Abstract
The use of platelet-rich plasma and mesenchymal stem cells has garnered much attention in orthopedic medicine, focusing on the biological aspects of cell function. However, shortly after systemic delivery, or even a local injection, few of the transplanted stem cells or platelets remain at the target site. Improvement in delivery, and the ability to track and monitor injected cells, would greatly improve clinical translation. Nanoparticles can effectively and quickly label most cells in vitro, and evidence to date suggests such labeling does not compromise the proliferation or differentiation of cells. A specific type of nanoparticle, the superparamagnetic iron oxide nanoparticle (SPION), is already employed as a magnetic resonance imaging (MRI) contrast agent. SPIONs can be coupled with cells or bioactive molecules (antibodies, proteins, drugs, etc.) to form an injectable complex for in vivo use. The biocompatibility, magnetic properties, small size, and custom-made surface coatings also enable SPIONs to be used for delivering and monitoring of small molecules, drugs, and cells, specifically to muscle, bone, or cartilage. Because SPIONs consist of cores made of iron oxides, targeting of SPIONs to a specific muscle, bone, or joint in the body can be enhanced with the help of applied gradient magnetic fields. Moreover, MRI has a high sensitivity to SPIONs and can be used for noninvasive determination of successful delivery and monitoring distribution in vivo. Gaps remain in understanding how the physical and chemical properties of nanomaterials affect biological systems. Nonetheless, SPIONs hold great promise for regenerative medicine, and progress is being made rapidly toward clinical applications in orthopedic medicine.
Keywords: : injury, muscle damage, nanoparticles, MRI, bone repair
Introduction
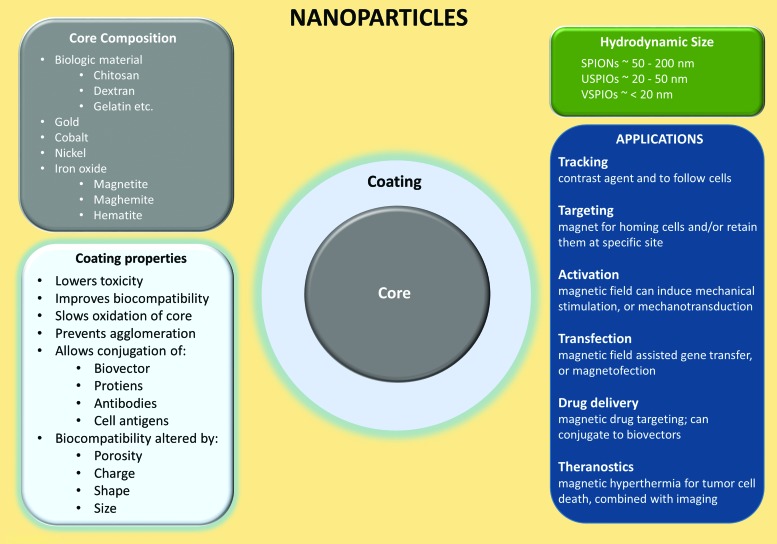
The integration of engineered nanoparticles in medicine as therapeutic products or as diagnostic tools is a rapidly growing field, with potential uses in regenerative medicine. Nanoparticles can have different chemical compositions, such as gold, iron oxide, cadmium selenide, nickel, and carbon (Fig. 1). Superparamagnetic iron oxide nanoparticles (SPIOs, or SPIONs) are commonly used engineered biocompatible nanoparticles, and are FDA-approved as contrast agents,1–3 iron replacement therapies,4 and tumor therapies using local tissue hyperthermia.5 There are many preclinical studies examining possible additional uses for SPIONs in medicine, such as delivery and tracking of induced pluripotent stem cells to the heart,6 driving macrophages and natural killer cells toward malignant tumors,7,8 improving gene therapy,9 steering stem cells to the liver,10 imaging the brain,11 and so on. The scope of this review will focus on the emerging role of SPIONs as therapeutics, diagnostics, and cellular tracking in muscle, bone, and cartilage.
FIG. 1.
Simplified schematic of nanoparticles. SPIONs are nanoparticles with an iron oxide core (maghemite, magnetite, or hematite core) and are categorized by their hydrodynamic size. Transitional metal oxides (copper, cobalt, nickel, and manganese) mixed with iron oxide also exhibit superparamagnetic properties and are considered members of the SPION family. SPIONs are one of the most employed contrast agents for the labeling of cells, and serve a variety of applications such as imaging, drug delivery, magnetic hyperthermia, and others. Bare SPIONs are cytotoxic, tend to aggregate/agglomerate, and undergo further oxidation, which makes an appropriate coating crucial. Various materials are used for coating, but most SPIONs intended for medical applications are coated with biocompatible derivatives of dextran. SPION, superparamagnetic iron oxide nanoparticle. Color images available online at www.liebertpub.com/teb
Nanoparticle Structure
Core
Ferromagnetic (i.e., iron) and ferrimagnetic (i.e., iron oxide) materials exhibit high magnetization with a low applied magnetic field and have a remnant magnetization with the elimination of the applied magnetic field.12 Small ferro- and ferrimagnetic materials (<20 nm diameter) exhibit superparamagnetism, in which the nanoparticles saturate with relatively high magnetization with a low applied magnetic field, but have no net magnetization with the removal of an applied magnetic field. While metals such as nickel and cobalt also exhibit superparamagnetism with small diameters, they are highly toxic.13 Iron oxide (usually magnetite and its oxidized forms, maghemite or hematite) is preferred for biological application because it is a naturally occurring metal in humans (e.g., ferritin in myoglobin and hemoglobin), allowing preexisting metabolic pathways to process the remaining iron from nanoparticles.
Proteins such as deoxymyoglobin and deoxyhemoglobin are paramagnetic and have some magnetization with a low applied magnetic field, although the magnetization is not at the high saturation level of superparamagnetic materials (∼50–60 emu gFe−1). They also have no remnant magnetization with the removal of the applied magnetic field. The high magnetization in presence of an applied magnetic field is crucial for SPIONs as relaxation-darkening (negative) magnetic resonance imaging (MRI) contrast agents, because this property eliminates background effects of biological paramagnetic materials (i.e., deoxymyoglobin, deoxyhemoglobin), allowing for a greatly reduced T2 signal.
SPIONs are composed of an iron oxide core, which is enveloped by a polymeric or polysaccharide coating, and categorized by their hydrodynamic diameters.12 Typically, standard SPIONs have a hydrodynamic diameter between 50 and 200 nm (Fig. 1), and can contain more than one iron oxide core per particle. Ultrasmall SPIONs (USPIOs) have a hydrodynamic diameter between 20 and 50 nm, and very small SPIONs (VSPIOs) have a hydrodynamic diameter less than 20 nm. Both USPIOs and VSPIOs behave as ferrofluids when suspended in solution (i.e., by not separating from the solution in the presence of a magnetic field). SPIONs can be targeted to a specific tissue area (e.g., delivering platelet-rich plasma [PRP] or stem cells to injured muscle, phagocytic cells to tumors, etc.) using a gradient magnetic field.14,15 Magnetic mediated hyperthermia in target regions can also be induced using an alternating magnetic field as well.16,17
Coating
The coatings of SPIONs serve many important roles, such as reducing iron oxide oxidation, preventing aggregation and agglomeration of extracellular SPIONs, increasing biocompatibility, improving targeting, increasing tracking duration, limiting nonspecific cell interactions, and improving localization by providing a chemical handle for conjugation of targeting ligands and drug molecules.18 The coating of SPIONs can change its size, shape, porosity, and surface charges, and it can be composed of polyethylene glycol, dextran, citrate, chitosan, polyethyleneimine, phosopholipids, or copolymers. SPION size (core plus coating) can affect their passage into tissues and cells, with most endothelial barriers allowing SPIONs <150 nm to pass.18 SPION size can also affect the rate of cellular uptake, with diameters between ∼30 and 150 nm having longer blood circulation duration, as they are not phagocytized as readily.19 When shape is altered such that the nanoparticle is rod-like and not spherical, the coating is anisotropic, resulting in increased in vivo blood circulation time.20 SPIONs can also be conjugated to a peptide to specifically target a ligand, or be conjugated to drug molecules, such as bisphosphonates for osteoporosis21 or Bcl2 (B cell lymphoma 2) to inhibit apoptosis and enhance bone regeneration,22 allowing for magnetic mediated drug delivery to the targeted tissue.14,23 Thus, careful consideration of coating parameters must be ensured for the specific therapeutic method.18
SPIONs as a contrast agent
At present, the most commonly used MRI contrast agents utilize paramagnetic gadolinium ions, which have a typical elimination half-life of ∼1.6 h.24 SPIONs have also been employed clinically as contrast agents for hepatic imaging.1–4 SPIONs have a unique advantage over previously developed contrast agents in that they can be tracked for a much longer duration. Human muscle progenitor cells (labeled with dextran-coated SPIONs and labeled with poly-L-lysine-coated SPIONs) can be tracked for over 4 weeks.25,26 The magnetic force experienced by a magnetic particle in a magnetic field is directly proportional to the magnetization of the particle, the gradient of the magnetic field, the volume of the particle, and the particle density.27 The magnetization of a SPION becomes saturated with a low applied magnetic field; thus the magnetic force is not dependent on the magnetization of the particle. During MRI, the magnetic force experienced by these nanoparticles is much less due to their small size (proportional to volume, in the order of ∼r3). The drag (proportional to r) experienced by SPIONs,27 together with the short time period of the applied gradient magnetic field,28 results in no movement during MRI. However, MRI systems have inherent magnetic field gradient coils that can, if desired, generate gradients for magnetic resonance targeting of SPIONs to target tissue.7
SPIONs in Musculoskeletal Therapies
SPIONs are absorbed by a variety of cells through spontaneous endocytosis or phagocytosis29 with no signs of toxicity at trackable loads of iron oxide in cells.25,30,31 Labeling of cells with these nanoparticles is simple, typically requiring no more than 1 h of incubation of nanoparticles with the cells. Some labeled cells can be frozen for storage, and replated after thawing, with viability identical to unlabeled cells.31 However, for nonphagocytic and slowly dividing cells, SPION uptake by cells requires transfection agents, which can impact cells negatively.32
Tracking and targeting platelets
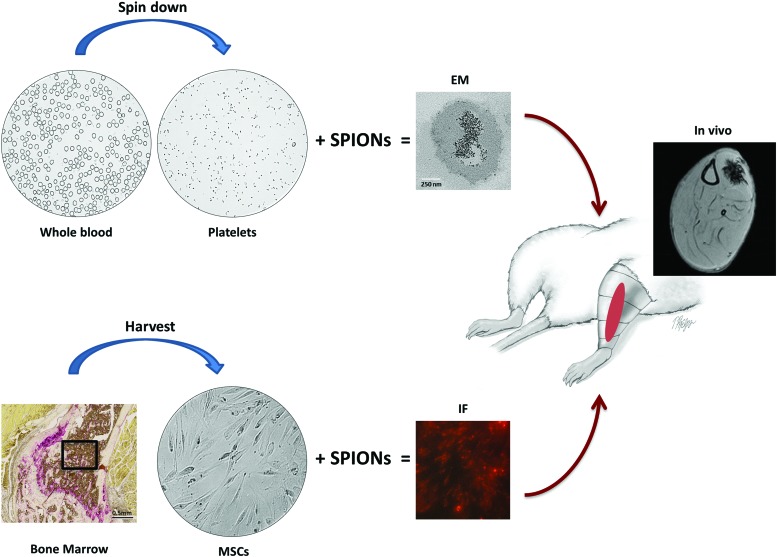
Platelets, which are small, anuclear, disk-shaped fragments are released into the vasculature from large bone marrow cells called megakaryocytes. A sizeable volume of new platelets (∼1 × 1011) is produced each day,33 and this yields a turnover of circulating platelets roughly every 10 days. Platelets contain α-granules, which are secretory vesicles that contain a range of growth factors34–36 associated with improved repair of damaged tissue, including cartilage, tendon, muscle, and bone.35,37–41 Plasma with a concentration of platelets above the concentration in whole blood is termed PRP (Fig. 2).42 PRP can be isolated using either centrifugation protocols, or a commercial system. PRP can have as high as an eightfold increase in the concentration of platelets found in whole blood.34 The resulting increase of growth factors, still present in physiological proportions to each other, is presumably an advantage compared to the use of isolated growth factors.
FIG. 2.
Isolation and in vivo magnetic cell targeting of PRP and MSCs. SPIONs are easily taken up by a variety of cells, reaching levels suitable for tracking, with labeled cells showing no signs of toxicity. They are internalized through spontaneous endocytosis or phagocytosis, and cell labeling is simple, chemically safe, and typically requires no more than 1 h of laboratory contact time. Top: Platelets were isolated from whole blood using a commercial system. Photographs show the whole blood and PRP (platelets) obtained after separation by centrifugation. Transmission electron microscopy shows example of SPIONs inside a platelet. The iron oxide core of the SPIONs is present as small dark spheres. Bottom: MSCs can be isolated from bone marrow and cultured. SPIONS can be tagged with a fluorophore, as in this example, for later detection during histological analysis. Red arrows: an internal magnet, external magnet (pictured here: red region on schematic of rat leg), or even a clinical MR scanner can be used to localize labeled platelets or MSCs at target locations and MRI can be used to track the SPION-containing platelets or MSCs in vivo (MRI shows rat leg with SPION-labeled cells targeted to tibialis anterior muscle). EM, electron microscopy; IF, immunofluorescent microscopy; MRI, magnetic resonance imaging; MSCs, mesenchymal stem cells; PRP, platelet-rich plasma. Color images available online at www.liebertpub.com/teb
A major challenge is sustaining PRP at the tissue damage site in vivo for the platelet lifespan (∼10 days), as the leakage of PRP from the region of tissue damage will likely limit its value. Alternatively, biosynthetic scaffolds can be employed for local activation of PRP over a prolonged period of time, but this has drawbacks as well.43 From a practical and comfort standpoint, recurring injections are unfavorable and are more likely to lead to undesirable systemic effects.42,44 Furthermore, storage of platelets from a single blood draw is challenging, with potential premature activation of platelets. PRP appears to be safe, but effectiveness remains to be proven.
PRP is currently applied to many musculoskeletal disorders,35,37,39,41,45 but with questionable efficacy as described above. A novel method has been described for injecting muscle with platelets containing SPIONs, which can be imaged in vivo by MRI and in vitro by fluorescence microscopy.15 Platelets endocytose SPIONs (without linkers or binding agents) with ∼98% efficiency.46 Use of an external magnet can be employed to target and retain the location of PRP with SPIONs.15,47 If translated from a preclinical notion to successful clinical studies, this technique allows for targeting of PRP to a desired site. It could also arrest premature loss of the platelets at damaged muscle, cartilage, tendon, or bone (Fig. 2).
Homing and monitoring stem cells
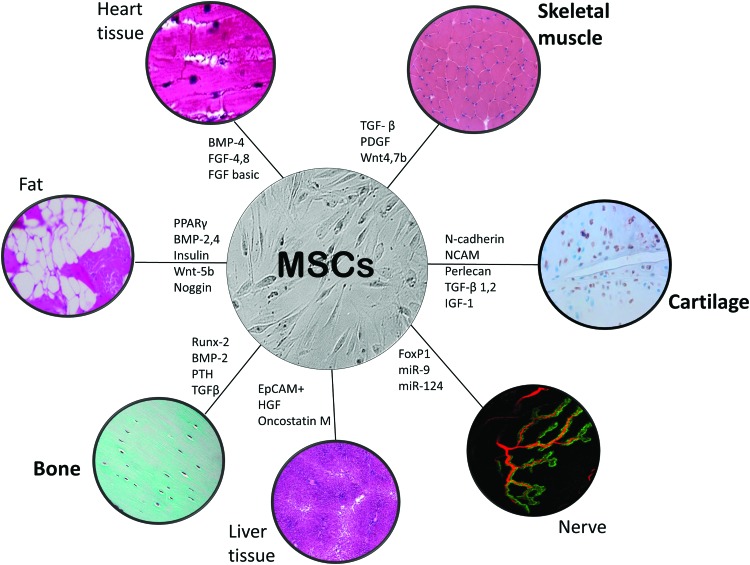
Regenerative medicine aims to repair or replace damaged human cells, tissues, or organs to restore normal function via stimulation of the body's own repair mechanisms.48 To translate stem cell therapies into clinical use, the long-term distribution, engraftment, and fate of stem cells must be monitored using a reliable and noninvasive tracking method. Mesenchymal stem cells (MSCs) are a cell population of undifferentiated cells isolated from adult tissue (Fig. 2). With the application of specific growth factors or bioactive molecules in vitro, they have the capacity to differentiate into mesodermal lineages, such as bone, cartilage, fat, muscle, and other tissues (Fig. 3).49,50 MSCs have the ability to respond to the local environment in vivo and have been used clinically in several fields to repair dysfunctional tissue.50–54 Although MSCs can be forced to differentiate into various cell types in vitro,55 when used in vivo, exogenous MSCs most likely do not directly incorporate, differentiate, and repair tissues.56 Instead, it is now evident their predominant mode of action is indirect via the secretion of “trophic” factors into the tissue microenvironment that permit the host tissue to regenerate and repair.57
FIG. 3.
Using various signaling conditions, MSCs can differentiate into different types of cells in vitro, or support endogenous cells in vivo. MSCs derived from bone marrow and can be isolated from most adults with the potential of autologous transplantation, not requiring immunosuppressive agents. They are easy to isolate, expand, and can be differentiated with various growth factors (transcription factors and signaling molecules participating in regulation of MSC differentiation; examples of molecules and factors participating in regulation of MSC differentiation are given, but by no means a complete list). MSCs can support tissue repair and regeneration either directly (direct differentiation) or indirectly (trophic factor secretion permitting endogenous progenitor cells), but with in vivo delivery the evidence points to the latter. MSCs can be successfully labeled with SPIONs, which can be conjugated with a chemical handle for differentiation. SPION-labeled MSCs can also be localized to target tissues with the use of a gradient magnetic field. Color images available online at www.liebertpub.com/teb
Transplantation of exogenous MSCs at the site of skeletal muscle injury can enhance regeneration58–60 and accelerate skeletal muscle repair.59,61 However, effective retention of transplanted MSCs following injection of MSCs into the area of injury has not been demonstrated. Articular cartilage has very little regeneration and healing (scar formation) is often inadequate, which could be augmented by the use of locally applied PRP39,40,43 or transplanted MSCs.51,62 Tissue-engineering has made great progress toward transplantation of ex vivo tissue-engineered cartilage growth, but implementing such methods involves multiple surgeries to harvest cartilage cells and then implant the newly grown autologous graft into the defect. Bone has regenerative capacity, but there are still conditions where MSC implantation could be useful, such as non-unions, bone grafts, bone disease, and potentially with osteoporosis.63–66 While, already there are methods to surgically address traumatic and degenerative conditions in bone and cartilage, the invasive nature of surgery, implant failure, immune rejection, infection, donor site morbidity, and others limit successful regeneration of these tissues. Noninvasive magnetic targeting of MSCs offers the possibility of regeneration without many of these issues, and the potential to track delivered cells over time.
Magnetic cell targeting is the use of a magnetic gradient with the goal of accumulating transplanted SPION-containing cells to a specific site (Fig. 2). Magnetic cell targeting with use of an external magnet provides a noninvasive means of enhancing tissue regeneration and has now been described for both platelets15 and MSCs67 (and a series of nonmusculoskeletal conditions).68 In preclinical studies, magnetic cell targeting has been employed to accumulate MSCs in bone and cartilage tissue,69–71 or targeting growth-promoting factors to such tissues.22 A localized magnetic field gradient could be achieved in deeper tissues using fixed internal magnets,72,73 but this necessitates invasive surgical implantation. Recent reports indicate that clinical MRI scanners can not only track the location of magnetically labeled cells, but also guide them into tissues that are inaccessible using an external magnet.7
The iron-positive signal in MRI can persist for as long as 2 months, however, this long lasting signal intensity can be the result of macrophages that have consumed the SPION bound to dead cells6 or generated by extracellular, instead of intracellular, iron particles.74 Thus, monitoring of platelet/cell viability, which is thought to correlate with the strength of the SPION signal, can present some challenges. Another major obstacle surrounding the SPION-labeling strategy is leakage of SPIONs into adjacent cells75 and, in the case of stem cells, the potential for dilution after cell division.76,77 Thus, one of the current drawbacks associated with SPION is its inability to distinguish between viable and nonviable cells, and long-term monitoring may provide an overestimation of cell survival and a false positive signal.25
SPIONs in muscular dystrophy/gene therapy
There are now a series of studies developing applications for nanoparticles in musculoskeletal tissues,21,78–86 but their use is not limited to orthopedic acute conditions. There are several preclinical studies describing the use of nanoparticles for diseases, for example, muscular dystrophy.87–89 Muscular dystrophies are a heterogeneous group of genetic disorders with progressive skeletal muscle weakness and degeneration. Duchenne muscular dystrophy (DMD), the most common form of muscular dystrophy, an X-linked disorder, was first described a century ago90 and is caused by the lack of dystrophin at the membrane of muscle fibers. Approximately 1 in 3500 newborn males worldwide are affected with DMD91–93 and patients develop progressive wasting of muscles and ultimately death, usually occurring by the ages of 20–30 due to cardiac or respiratory decline.94 Muscle regeneration, typically occurring after damage in healthy skeletal muscle, is lost in patients with DMD.95–97 Thus, magnetic cell targeting of MSCs could have potential use in treating muscular dystrophies. However, the limitation of SPIONs in long-term monitoring due to dilution with cell division, leakage to adjacent cells, and macrophage uptake, pose a challenge in successful use of SPIONs to track MSCs survival in DMD patients.
In DMD and in mdx mice, the murine homolog of DMD (mdx also lacks dystrophin), an ideal treatment would be to restore dystrophin. To date, the efforts to treat dystrophies have focused on gene therapy, however, delivery of such a large gene, and to all muscles throughout body, presents challenges. The dystrophin gene is 2.4 Mb in size and is not easily inserted into available vectors. Dystrophin can retain a large part of its function even when missing much of its middle region, and so use of “mini-dystrophin,” containing the N-terminal and C-terminal sequences responsible for binding, has shown promising results.98–101 Other therapeutic options include nonviral carriers (e.g., polymers) to deliver the dystrophin cDNA to muscle.102 A potential therapy could involve stem cell therapy. MSCs injected intravenously in the mdx mouse can move into muscle, differentiate, and result in partial, although transient, restoration of dystrophin.103,104 The potential of MSCs as an antiapoptotic agent105 and inhibitor of inflammation106 is enticing, yet even though animal studies yield successful outcomes, clinical trials have failed to yield significant benefits for patients with DMD, and the problem of delivery efficiency remains a challenge.
SPION containing MSCs appear to differentiate and mature into muscle cells, and studies in mdx mice show the ability to track SPION-labeled MSCs in muscle noninvasively with MRI.89 SPION-labeled mesangioblasts and magnetodendrimers-labeled MSCs also exhibit normal differentiation and growth and have been tracked after implantation in mdx mice.87–89 Such studies hold great promise for imaging and tracking stem cells in DMD.
Magnetofection is the term given to strategically introducing DNA into cells using coated magnetic nanoparticles, coupled with the influence of an external magnetic field.107–109 This magnetic field-assisted gene transfer is especially useful in cells that are difficult to transfect and has been used to efficiently overcome transduction resistance in skeletal muscle cells.9 There are some obvious biological barriers in terms of delivering nucleic acids into cells, such as membranes that surround the nucleus, cell vesicles, and the cell itself. Methods to overcome such barriers have been tried, such as cell bombardment methods (the “gene gun approach”) and application of an electric field (electroporation),110 but the efficacy is variable and they can even result in cell damage.111 With magnetofection, the vector can be coupled to SPIONs and accumulated on target cells by the application of a gradient magnetic field (magnetic cell therapy, but for nucleic acids). Magnetofection has been reported to improve viral and nonviral mediated gene delivery into cells, such as muscle,9,112 and it may provide a reliable method to deliver gene therapy to cells that are resistant to transfection, such a musculoskeletal tissues.
SPION removal
Following their internalization, SPIONs are eventually metabolized by the lysosomal pathway and the iron oxide core is gradually incorporated into the body's iron store.3,113 Thereafter, it is eliminated in the same manner as endogenous iron through the feces. Coating degradation is determined by its composition. For example, dextran and its derivatives are degraded by enzymes and are eliminated by renal clearance.113 Size and coating of the SPIONs can be further tuned for faster or slower clearance time. Since iron-based agents will not cause nephrogenic systemic fibrosis in patients with compromised renal function, SPIONs are a viable option to gadolinium-based contrast agents. Macrophages/the reticuloendothelial system in the liver, spleen, and lymph nodes, absorb the iron and this pathway could potentially be utilized to image vascular lesions, tumors, and lymph nodes.114
Limitations for Clinical Use
SPIONs are reported to be highly biocompatible nanomaterials with little to no toxicity, but recent research sheds doubt on the benign nature of these nanoparticles in biology.115,116 Although high SPION uptake is desirable for improving imaging contrast and therapeutic delivery, high SPIONs loads can be cytotoxic.25,117 SPIONs could have a clinical role someday in musculoskeletal medicine for uses such as diagnostics, cell labeling, drug targeting, and gene delivery, but some studies point to adverse effects on cells, including mitochondria damage, oxidative stress, chromosomal and oxidative DNA damage, altered cell cycle regulation, and protein denaturation.118,119 Whether SPIONs can act as a mutagen is unclear, but there is some experimental evidence of nanoparticles having potential mutagenic interactions on human cell lines.116 Thus, SPION toxicity, although usually reported as low, has not been completely established.120 Despite the numerous SPION uses being explored, currently available information on their potential toxicity is scarce and controversial data have been reported. Hence, concerns such as toxicity, stability, and resident time still need to be addressed.
Still undetermined variables for using SPIONs include proper selection, dose, and biophysical parameters to enhance binding, specific tissue compatibility, and others. The use of magnetic cell targeting is exciting, but there are many variables that still need to be optimized, such as optimal magnetic field strength, duration of exposure, and obtaining adequate depth, which are all likely dependent on the makeup of the SPION and the type and state of the tissue being targeted. While SPIONs such as Feridex®, GastroMARK,® and Resovist® have received FDA approval as contrast agents, today only Resovist is available in a few countries.1,4 A USPIO, Feraheme™, has received regulatory approval for ameliorating iron deficiency in chronic kidney disease, and potentially could be used as an imaging agent for lymph nodes and hepatocellular carcinomas. Nanotherm® has European Union-wide regulatory approval and is awaiting FDA approval, with phase II clinical trials for use of magnetic hyperthermia for glioblastomas. Thus, many hurdles remain in transitioning the use of SPIONs into orthopedic clinical use.
Summary
There remains a lack of standardization for isolation and delivery of PRP/MSCs, and the efficacy and optimization of such therapies remain unclear, but the use of such therapies to facilitate musculoskeletal tissue healing appears quite promising. SPIONs can be manufactured from a variety of synthetic or biological materials and they effectively label platelets and cells without compromising capacity for cell proliferation or differentiation. Moreover, studies show that cells loaded with SPIONs can be injected locally or systemically and can be attracted to a target tissue by the application of a gradient magnetic field, such as with an external magnet. However, almost all of the work for such musculoskeletal magnetic cell targeting, and/or subsequent monitoring, is preclinical, involving only animal studies (Table 1). SPIONs can have other roles in musculoskeletal tissues besides regenerative medicine, such as coatings for prevention of biofilm formation121 (not discussed). To effectively use these nanomaterials in clinical applications, the physiochemical properties and the effects of these properties on physiological processes need to be fully characterized.122
Table 1.
Example Studies Using Superparamagnetic Iron Oxide Nanoparticles in Musculoskeletal Tissues
| Author | Tissue | Method | SPION | Magnetic field strength for targeting | Species | In vitro | In vivo |
|---|---|---|---|---|---|---|---|
| Pereyra et al.9 | Skeletal muscle | Magnetic field to enhance magnetic nanoparticle adenovector transduction | Magnetite core coated with polyethylenimine and ZONYL®FSA-adenovrial vector ∼600–940 nm | 0.430 T at surface | Mouse | X | X |
| Talaie et al.15 | Skeletal muscle | Platelet-rich plasma labeled with SPIONs injected into tibialis anterior muscle, and targeted using external magnets | Molday ION, carboxyl terminated, ∼30 nm | 6 external magnets (0.15 T each) in a Halbach array | Rat | X | |
| Azzabi et al.25 | Skeletal muscle | Human muscle precursor cells labeled with increasing concentrations of SPIONs to analyze cell viability, differentiation capacity. In vivo detection of transplanted cells for 4 weeks via MRI | Endorem®, dextran coated ∼120–180 nm | Not applicable | Mouse | X | X |
| Elmi et al.79 | Skeletal muscle | Anal sphincterotomy followed by injection of muscle progenitor cells labeled with USPIOs. In vivo MRI, electromyography, manometry followed by histological analysis. Viability and differentiation analyzed in vitro | Nanomag®-D-spio dextran coated USPIO (size not given) | Not Applicable | Rabbit | X | X |
| Pacak et al.78 | Skeletal/cardiac muscle | Primary skeletal myoblasts labeled with SPIONs with PLL and embedded in fibrin sealant imaged in vitro and in vivo following implantation in heart. Compared MRI/uCT | Feridex IV®, (Ferumoxide), dextran coated with PLL ∼120–180 nm | Not applicable | Rat | X | X |
| Oshima et al.67 | Skeletal muscle | hMSC labeled with SPIONs transplanted following laceration of tibiailis anterior muscle. Test duration and strength of external magnet field needed | Resovist®, carboxydextrane coated ∼45–60 nm | 0.6–3 T for 1–60 min | Rat | X | |
| Libani et al.80 | Skeletal muscle | Comparison of labeling of human skeletal muscle cells with different transfection methods. Labeling of human skeletal muscle cells (with SPIONs and lentiviral vector infection) injected in mice and tracked via MRI/bioluminesce imaging | Endorem with PLL, polybrene, or protamine sulfate transfection agents | Not applicable | Mouse | X | X |
| Odintsov et al.88 | Skeletal/cardiac muscle | Mesangioblasts labeled with SPIONs, analyzed in vitro for growth and differentiation. Injected locally into mdx mice in both gastrocnemius muscle, heart wall, and systemically into left ventricle, and tracked via MRI | Feridex IV | Not applicable | Mouse | X | X |
| Winkler et al.59 | Skeletal muscle | Labeled MSCs with VSPIO analyzed for vitality and proliferation. MSCs transplanted into soleus following crush injury and tracked via MRI, compared to histology | VSPIO, Ferropharm C-200, core diameter 5 nm, citrate coating 2 nm diameter | Not applicable | Rat | X | X |
| Cahill et al.89 | Skeletal muscle | MC13 labeled with SPIONS with PLL transplanted into mdx with labeling, and differentiation of cells monitored both in vitro and in vivo following intramuscular injection in hind limb, or arterial cell delivery | Ferumoxides, dextran coated with PLL ∼120–180 nm | Not applicable | Mouse | X | X |
| Cahill et al.26 | Skeletal/cardiac muscle | Limb musculature myoblasts labeled with ferumoxides with PLL, assessed for viability and differentiation. Injected into myocardium and tracked via MRI | Ferumoxides, dextran coated with PLL ∼120–180 nm | Not applicable | Rat | X | X |
| Walter et al.87 | Skeletal muscle | Magnetodendrimers labeled muscle-derived stem cells/C2C12 (immortalized) myoblasts, and analyzed for viability, growth, and differentiation. Labeled cells transplanted into mdx plantar flexors via intraperitoneal injections, and tracked via MRI | Magnetodendrimer (MD-100) | Not applicable | Mouse | X | X |
| Henning et al.83 | Cartilage | Labeled human MSCs with SPIONs with and without tranfection agents. MRI tracking of viable and nonviable MSCs in scaffold labeled with SPIONs using in vitro gelatin (determination of incubation time of 24 h), ex vivo porcine knee (determination of incubation of MSCs with only SPIONs without transfection agents with signal-to-noise ratio analysis) and in vivo rat knee | Endorem labeling with and without protamine or lipofectin transfection | Not applicable | In vitro: gelatin, ex vivo: porcine knee with osteochondrol defect, in vivo: rat knee joint with osteochondral defect | X | X |
| Feng et al.82 | Cartilage | Labeled MSCs administered to human osteochondral defects in vitro (in flask). Used external magnet to target cells | N-dodecyl-polyethylenimine coated SPION ∼50–110 nm | External magnet 0.57 T | Ex vivo: human osteochondral defects | X | |
| Nedopil et al.81 | Cartilage | Viable and nonviable hMSCS labeled/unlabeled with SPIONs implanted in ex vivo porcine knee osteochondral defects and imaged via MRI to measure assessment of implants by differentiation viable and nonviable MSCs | Feridex IV, endorem | Not applicable | Ex vivo porcine knee osteochondral defect implanted with hMSCs | X | |
| Kobayashi et al.69 | Cartilage | Labeled MSCs into patella defect in rabbit, swine with external magnet targeting cells. Rabbit patellae viewed macroscopically and histologically, pig patellae viewed arthroscopically | Ferumoxides, dextran coated with PLL ∼120–180 nm | 0.6 T, 4 h | Rabbit/swine | X | |
| Kobayashi et al.71 | Cartilage | Labeled MSCs administered to human osteochondral defects in vitro (in flask). Used external magnet to target cells | Felidex®, (Ferumoxides), dextran coated with PLL ∼120–180 nm | 0.4 or 0.6 T, 2–4 h | Ex vivo: human osteochondral defect | X | |
| Brett et al.22 | Bone | Scaffold integrated with SPIONs to upregulate B cell lymphoma 2 expression (in implanted human adipose-derived stromal cells for bone regeneration) | Iron oxide nanoparticles coated with polyethyleneimine+DNA+poly-β-amino ester ∼300 nm | 1.2 T external magnet | Mouse | X | X |
| Jiang et al.85 | Bone | Rat bone-derived MSCs loaded with increasing concentrations of iron oxide nanoparticles-loaded BSA placed with magnetic field enhances osteogenic differentiation (increased mRNA, protein levels of collagen type I, osteocalcin, increased calcium deposition, alkaline phosphatase activity) | Sodium oleate modified iron oxide nanoparticles-loaded BSA ∼190–210 nm | Static magnetic field 1 T | Rat bone-derived MSCs | X | |
| Lee et al.21 | Bone | Development of bisphosphonate-loaded SPION with radio frequency-induced thermogenic properties for further reduction of osteoclast viability (in vitro only) and delivery of bisphosphonate and tracked via MRI | Bisphosphonate-conjugated dextran-coated iron oxide nanoparticle (∼20 nm) | Not applicable | In vitro: mouse osteoblasts & macrophages (osteoclast precursors); in vivo: rat | X | X |
| Wang et al.86 | Bone | Gene microarray, bioinformatics analysis performed on human bone-derived MSCs labeled with SPIONs of increasing concentration to analyze molecular mechanisms of cellular responses to SPIONs in regards to osteogenic differentiation | Maghemite core coated with polyglucose-sorbitol-carboxymethyether ∼30 nm | Not applicable | Human bone-derived MSCs | X | |
| Oshima et al.70 | Bone | Interconnected porous calcium hydroxyapatite ceramic to bridge a rabbit ulnar defect. SPION-labeled MSCS injected 2 weeks postdefect and external magnetic devices used to attract cells into ceramic | Feridex IV | Maximum magnetic field: 0.6 T, 1 h | Rabbit | X | |
| Sugioka et al.84 | Bone | Cultured and labeled rat MSCs with osteogenic differentiation media; examined “complexes” with and without external magnet | CD44 antibody-immobilized on magnetic beads (Ferri Sphere 100C®) | 0.43 T | Rat MSCs | X |
Representative publications using SPIONs in muscle, cartilage, and bone (superior number corresponds to reference number; ‘X’ indicates when performed in vitro or in vivo, or both). Note that, although SPIONs have been used clinically as a hepatic contrast agent, studies in musculoskeletal applications are still in the preclinical stages.
BSA, bovine serum albumin; hMSCs, human mesenchymal stem cells; PLL, poly-L-lysine; MRI, magnetic resonance imaging; SPION, superparamagnetic iron oxide nanoparticle; uCT, micro-computed tomography scan.
Acknowledgments
This work was supported by grants from the National Institutes of Health, including training grant T32 AR-007592 (S.R.I.) and research grant R21-AR067872 (R.M.L.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Wang Y.X. Current status of superparamagnetic iron oxide contrast agents for liver magnetic resonance imaging. World J Gastroenterol 21, 13400, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashir M.R., Bhatti L., Marin D., and Nelson R.C. Emerging applications for ferumoxytol as a contrast agent in MRI. J Magn Reson Imaging 41, 884, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Reimer P., and Balzer T. Ferucarbotran (Resovist): a new clinically approved RES-specific contrast agent for contrast-enhanced MRI of the liver: properties, clinical development, and applications. Eur Radiol 13, 1266, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bobo D., Robinson K.J., Islam J., Thurecht K.J., and Corrie S.R. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm Res 33, 2373, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Maier-Hauff K., et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol 103, 317, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoso M.R., and Yang P.C. Magnetic nanoparticles for targeting and imaging of stem cells in myocardial infarction. Stem Cells Int 2016, 4198790, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthana M., et al. Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat Commun 6, 8009, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakashima Y., Deie M., Yanada S., Sharman P., and Ochi M. Magnetically labeled human natural killer cells, accumulated in vitro by an external magnetic force, are effective against HOS osteosarcoma cells. Int J Oncol 27, 965, 2005 [PubMed] [Google Scholar]
- 9.Pereyra A.S., et al. Magnetofection enhances adenoviral vector-based gene delivery in skeletal muscle cells. J Nanomed Nanotechnol 7, pii: , 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbab A.S., et al. In vivo trafficking and targeted delivery of magnetically labeled stem cells. Hum Gene Ther 15, 351, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Fu T., Kong Q., Sheng H., and Gao L. Value of functionalized superparamagnetic iron oxide nanoparticles in the diagnosis and treatment of acute temporal lobe epilepsy on MRI. Neural Plast 2016, 2412958, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demas V., and Lowery T.J. Magnetic resonance for in vitro medical diagnostics: superparamagnetic nanoparticle-based magnetic relaxation switches. New J Phys 5, 182ra54, 2011 [Google Scholar]
- 13.Mahmoudi M., Sant S., Wang B., Laurent S., and Sen T. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev 63, 24, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Wilson M.W., et al. Hepatocellular carcinoma: regional therapy with a magnetic targeted carrier bound to doxorubicin in a dual MR imaging/conventional angiography suite—initial experience with four patients. Radiology 230, 287, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Talaie T., et al. Site-specific targeting of platelet-rich plasma via superparamagnetic nanoparticles. Orthop J Sports Med 3, 2015; DOI: 10.1177/2325967114566185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moroz P., Jones S.K., and Gray B.N. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia 18, 267, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z.Q., and Song S.C. Thermosensitive/superparamagnetic iron oxide nanoparticle-loaded nanocapsule hydrogels for multiple cancer hyperthermia. Biomaterials 106, 13, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Veiseh O., Gunn J.W., and Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev 62, 284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias A., and Tsourkas A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. Hematol Am Soc Hematol Educ Program 720, 2009; DOI: 10.1182/asheducation-2009.1.720 [DOI] [PubMed] [Google Scholar]
- 20.Geng Y., et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol 2, 249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee M.S., et al. Synthesis of composite magnetic nanoparticles Fe3O4 with alendronate for osteoporosis treatment. Int J Nanomedicine 11, 4583, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brett E., et al. Magnetic nanoparticle-based upregulation of B-cell lymphoma 2 enhances bone regeneration. Stem Cells Transl Med 2016. [Epub ahead of print]; DOI: 10.5966/sctm.2016-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C., et al. In vivo MRI detection of gliomas by chlorotoxin-conjugated superparamagnetic nanoprobes. Small 4, 372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinstein J.S., et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 30, 15, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Azzabi F., et al. Viability, differentiation capacity, and detectability of super-paramagnetic iron oxide-labeled muscle precursor cells for magnetic-resonance imaging. Tissue Eng Part C Methods 21, 182, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cahill K.S., Germain S., Byrne B.J., and Walter G.A. Non-invasive analysis of myoblast transplants in rodent cardiac muscle. Int J Cardiovasc Imaging 20, 593, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Rogers H.B., Anani T., Choi Y.S., Beyers R.J., and David A.E. Exploiting size-dependent drag and magnetic forces for size-specific separation of magnetic nanoparticles. Int J Mol Sci 16, 20001, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chanu A., Felfoul O., Beaudoin G., and Martel S. Adapting the clinical MRI software environment for real-time navigation of an endovascular untethered ferromagnetic bead for future endovascular interventions. Magn Reson Med 59, 1287, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Kolosnjaj-Tabi J., Wilhelm C., Clement O., and Gazeau F. Cell labeling with magnetic nanoparticles: opportunity for magnetic cell imaging and cell manipulation. J Nanobiotechnology 11 Suppl 1, S7, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., and Zhang J. Surface modification of monodisperse magnetite nanoparticles for improved intracellular uptake to breast cancer cells. J Colloid Interface Sci 283, 352, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Shen W.B., et al. Human neural progenitor cells retain viability, phenotype, proliferation, and lineage differentiation when labeled with a novel iron oxide nanoparticle, Molday ION Rhodamine B. Int J Nanomedicine 8, 4593, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arbab A.S., et al. Intracytoplasmic tagging of cells with ferumoxides and transfection agent for cellular magnetic resonance imaging after cell transplantation: methods and techniques. Transplantation 76, 1123, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Geddis A.E., and Kaushansky K. Immunology. The root of platelet production. Science 317, 1689, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Creaney L., and Hamilton B. Growth factor delivery methods in the management of sports injuries: the state of play. Br J Sports Med 42, 314, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Schnabel L.V., et al. Platelet rich plasma (PRP) enhances anabolic gene expression patterns in flexor digitorum superficialis tendons. J Orthop Res 25, 230, 2007 [DOI] [PubMed] [Google Scholar]
- 36.El-Sharkawy H., et al. Platelet-rich plasma: growth factors and pro- and anti-inflammatory properties. J Periodontol 78, 661, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Hammond J.W., Hinton R.Y., Curl L.A., Muriel J.M., and Lovering R.M. Use of autologous platelet-rich plasma to treat muscle strain injuries. Am J Sports Med 37, 1135, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., and Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 37, 2259, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Sakata R., and Reddi A.H. Platelet-rich plasma modulates actions on articular cartilage lubrication and regeneration. Tissue Eng Part B Rev 22, 408, 2016 [DOI] [PubMed] [Google Scholar]
- 40.Sakata R., et al. Stimulation of the superficial zone protein and lubrication in the articular cartilage by human platelet-rich plasma. Am J Sports Med 43, 1467, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonello G.D., et al. Evaluation of the effects of the use of platelet-rich plasma (PRP) on alveolar bone repair following extraction of impacted third molars: prospective study. J Craniomaxillofac Surg 41, e70, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Schippinger G., Fankhauser F., Oettl K., Spirk S., and Hofmann P. Does single intramuscular application of autologous conditioned plasma influence systemic circulating growth factors? J Sports Sci Med 11, 551, 2012 [PMC free article] [PubMed] [Google Scholar]
- 43.Getgood A., Henson F., Brooks R., Fortier L.A., and Rushton N. Platelet-rich plasma activation in combination with biphasic osteochondral scaffolds-conditions for maximal growth factor production. Knee Surg Sports Traumatol Arthrosc 19, 1942, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Wasterlain A.S., Braun H.J., Harris A.H., Kim H.J., and Dragoo J.L. The systemic effects of platelet-rich plasma injection. Am J Sports Med 41, 186, 2013 [DOI] [PubMed] [Google Scholar]
- 45.Halpern B.C., Chaudhury S., and Rodeo S.A. The role of platelet-rich plasma in inducing musculoskeletal tissue healing. HSS J 8, 137, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aurich K., et al. Development of a method for magnetic labeling of platelets. Nanomedicine 8, 537, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Sarwar A., Nemirovski A., and Shapiro B. Optimal Halbach permanent magnet designs for maximally pulling and pushing nanoparticles. J Magn Magn Mater 324, 742, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gonzalez-Bejar M., Frances-Soriano L., and Perez-Prieto J. Upconversion nanoparticles for bioimaging and regenerative medicine. Front Bioeng Biotechnol 4, 47, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pittenger M.F., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y., et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41, 2002 [DOI] [PubMed] [Google Scholar]
- 51.Agung M., et al. Mobilization of bone marrow-derived mesenchymal stem cells into the injured tissues after intraarticular injection and their contribution to tissue regeneration. Knee Surg Sports Traumatol Arthrosc 14, 1307, 2006 [DOI] [PubMed] [Google Scholar]
- 52.Surder D., et al. Cell-based therapy for myocardial repair in patients with acute myocardial infarction: rationale and study design of the SWiss Multicenter Intracoronary Stem cells Study in Acute Myocardial Infarction (SWISS-AMI). Am Heart J 160, 58, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Kim B.G., Hwang D.H., Lee S.I., Kim E.J., and Kim S.U. Stem cell-based cell therapy for spinal cord injury. Cell Transplant 16, 355, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Garbayo E., et al. Neuroprotective properties of marrow-isolated adult multilineage-inducible cells in rat hippocampus following global cerebral ischemia are enhanced when complexed to biomimetic microcarriers. J Neurochem 119, 972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caplan A.I. Mesenchymal stem cells. J Orthop Res 9, 641, 1991 [DOI] [PubMed] [Google Scholar]
- 56.Murphy M.B., Moncivais K., and Caplan A.I. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45, e54, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofer H.R., and Tuan R.S. Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 7, 131, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yokoya S., et al. Rotator cuff regeneration using a bioabsorbable material with bone marrow-derived mesenchymal stem cells in a rabbit model. Am J Sports Med 40, 1259, 2012 [DOI] [PubMed] [Google Scholar]
- 59.Winkler T., et al. Immediate and delayed transplantation of mesenchymal stem cells improve muscle force after skeletal muscle injury in rats. J Tissue Eng Regen Med 6 Suppl 3, s60, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Winkler T., et al. Dose-response relationship of mesenchymal stem cell transplantation and functional regeneration after severe skeletal muscle injury in rats. Tissue Eng Part A 15, 487, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Winkler T., et al. In vivo visualization of locally transplanted mesenchymal stem cells in the severely injured muscle in rats. Tissue Eng Part A 14, 1149, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi T., Adachi N., Deie M., and Ochi M. [Regeneration of articular cartilage]. Nihon Rinsho 66, 966, 2008 [PubMed] [Google Scholar]
- 63.Qin Y., Guan J., and Zhang C. Mesenchymal stem cells: mechanisms and role in bone regeneration. Postgrad Med J 90, 643, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Labibzadeh N., et al. Mesenchymal stromal cells implantation in combination with platelet lysate product is safe for reconstruction of human long bone nonunion. Cell J 18, 302, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watanabe Y., et al. Stem cell therapy: is there a future for reconstruction of large bone defects? Injury 47 Suppl 1, S47, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Sui B., et al. Allogeneic mesenchymal stem cell therapy promotes osteoblastogenesis and prevents glucocorticoid-induced osteoporosis. Stem Cells Transl Med 5, 1238, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oshima S., Kamei N., Nakasa T., Yasunaga Y., and Ochi M. Enhancement of muscle repair using human mesenchymal stem cells with a magnetic targeting system in a subchronic muscle injury model. J Orthop Sci 19, 478, 2014 [DOI] [PubMed] [Google Scholar]
- 68.Wimpenny I., Markides H., and El Haj A.J. Orthopaedic applications of nanoparticle-based stem cell therapies. Stem Cell Res Ther 3, 13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kobayashi T., et al. A novel cell delivery system using magnetically labeled mesenchymal stem cells and an external magnetic device for clinical cartilage repair. Arthroscopy 24, 69, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Oshima S., et al. Enhancement of bone formation in an experimental bony defect using ferumoxide-labelled mesenchymal stromal cells and a magnetic targeting system. J Bone Joint Surg Br 92, 1606, 2010 [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T., et al. Augmentation of degenerated human cartilage in vitro using magnetically labeled mesenchymal stem cells and an external magnetic device. Arthroscopy 25, 1435, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Polyak B., et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci U S A 105, 698, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yellen B.B., Hovorka O., and Friedman G. Arranging matter by magnetic nanoparticle assemblers. Proc Natl Acad Sci U S A 102, 8860, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Z., et al. Magnetic resonance hypointensive signal primarily originates from extracellular iron particles in the long-term tracking of mesenchymal stem cells transplanted in the infarcted myocardium. Int J Nanomedicine 10, 1679, 2015. 25767388 [Google Scholar]
- 75.Sakhtianchi R., et al. Exocytosis of nanoparticles from cells: role in cellular retention and toxicity. Adv Colloid Interface Sci 201–202, 18, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Amsalem Y., et al. Iron-oxide labeling and outcome of transplanted mesenchymal stem cells in the infarcted myocardium. Circulation 116, I38, 2007 [DOI] [PubMed] [Google Scholar]
- 77.Terrovitis J., et al. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation 117, 1555, 2008 [DOI] [PubMed] [Google Scholar]
- 78.Pacak C.A., et al. Superparamagnetic iron oxide nanoparticles function as a long-term, multi-modal imaging label for non-invasive tracking of implanted progenitor cells. PLoS One 9, e108695, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Elmi A., et al. Anal sphincter repair with muscle progenitor cell transplantation: serial assessment with iron oxide-enhanced MRI. AJR Am J Roentgenol 202, 619, 2014 [DOI] [PubMed] [Google Scholar]
- 80.Libani I.V., et al. Labeling protocols for in vivo tracking of human skeletal muscle cells (HSkMCs) by magnetic resonance and bioluminescence imaging. Mol Imaging Biol 14, 47, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Nedopil A., et al. MR signal characteristics of viable and apoptotic human mesenchymal stem cells in matrix-associated stem cell implants for treatment of osteoarthritis. Invest Radiol 45, 634, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng Y., et al. In vitro targeted magnetic delivery and tracking of superparamagnetic iron oxide particles labeled stem cells for articular cartilage defect repair. J Huazhong Univ Sci Technolog Med Sci 31, 204, 2011 [DOI] [PubMed] [Google Scholar]
- 83.Henning T.D., et al. Magnetic resonance imaging of ferumoxide-labeled mesenchymal stem cells in cartilage defects: in vitro and in vivo investigations. Mol Imaging 11, 197, 2012 [PMC free article] [PubMed] [Google Scholar]
- 84.Sugioka T., Ochi M., Yasunaga Y., Adachi N., and Yanada S. Accumulation of magnetically labeled rat mesenchymal stem cells using an external magnetic force, and their potential for bone regeneration. J Biomed Mater Res A 85, 597, 2008 [DOI] [PubMed] [Google Scholar]
- 85.Jiang P., et al. Fe3O4/BSA particles induce osteogenic differentiation of mesenchymal stem cells under static magnetic field. Acta Biomater 46, 141, 2016 [DOI] [PubMed] [Google Scholar]
- 86.Wang Q., et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials 86, 11, 2016 [DOI] [PubMed] [Google Scholar]
- 87.Walter G.A., et al. Noninvasive monitoring of stem cell transfer for muscle disorders. Magn Reson Med 51, 273, 2004 [DOI] [PubMed] [Google Scholar]
- 88.Odintsov B., Chun J.L., Mulligan J.A., and Berry S.E. 14.1 T whole body MRI for detection of mesoangioblast stem cells in a murine model of Duchenne muscular dystrophy. Magn Reson Med 66, 1704, 2011 [DOI] [PubMed] [Google Scholar]
- 89.Cahill K.S., et al. Noninvasive monitoring and tracking of muscle stem cell transplants. Transplantation 78, 1626, 2004 [DOI] [PubMed] [Google Scholar]
- 90.Duchenne G. I'Electrisation Localisee at de son Application a la Pathologie at a la Therapeutique. Paris: Bailliere et Fils, 1861 [Google Scholar]
- 91.Biggar W.D., Gingras M., Fehlings D.L., Harris V.A., and Steele C.A. Deflazacort treatment of Duchenne muscular dystrophy. J Pediatr 138, 45, 2001 [DOI] [PubMed] [Google Scholar]
- 92.Metules T. Duchenne muscular dystrophy. RN 65, 39, 47, 2002 [PubMed] [Google Scholar]
- 93.Wagner K.R. Genetic diseases of muscle. Neurol Clin 20, 645, 2002 [DOI] [PubMed] [Google Scholar]
- 94.Emery A.E. The muscular dystrophies. BMJ 317, 991, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Blau H.M., Webster C., and Pavlath G.K. Defective myoblasts identified in Duchenne muscular dystrophy. Proc Natl Acad Sci U S A 80, 4856, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Webster C., and Blau H.M. Accelerated age-related decline in replicative life-span of Duchenne muscular dystrophy myoblasts: implications for cell and gene therapy. Somat Cell Mol Genet 16, 557, 1990 [DOI] [PubMed] [Google Scholar]
- 97.Bockhold K.J., Rosenblatt J.D., and Partridge T.A. Aging normal and dystrophic mouse muscle: analysis of myogenicity in cultures of living single fibers. Muscle Nerve 21, 173, 1998 [DOI] [PubMed] [Google Scholar]
- 98.Harper S.Q., et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8, 253, 2002 [DOI] [PubMed] [Google Scholar]
- 99.Crawford G.E., et al. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J Cell Biol 150, 1399, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.DelloRusso C., et al. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc Natl Acad Sci U S A 99, 12979, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jung D., Yang B., Meyer J., Chamberlain J.S., and Campbell K.P. Identification and characterization of the dystrophin anchoring site on beta-dystroglycan. J Biol Chem 270, 27305, 1995 [DOI] [PubMed] [Google Scholar]
- 102.van Deutekom J.C., and van Ommen G.J. Advances in Duchenne muscular dystrophy gene therapy. Nat Rev Genet 4, 774, 2003 [DOI] [PubMed] [Google Scholar]
- 103.Ferrari G., et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science 279, 1528, 1998 [DOI] [PubMed] [Google Scholar]
- 104.Gussoni E., et al. Dystrophin expression in the mdx mouse restored by stem cell transplantation. Nature 401, 390, 1999 [DOI] [PubMed] [Google Scholar]
- 105.Cselenyak A., Pankotai E., Horvath E.M., Kiss L., and Lacza Z. Mesenchymal stem cells rescue cardiomyoblasts from cell death in an in vitro ischemia model via direct cell-to-cell connections. BMC Cell Biol 11, 29, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ichim T.E., et al. Mesenchymal stem cells as anti-inflammatories: implications for treatment of Duchenne muscular dystrophy. Cell Immunol 260, 75, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Plank C., et al. The magnetofection method: using magnetic force to enhance gene delivery. Biol Chem 384, 737, 2003 [DOI] [PubMed] [Google Scholar]
- 108.Plank C., Scherer F., Schillinger U., Bergemann C., and Anton M. Magnetofection: enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J Liposome Res 13, 29, 2003 [DOI] [PubMed] [Google Scholar]
- 109.Scherer F., et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther 9, 102, 2002 [DOI] [PubMed] [Google Scholar]
- 110.Somiari S., et al. Theory and in vivo application of electroporative gene delivery. Mol Ther 2, 178, 2000 [DOI] [PubMed] [Google Scholar]
- 111.Roche J.A., et al. Physiological and histological changes in skeletal muscle following in vivo gene transfer by electroporation. Am J Physiol Cell Physiol 301, C1239, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou X.F., et al. Using magnetic force to enhance immune response to DNA vaccine. Small 3, 1707, 2007 [DOI] [PubMed] [Google Scholar]
- 113.Bietenbeck M., Florian A., Faber C., Sechtem U., and Yilmaz A. Remote magnetic targeting of iron oxide nanoparticles for cardiovascular diagnosis and therapeutic drug delivery: where are we now? Int J Nanomedicine 11, 3191, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Y.X. Superparamagnetic iron oxide based MRI contrast agents: current status of clinical application. Quant Imaging Med Surg 1, 35, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sadeghi L., Tanwir F., and Yousefi B. V In vitro toxicity of iron oxide nanoparticle: oxidative damages on Hep G2 cells. Exp Toxicol Pathol 67, 197, 2015 [DOI] [PubMed] [Google Scholar]
- 116.Dissanayake N.M., Current K.M., and Obare S.O. Mutagenic effects of iron oxide nanoparticles on biological cells. Int J Mol Sci 16, 23482, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valdiglesias V., et al. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J Trace Elem Med Biol 38, 53, 2016 [DOI] [PubMed] [Google Scholar]
- 118.Nel A., Xia T., Madler L., and Li N. Toxic potential of materials at the nanolevel. Science 311, 622, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Carlson C., et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B 112, 13608, 2008 [DOI] [PubMed] [Google Scholar]
- 120.Laurent S., Saei A.A., Behzadi S., Panahifar A., and Mahmoudi M. Superparamagnetic iron oxide nanoparticles for delivery of therapeutic agents: opportunities and challenges. Expert Opin Drug Deliv 11, 1449, 2014 [DOI] [PubMed] [Google Scholar]
- 121.Taylor E.N., and Webster T.J. The use of superparamagnetic nanoparticles for prosthetic biofilm prevention. Int J Nanomedicine 4, 145, 2009 [PMC free article] [PubMed] [Google Scholar]
- 122.Nel A.E., et al. Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater 8, 543, 2009 [DOI] [PubMed] [Google Scholar]