and design, data interpretation, and drafting of the manuscript, M.C.
Editor: David Warshaw.
Footnotes
Supporting Materials and Methods, one figure, and two tables are available at http://www.biophysj.org/biophysj/supplemental/S0006-3495(17)30752-X.
Supporting Citations
References (44, 45, 46, 47, 48, 49, 50, 51) appear in the Supporting Material.
Supporting Material
References
- 1.Cleland J.G., Teerlink J.R., Malik F.I. The effects of the cardiac myosin activator, omecamtiv mecarbil, on cardiac function in systolic heart failure: a double-blind, placebo-controlled, crossover, dose-ranging phase 2 trial. Lancet. 2011;378:676–683. doi: 10.1016/S0140-6736(11)61126-4. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y.T., Malik F.I., Vatner S.F. Improvement of cardiac function by a cardiac Myosin activator in conscious dogs with systolic heart failure. Circ Heart Fail. 2010;3:522–527. doi: 10.1161/CIRCHEARTFAILURE.109.930321. [DOI] [PubMed] [Google Scholar]
- 3.Teerlink J.R., Clarke C.P., Wolff A.A. Dose-dependent augmentation of cardiac systolic function with the selective cardiac myosin activator, omecamtiv mecarbil: a first-in-man study. Lancet. 2011;378:667–675. doi: 10.1016/S0140-6736(11)61219-1. [DOI] [PubMed] [Google Scholar]
- 4.Malik F.I., Hartman J.J., Morgans D.J. Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science. 2011;331:1439–1443. doi: 10.1126/science.1200113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamidi R., Gresham K.S., Stelzer J.E. Molecular effects of the myosin activator omecamtiv mecarbil on contractile properties of skinned myocardium lacking cardiac myosin binding protein-C. J. Mol. Cell. Cardiol. 2015;85:262–272. doi: 10.1016/j.yjmcc.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy L., Kovács Á., Papp Z. The novel cardiac myosin activator omecamtiv mecarbil increases the calcium sensitivity of force production in isolated cardiomyocytes and skeletal muscle fibres of the rat. Br. J. Pharmacol. 2015;172:4506–4518. doi: 10.1111/bph.13235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swenson A.M., Tang W., Yengo C.M. Omecamtiv mecarbil enhances the duty ratio of human β-cardiac myosin resulting in increased calcium sensitivity and slowed force development in cardiac muscle. J. Biol. Chem. 2017;292:3768–3778. doi: 10.1074/jbc.M116.748780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Utter M.S., Ryba D.M., Solaro R.J. Omecamtiv mecarbil, a cardiac myosin activator, increases Ca2+ sensitivity in myofilaments with a dilated cardiomyopathy mutant tropomyosin E54K. J. Cardiovasc. Pharmacol. 2015;66:347–353. doi: 10.1097/FJC.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen D.G., Kentish J.C. The cellular basis of the length-tension relation in cardiac muscle. J. Mol. Cell. Cardiol. 1985;17:821–840. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 10.Konhilas J.P., Irving T.C., de Tombe P.P. Length-dependent activation in three striated muscle types of the rat. J. Physiol. 2002;544:225–236. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y.P., Fuchs F. Length, force, and Ca2+-troponin C affinity in cardiac and slow skeletal muscle. Am. J. Physiol. 1994;266:C1077–C1082. doi: 10.1152/ajpcell.1994.266.4.C1077. [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimons D.P., Moss R.L. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ. Res. 1998;83:602–607. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- 13.Moss R.L., Fitzsimons D.P. Frank-Starling relationship: long on importance, short on mechanism. Circ. Res. 2002;90:11–13. [PubMed] [Google Scholar]
- 14.Razumova M.V., Bukatina A.E., Campbell K.B. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys. J. 2000;78:3120–3137. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith L., Tainter C., Martyn D.A. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys. J. 2009;96:3692–3702. doi: 10.1016/j.bpj.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abraham D.M., Davis R.T., 3rd, Rockman H.A. β-Arrestin mediates the Frank-Starling mechanism of cardiac contractility. Proc. Natl. Acad. Sci. USA. 2016;113:14426–14431. doi: 10.1073/pnas.1609308113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arteaga G.M., Palmiter K.A., Solaro R.J. Attenuation of length dependence of calcium activation in myofilaments of transgenic mouse hearts expressing slow skeletal troponin I. J. Physiol. 2000;526:541–549. doi: 10.1111/j.1469-7793.2000.t01-1-00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Layland J., Grieve D.J., Shah A.M. Essential role of troponin I in the positive inotropic response to isoprenaline in mouse hearts contracting auxotonically. J. Physiol. 2004;556:835–847. doi: 10.1113/jphysiol.2004.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nowak G., Peña J.R., Wolska B.M. Correlations between alterations in length-dependent Ca2+ activation of cardiac myofilaments and the end-systolic pressure-volume relation. J. Muscle Res. Cell Motil. 2007;28:415–419. doi: 10.1007/s10974-008-9136-y. [DOI] [PubMed] [Google Scholar]
- 20.Ford S.J., Mamidi R., Chandra M. Effects of R92 mutations in mouse cardiac troponin T are influenced by changes in myosin heavy chain isoform. J. Mol. Cell. Cardiol. 2012;53:542–551. doi: 10.1016/j.yjmcc.2012.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sequeira V., Wijnker P.J., van der Velden J. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ. Res. 2013;112:1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dijk S.J., Paalberends E.R., van der Velden J. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ Heart Fail. 2012;5:36–46. doi: 10.1161/CIRCHEARTFAILURE.111.963702. [DOI] [PubMed] [Google Scholar]
- 23.Schwinger R.H., Böhm M., Erdmann E. The failing human heart is unable to use the Frank-Starling mechanism. Circ. Res. 1994;74:959–969. doi: 10.1161/01.res.74.5.959. [DOI] [PubMed] [Google Scholar]
- 24.Ford S.J., Chandra M., Campbell K.B. Model representation of the nonlinear step response in cardiac muscle. J. Gen. Physiol. 2010;136:159–177. doi: 10.1085/jgp.201010467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Campbell K.B., Chandra M., Hunter W.C. Interpreting cardiac muscle force-length dynamics using a novel functional model. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1535–H1545. doi: 10.1152/ajpheart.01029.2003. [DOI] [PubMed] [Google Scholar]
- 26.Chandra V., Gollapudi S.K., Chandra M. Rat cardiac troponin T mutation (F72L)-mediated impact on thin filament cooperativity is divergently modulated by α- and β-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H1260–H1270. doi: 10.1152/ajpheart.00519.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reda S.M., Gollapudi S.K., Chandra M. L71F mutation in rat cardiac troponin T augments crossbridge recruitment and detachment dynamics against α-myosin heavy chain, but not against β-myosin heavy chain. J. Muscle Res. Cell Motil. 2016;37:215–223. doi: 10.1007/s10974-016-9460-6. [DOI] [PubMed] [Google Scholar]
- 28.de Tombe P.P., Mateja R.D., Irving T.C. Myofilament length dependent activation. J. Mol. Cell. Cardiol. 2010;48:851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Campbell K.B., Chandra M. Functions of stretch activation in heart muscle. J. Gen. Physiol. 2006;127:89–94. doi: 10.1085/jgp.200509483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stelzer J.E., Larsson L., Moss R.L. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J. Gen. Physiol. 2006;127:95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys. J. 1997;72:254–262. doi: 10.1016/S0006-3495(97)78664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y., White H.D., Forgacs E. Omecamtiv mecarbil modulates the kinetic and motile properties of porcine β-cardiac myosin. Biochemistry. 2015;54:1963–1975. doi: 10.1021/bi5015166. [DOI] [PubMed] [Google Scholar]
- 33.Winkelmann D.A., Forgacs E., Stock A.M. Structural basis for drug-induced allosteric changes to human β-cardiac myosin motor activity. Nat. Commun. 2015;6:7974. doi: 10.1038/ncomms8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandra M., Tschirgi M.L., Campbell K.B. Troponin T modulates sarcomere length-dependent recruitment of cross-bridges in cardiac muscle. Biophys. J. 2006;90:2867–2876. doi: 10.1529/biophysj.105.076950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ford S.J., Chandra M. Length-dependent effects on cardiac contractile dynamics are different in cardiac muscle containing α- or β-myosin heavy chain. Arch. Biochem. Biophys. 2013;535:3–13. doi: 10.1016/j.abb.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korte F.S., McDonald K.S. Sarcomere length dependence of rat skinned cardiac myocyte mechanical properties: dependence on myosin heavy chain. J. Physiol. 2007;581:725–739. doi: 10.1113/jphysiol.2007.128199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adhikari B.B., Regnier M., Martyn D.A. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys. J. 2004;87:1784–1794. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner B., Eisenberg E. Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. USA. 1986;83:3542–3546. doi: 10.1073/pnas.83.10.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stelzer J.E., Moss R.L. Contributions of stretch activation to length-dependent contraction in murine myocardium. J. Gen. Physiol. 2006;128:461–471. doi: 10.1085/jgp.200609634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davis J.S., Hassanzadeh S., Epstein N.D. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 41.Stelzer J.E., Fitzsimons D.P., Moss R.L. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys. J. 2006;90:4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stelzer J.E., Patel J.R., Moss R.L. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ. Res. 2006;99:884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 43.Greenberg B.H., Chou W., Shaburishvili T. Safety and tolerability of omecamtiv mecarbil during exercise in patients with ischemic cardiomyopathy and angina. JACC Heart Fail. 2015;3:22–29. doi: 10.1016/j.jchf.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 44.Chandra M., Tschirgi M.L., Campbell K.B. Interaction between myosin heavy chain and troponin isoforms modulate cardiac myofiber contractile dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1595–R1607. doi: 10.1152/ajpregu.00157.2007. [DOI] [PubMed] [Google Scholar]
- 45.de Tombe P.P., Stienen G.J. Protein kinase A does not alter economy of force maintenance in skinned rat cardiac trabeculae. Circ. Res. 1995;76:734–741. doi: 10.1161/01.res.76.5.734. [DOI] [PubMed] [Google Scholar]
- 46.Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J. Physiol. (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 47.Gollapudi S.K., Gallon C.E., Chandra M. The tropomyosin binding region of cardiac troponin T modulates crossbridge recruitment dynamics in rat cardiac muscle fibers. J. Mol. Biol. 2013;425:1565–1581. doi: 10.1016/j.jmb.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gollapudi S.K., Mamidi R., Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys. J. 2012;103:940–948. doi: 10.1016/j.bpj.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gollapudi S.K., Tardiff J.C., Chandra M. The functional effect of dilated cardiomyopathy mutation (R144W) in mouse cardiac troponin T is differently affected by α- and β-myosin heavy chain isoforms. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H884–H893. doi: 10.1152/ajpheart.00528.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mamidi R., Chandra M. Divergent effects of α- and β-myosin heavy chain isoforms on the N terminus of rat cardiac troponin T. J. Gen. Physiol. 2013;142:413–423. doi: 10.1085/jgp.201310971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stienen G.J., Zaremba R., Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J. Physiol. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.