Abstract
Clinical and animal studies have indicated that propofol has potential for abuse, but the specific neurobiological mechanism underlying propofol reward is not fully understood. The purpose of this study was to investigate the role of extracellular signal-regulated kinase (ERK) signal transduction pathways in the nucleus accumbens (NAc) in propofol self-administration. We tested the expression of p-ERK in the NAc following the maintenance of propofol self-administration in rats. We also assessed the effect of administration of SCH23390, an antagonist of the D1 dopamine receptor, on the expression of p-ERK in the NAc in propofol self-administering rats, and examined the effects of intra-NAc injection of U0126, an MEK inhibitor, on propofol reinforcement in rats. The results showed that the expression of p-ERK in the NAc increased significantly in rats maintained on propofol, and pre-treatment with SCH23390 inhibited the propofol self-administration and diminished the expression of p-ERK in the NAc. Moreover, intra-NAc injection of U0126 (4 µg/side) attenuated the propofol self-administration. The data suggest that ERK signal transduction pathways coupled with D1 dopamine receptors in the NAc may be involved in the maintenance of propofol self-administration and its rewarding effects.
Keywords: Dopamine receptor, Drug reward, ERK, Anesthesiology, Nucleus accumbens
Introduction
Propofol is a short-acting intravenous anesthetic, widely used for anesthesia and sedation. Apart from its medical uses, propofol also has potential for dependence and abuse and has been reported to be abused for entertainment [1, 2]. The evidence shows that propofol induces feelings of well-being, relaxation, sexual hallucinations, and euphoria. An anesthesiologist became psychologically dependent on propofol because he initially self-administered it to relieve stress [3, 4]. Furthermore, animal studies suggest that propofol has abuse potential, as assessed using the conditioned place preference test [5, 6] and an intravenous self-administration model [7, 8]. Our previous studies have shown that dopamine (DA) receptors and γ-aminobutyric acid receptors are involved in intravenous propofol self-administration in rats [7]. Though the clinical and preclinical data indicate that propofol has psychic dependence and abuse potential, the specific neurobiological mechanism underlying propofol reward is not fully understood.
Extracellular signal-regulated kinase (ERK) has been widely implicated as the central mediator of signal transduction in neuroadaptation, morphological plasticity, behavioral performance [9], and even in mood modulation [10]. ERK is expressed in the brain [11], and is especially abundant in the mesocorticolimbic DA system. Recently, accumulating evidence has indicated that ERK signal transduction pathways in mesocorticolimbic areas are involved in drug dependence and addiction [12, 13]. Acute or repeated administration of cocaine, nicotine, and morphine enhances ERK phosphorylation in the nucleus accumbens (NAc), which is a major terminal area of the mesolimbic DA projection systems and a crucial component of the neuronal circuitry mediating reward-related behaviors. In addition, microinjections of the MEK inhibitors PD98059 or U0126 into the NAc impair the acquisition of morphine or tetrahydrocannabinol (THC) conditioned place preference in mice [14, 15]. Moreover, activation of ERK signal transduction pathways in the mesocorticolimbic area also mediates the rewarding effect of cocaine and cocaine-induced locomotor sensitization [16, 17]. Thus, here we hypothesized that ERK signal transduction pathways in the NAc may be involved in propofol self-administration.
To test this hypothesis, we assessed the expression of p-ERK in the NAc of rats with maintained propofol self-administration, and investigated whether administration of SCH23390, an antagonist of the D1 DA receptor, affected p-ERK expression in the NAc of these rats. Specifically, we also investigated the effects of micro-injection of U0126, an MEK inhibitor, into the NAc in such rats.
Materials and Methods
Animals
Forty-two adult male Sprague–Dawley rats (280–300 g, from the Experimental Animal Center of Zhejiang Province, Hangzhou, China) were housed individually in home cages in a temperature-controlled ventilated colony room with a reversed 12-h light/dark cycle (lights on at 19:00). All animals were weighed each day. Ad libitum food and water were provided in the home cage. All procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of Zhejiang Province and the Ningbo Addiction Research and Treatment Center. After completion of experiments, some rats were sacrificed immediately after pentobarbital anesthesia and their brains removed for analysis of cannula placement and Western blotting.
Reagents
Propofol (10 mg/mL; Diprivan) was from AstraZeneca (Wuxi, China). A dose of 1.7 mg/kg per infusion was chosen for the intravenous propofol self-administration based on a previous study [7]. R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390), a D1 DA receptor antagonist from Sigma Chemical Co. (St. Louis, MO), was dissolved in sterile saline and adjusted to pH 4.5 with 0.1 mol/L NaOH. An injection volume of 1 mL was then delivered to each rat.
1,4-Diamino-2,3-dicyano-1,4-bis(o-aminophenylmercapto)butadiene (U0126), an ERK1/2-selective inhibitor, was from Sigma Chemical Co. and dissolved in 20% dimethyl sulfoxide. The volume of intracerebral infusion was ~0.5 μL/side.
Surgical Procedures
Rats were surgically implanted with chronic indwelling intravenous catheters or/and a guide cannula into the NAc as described previously [7, 18]. The catheters were flushed daily with 0.3 mL saline–heparin (25 U/mL heparin). The rats were allowed to recover for at least 7 days and treated with penicillin B to prevent infection. In some experiments, guide cannulae (20-gauge, Small Parts Inc., Roanoke, VA) were implanted bilaterally into the NAc (AP: 1.5 mm, ML: 2.0 mm, and DV: –6.7 mm) based on a previous study [18], and the cannulae were attached to the skull with dental acrylic.
Microinjection Procedure
On day 15, well-trained rats received microinjections via a microinjection pump (MD-1001, Bioanalytical System Inc., West Lafayette, IN). All injections into the NAc were delivered through infusion cannulae in a volume of 0.5 μL per site as described previously [7].
Propofol Self-Administration Training
Rats were trained to self-administer propofol in an Operant Behavioral Apparatus (Ningbo Addiction Research and Treatment Center, Ningbo, China) for 14 daily 3-h training sessions. Prior to each training session, the rats were moved to the operant chamber and attached to the infusion lines through connectors. The training session was as described previously [7, 18]. Briefly, rats received an infusion of propofol (1.7 mg/kg per infusion) under a fixed ratio of 1 by triggering the active nose-poke hole when a green light inside was switched on. Each infusion was paired with a 5-s illumination of the room lights. This was followed by a 30-s time-out, during which a response had no consequences but was still recorded. Illumination of the green light in the active nose-poke hole signaled the end of the 30-s time-out period. No programmed infusions were given when rats touched the inactive nose-poke hole. The sessions were ended after 3 h or 50 propofol infusions. The rats were returned to their individual home cages shortly after each session.
Animal Treatments
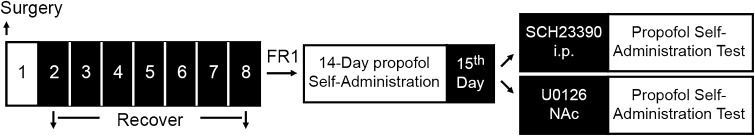
A schematic representation of the time-line of experiments is shown in Fig. 1.
Fig. 1.
Schematic of the time line of all experiments. FR1, fixed ratio = 1.
Experiment 1: Effect of systemic administration of SCH23390 on the maintenance of propofol self-administration.
Rats completed propofol self-administration in daily 3-h sessions for a total of 14 days. Twenty-four rats (6/group) were tested for the effects of SCH23390, an antagonist of the D1 DA receptor given systemically before propofol rewarding. Ten minutes prior to the session on day 15, the well-trained rats received an intraperitoneal injection of SCH23390 at 0, 10, or 30 μg/kg. And 6 naïve rats were chosen as the control group.
Experiment 2: Effect of intra-NAc injection of U0126 on the maintenance of propofol self-administration.
Eighteen rats with guide cannulae implanted bilaterally were trained to self-administer propofol for 14 days. On day 15, 18 well-trained rats (6/group) had the MEK inhibitor U0126 microinjected into the NAc at 0, 2, or 4 μg/side. Ten minutes later, the rats were moved into the operant chambers and allowed to self-administer propofol.
Western Blot Analysis
After the final test session in Experiment 1, the rats were anesthetized with pentobarbital (80 mg/kg) and decapitated. The brain was rapidly isolated and the entire NAc was dissected on ice from a 2-mm coronal slice using a brain matrix. Briefly, the NAc tissue samples were homogenized in cold lysis buffer (Beyotime, Nantong, China) plus 1:100 phenylmethylsulfonyl fluoride and phosphatase inhibitors. The proteins in the samples were extracted by centrifugation at 12,000 rpm for 30 min in 4 °C to remove debris. The protein level was measured with a BCA kit (Beyotime), and samples were loaded onto 10% sodium dodecyl sulfate (SDS) polyacrylamide gel and subjected to electrophoresis in a Bio-Rad Mini Western blotting apparatus (Pleasanton, CA) with Tris/glycine/SDS buffer at 150 V for 1.5 h. Following SDS-electrophoresis, protein was transferred from the gels onto a nitrocellulose membrane (Bio-Rad) at 300 mA for 1 h. The membrane was blocked in 5% nonfat milk in TBST for 1 h at room temperature with shaking. After blocking, the membranes were incubated with anti p-ERK1/2 antibodies (1:1000, Santa Cruz Biotechnology Inc., Santa Cruz, CA) and glyceraldehyde-3-phosphate dehydrogenase as a loading control (1:2000) in 5% milk-TBST overnight at 4 °C. Blots were washed 3 times in TBST for 30 min. Then blots were left to incubate for 2 h at room temperature in 5% dried nonfat milk in TBST and the horseradish peroxidase-conjugated secondary antibody (1:5000). The protein of interest was detected using an ECL chemiluminescence kit (Sangon Biotech, Shanghai, China) after 3 washes in TBST. The blots were then stripped and re-probed for total ERK (1:1000, Santa Cruz Biotechnology Inc.). The immunoblotting bands were analyzed by integrative densitometry using GeneSnap and GeneTools (Chemigenius Gel Documentation System, Syngene, Cambridge, UK) [19].
Histological Analysis
After the end of testing, anesthetized rats were perfused with 0.01 mol/L PBS followed by 4% paraformaldehyde. Coronal sections were cut in the coronal plane at 50 µm on a cryostat microtome (Leica CM1850; Leica, Wetzlar, Germany). The placement of each cannula tip was verified by light microscopy and mapped onto a schematic diagram of the rat brain [7].
Statistical Analysis
The numbers of active and inactive responses and infusions during self-administration were analyzed using one-way ANOVA or two-way ANOVA with repeated measures. A Newman-Keuls multiple comparison with an alpha level of 0.05 was used for post hoc comparisons.
Results
Maintained Propofol Self-Administration in Rats
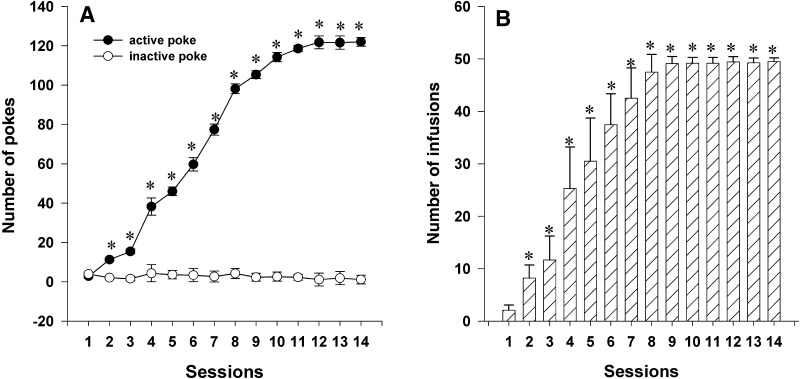
Rats developed reliable self-administration of propofol after 14 days of training under a fixed ratio of 1 (Fig. 2). Repeated measures showed an increase in the number of active responses for propofol (F(1,34) = 2509.32, P < 0.01), while it did not alter the number of inactive responses. The number of propofol infusions increased significantly after 2–3 days of training (Fig. 2B). These results showed that rats acquired and maintained the propofol self-administration.
Fig. 2.
Training rats for propofol self-administration. A Number of active (nose-pokes) and inactive responses during training (mean ± SEM; *P < 0.05 active versus inactive responses at the same time points). B Number of infusions of propofol (mean ± SEM; *P < 0.05 versus beginning of training).
D1 Receptor Antagonist SCH23390 Reduces ERK Expression in the NAc
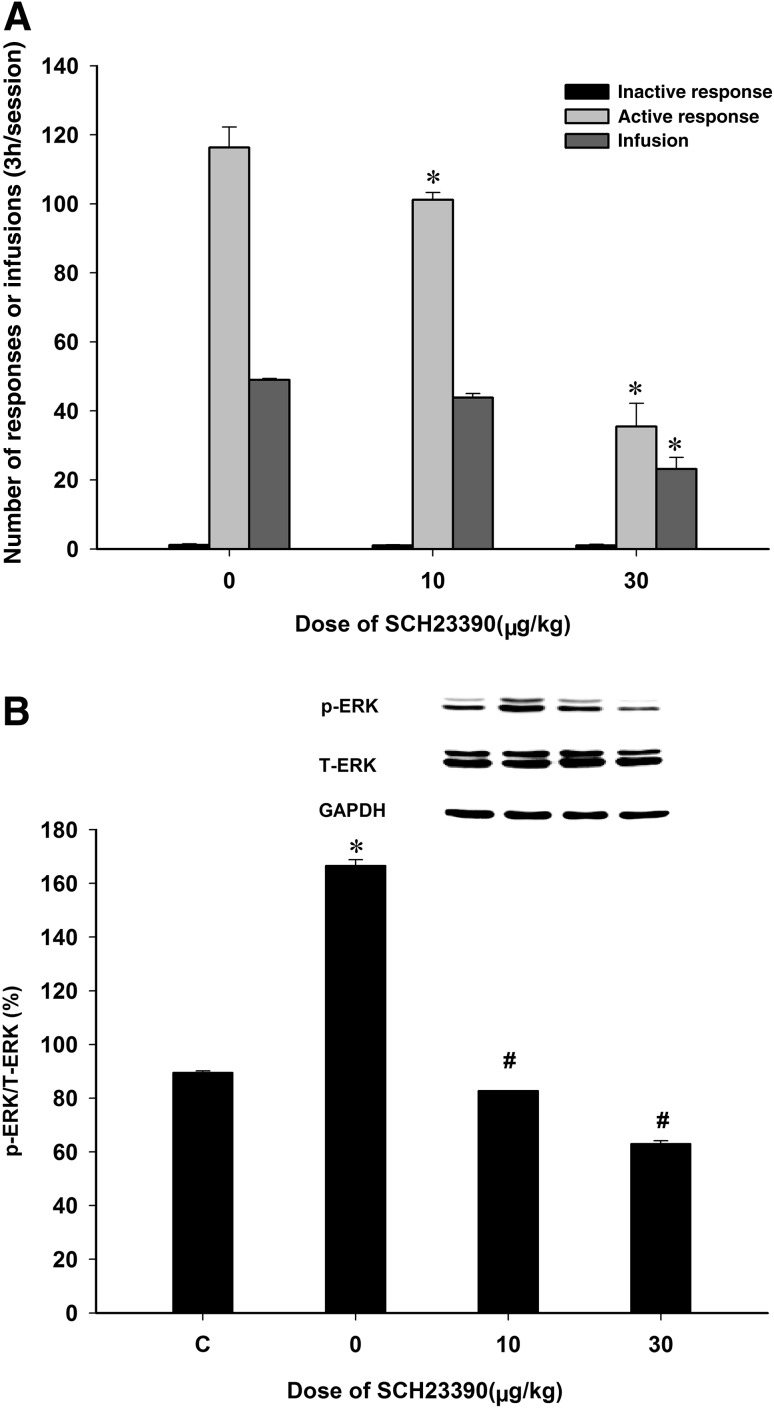
On day 15 of propofol self-administration, rats were pretreated with either saline (control) or SCH23390 at 10 or 30 μg/kg, 10 min prior to propofol self-administration. The results showed that SCH23390 dose-dependently inhibited the active responses (Fig. 3A; F(2,17) = 65.21, P < 0.01) and the self-administered infusions of propofol (Fig. 3A; F(2,17) = 43.16, P < 0.01) compared to the vehicle group. Similarly, p-ERK/ERK was higher in the propofol self-administering rats than in naïve rats (Fig. 3B, F(3,11) = 1350.21, P < 0.001). Compared to the vehicle group, the p-ERK/ERK levels in the NAc were lower in rats pretreated with either 10 or 30 µg/kg SCH23390 (Fig. 3B; both P < 0.05). Clearly, pretreatment with SCH23390 dose-dependently decreased the expression of p-ERK in the NAc, which correlated with its inhibitory action on the maintained propofol self-administration. These results indicated that blockade of the D1 DA receptor inhibits propofol self-administration via reducing p-ERK expression in the NAc.
Fig. 3.
Systemic administration of SCH23390 inhibited propofol self-administration and reduced ERK expression in the NAc. A Numbers of active and inactive responses, and the number of infusions (*P < 0.05 versus vehicle). B Compared to normal controls (C), p-ERK in the NAc was increased in propofol-maintained rats, while it was decreased in rats pretreated with SCH23390 (mean ± SEM; *P < 0.05 versus controls; # P < 0.05 versus vehicle).
MEK Inhibitor U0126 Attenuates Propofol Self-Administration
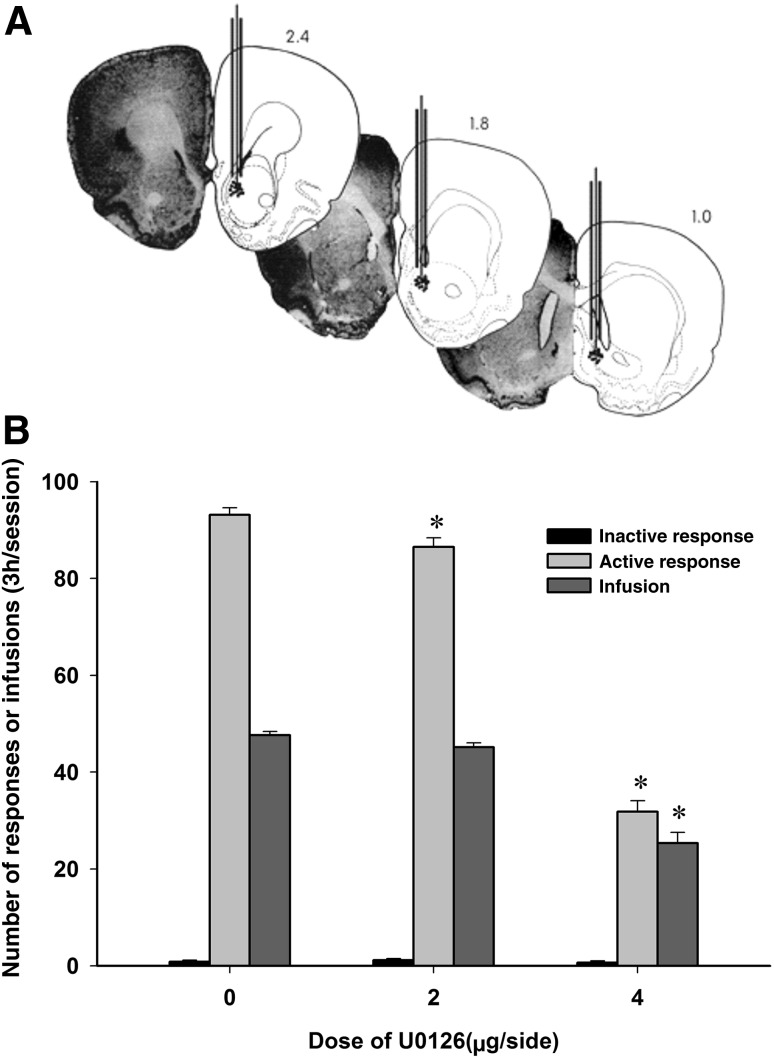
ANOVA revealed that pre-microinjection of U0126 into the NAc attenuated the active responses (F(2,17) = 315.38, P < 0.01) and infusions (F(2,17) = 71.87, P < 0.01), but failed to affect the inactive responses (F(2,17) = 0.65, P = 0.54) (Fig. 4). Multiple comparisons showed that pretreatment with U0126 at 2 or 4 μg/side decreased the active responses during propofol self-administration. In contrast, only 4 μg/side of U0126 reduced the infusions of propofol during self-administration (P < 0.05).
Fig. 4.
Effects of intra-NAc injection of U0126 on the maintenance of propofol self-administration. A Histological reconstruction showing injection sites in the NAc. B The numbers of active responses and infusions decreased after intra-NAc injection of U0126 (mean ± SEM. *P < 0.05 versus vehicle).
Discussion
The chronic administration of addictive drugs such as cocaine, amphetamine, THC, nicotine, morphine, and alcohol induces a specific ERK activity pattern in mesocorticolimbic regions [20–27]. Several studies have indicated that NAc may be an important locus for propofol self-administration. For example, in vivo infusion showed that sub-anesthetic and anesthetic doses of propofol increases extracellular DA levels in the NAc [28]. In the present study, we found that propofol, as a positive reinforcer, maintained the intravenous self-administration, and increased the expression of p-ERK in the NAc. These results are consistent with several previous studies showing increased p-ERK expression in the NAc following cocaine and ethanol self-administration [16, 20, 29]. Thus, the data indicated that activation of ERK transduction pathways in the NAc is involved in propofol self-administration.
A previous study demonstrated that D1, but not D2, DA receptors in the NAc mediate the maintenance of propofol self-administration. In the present study, pretreatment with SCH23390 not only inhibited the propofol self-administration, but also decreased the expression of p-ERK in the NAc in propofol-maintained rats. This scenario is supported by the report that progressive activation of ERK in the dorsal striatum and NAc of rats induced by acute administration of THC is blocked by the D1 receptor antagonist SCH23390 [30]. In addition, pretreatment with SCH23390 completely reverses the activation of ERK pathways induced by acute treatment with cocaine [16]. D1 DA receptors activate the ERK pathway via activation of the small Ras-related G protein Rap1 [31–33] and calcyon [34]. These results suggest that activation of the D1 DA receptor and its coupled ERK signal transduction pathway following DA release induced by propofol may contribute to the propofol self-administration and rewarding effect.
In the present study, we demonstrated an effect of intra-NAc injection of the MEK inhibitor U0126 on propofol self-administration. The results showed that pretreatment with U0126 attenuated the propofol-maintained self-administration, consistent with several other studies. For example, inhibition of ERK activity by intracerebroventricular pre-treatment with either PD98059 or U0126 decreases morphine-induced place preference [14]; ERK inhibition blocks THC-induced reward properties in the place-preference paradigm [15]; Amphetamine increases the levels of ERK, and NAc injections of the ERK inhibitor PD98059 dose-dependently impair amphetamine-induced place-preference [35]; and overexpressing ERK2 by virus-mediated gene transfer increases the preference for environments previously paired with low doses of cocaine, whereas blocking ERK2 activity inhibits this cocaine-induced place conditioning [36]. Therefore, ERK signaling is critical for the downstream regulation of DA signal transmission in the NAc mediating the propofol self-administration and rewarding effects. Given the inhibition of both active responses and infusions by U0126 or SCH23390 at the highest dose in the present study, further studies are warranted to address how MEK inhibitors modify ERK expression and the functional responses to propofol in the NAc.
In conclusion, the present results demonstrated that expression of p-ERK in the NAc increased following propofol self-administration, and both blockade of D1 DA receptors and inhibition of the ERK signal pathway attenuated the self-administration. The results suggested that the ERK signal transduction pathways coupled with D1 receptors in the NAc are involved in the maintenance of propofol self-administration and its rewarding effects.
Acknowledgments
This work was supported in part by the National Natural Science Foundation of China (81271469 and 81471350), the Natural Science Foundation of Zhejiang Province, China (Z2101211 and Y20140692), and a Medical Health Project of Zhejiang Province, China (2014KYB161).
Contributor Information
Wenhua Zhou, Email: whzhou@vip.163.com.
Qingquan Lian, Email: lianqingquanmz@163.com.
References
- 1.Roussin A, Montastruc JL, Lapeyre-Mestre M. Pharmacological and clinical evidences on the potential for abuse and dependence of propofol: a review of the literature. Fundam Clin Pharmacol. 2007;21:459–466. doi: 10.1111/j.1472-8206.2007.00497.x. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet U, Harkener J, Scherbaum N. A case report of propofol dependence in a physician. J Psychoactive Drugs. 2008;40:215–217. doi: 10.1080/02791072.2008.10400634. [DOI] [PubMed] [Google Scholar]
- 3.Fritz GA, Niemczyk WE. Propofol dependency in a lay person. Anesthesiology. 2002;96:505–506. doi: 10.1097/00000542-200202000-00039. [DOI] [PubMed] [Google Scholar]
- 4.Schneider U, Rada D, Rollnik JD, Passie T, Emrich HM. Propofol dependency after treatment of tension headache. Addict Biol. 2001;6:263–265. doi: 10.1080/13556210120056607. [DOI] [PubMed] [Google Scholar]
- 5.Pain L, Oberling P, Sandner G, Di Scala G. Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology. 1996;85:121–128. doi: 10.1097/00000542-199607000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Pain L, Oberling P, Sandner G, Di Scala G. Effect of midazolam on propofol-induced positive affective state assessed by place conditioning in rats. Anesthesiology. 1997;87:935–943. doi: 10.1097/00000542-199710000-00029. [DOI] [PubMed] [Google Scholar]
- 7.Lian Q, Wang B, Zhou W, Jin S, Xu L, Huang Q, et al. Self-administration of propofol is mediated by dopamine D1 receptors in nucleus accumbens in rats. Neuroscience. 2013;231:373–383. doi: 10.1016/j.neuroscience.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 8.LeSage MG, Stafford D, Glowa JR. Abuse liability of the anesthetic propofol: self-administration of propofol in rats under fixed-ratio schedules of drug delivery. Psychopharmacology (Berl) 2000;153:148–154. doi: 10.1007/s002130000430. [DOI] [PubMed] [Google Scholar]
- 9.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, et al. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. J Neurosci. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- 14.Ozaki S, Narita M, Narita M, Ozaki M, Khotib J, Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J Neurochem. 2004;88:1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- 15.Valjent E, Caboche J, Vanhoutte P. Mitogen-activated protein kinase/extracellular signal-regulated kinase induced gene regulation in brain: a molecular substrate for learning and memory? Mol Neurobiol. 2001;23:83–99. doi: 10.1385/MN:23:2-3:083. [DOI] [PubMed] [Google Scholar]
- 16.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Valjent E, Aubier B, Corbille AG, Brami-Cherrier K, Caboche J, Topilko P, et al. Plasticity-associated gene Krox24/Zif268 is required for long-lasting behavioral effects of cocaine. J Neurosci. 2006;26:4956–4960. doi: 10.1523/JNEUROSCI.4601-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, et al. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144:1209–1218. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, et al. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. Int J Neuropsychopharmacol. 2014;17:1397–1407. doi: 10.1017/S1461145714000595. [DOI] [PubMed] [Google Scholar]
- 20.Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/S0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- 21.Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–11255. doi: 10.1523/JNEUROSCI.1040-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giorgi O, Corda MG, Sabariego M, Giugliano V, Piludu MA, Rosas M, et al. Differential effects of cocaine on extracellular signal-regulated kinase phosphorylation in nuclei of the extended amygdala and prefrontal cortex of psychogenetically selected Roman high- and low-avoidance rats. J Neurosci Res. 2015;93:714–721. doi: 10.1002/jnr.23526. [DOI] [PubMed] [Google Scholar]
- 23.Voyer D, Levesque D, Rompre PP. Repeated ventral midbrain neurotensin injections sensitize to amphetamine-induced locomotion and ERK activation: A role for NMDA receptors. Neuropharmacology 2016. doi:10.1016/j.neuropharm.2016.06.005. [DOI] [PMC free article] [PubMed]
- 24.Mao LM, Reusch JM, Fibuch EE, Liu Z, Wang JQ. Amphetamine increases phosphorylation of MAPK/ERK at synaptic sites in the rat striatum and medial prefrontal cortex. Brain Res. 2013;1494:101–108. doi: 10.1016/j.brainres.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fishbein M, Gov S, Assaf F, Gafni M, Keren O, Sarne Y. Long-term behavioral and biochemical effects of an ultra-low dose of Delta9-tetrahydrocannabinol (THC): neuroprotection and ERK signaling. Exp Brain Res. 2012;221:437–448. doi: 10.1007/s00221-012-3186-5. [DOI] [PubMed] [Google Scholar]
- 26.Gomez AM, Sun WL, Midde NM, Harrod SB, Zhu J. Effects of environmental enrichment on ERK1/2 phosphorylation in the rat prefrontal cortex following nicotine-induced sensitization or nicotine self-administration. Eur J Neurosci. 2015;41:109–119. doi: 10.1111/ejn.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haghparast A, Fatahi Z, Alamdary SZ, Reisi Z, Khodagholi F. Changes in the levels of p-ERK, p-CREB, and c-fos in rat mesocorticolimbic dopaminergic system after morphine-induced conditioned place preference: the role of acute and subchronic stress. Cell Mol Neurobiol. 2014;34:277–288. doi: 10.1007/s10571-013-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pain L, Gobaille S, Schleef C, Aunis D, Oberling P. In vivo dopamine measurements in the nucleus accumbens after nonanesthetic and anesthetic doses of propofol in rats. Anesth Analg. 2002;95:915–919. doi: 10.1097/00000539-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 29.Faccidomo S, Salling MC, Galunas C, Hodge CW. Operant ethanol self-administration increases extracellular-signal regulated protein kinase (ERK) phosphorylation in reward-related brain regions: selective regulation of positive reinforcement in the prefrontal cortex of C57BL/6 J mice. Psychopharmacology (Berl) 2015;232:3417–3430. doi: 10.1007/s00213-015-3993-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valjent E, Pages C, Rogard M, Besson MJ, Maldonado R, Caboche J. Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission. Eur J Neurosci. 2001;14:342–352. doi: 10.1046/j.0953-816x.2001.01652.x. [DOI] [PubMed] [Google Scholar]
- 31.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/S0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 32.Yao H, York RD, Misra-Press A, Carr DW, Stork PJ. The cyclic adenosine monophosphate-dependent protein kinase (PKA) is required for the sustained activation of mitogen-activated kinases and gene expression by nerve growth factor. J Biol Chem. 1998;273:8240–8247. doi: 10.1074/jbc.273.14.8240. [DOI] [PubMed] [Google Scholar]
- 33.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, et al. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 34.Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C. Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science. 2000;287:1660–1664. doi: 10.1126/science.287.5458.1660. [DOI] [PubMed] [Google Scholar]
- 35.Gerdjikov TV, Ross GM, Beninger RJ. Place preference induced by nucleus accumbens amphetamine is impaired by antagonists of ERK or p38 MAP kinases in rats. Behav Neurosci. 2004;118:740–750. doi: 10.1037/0735-7044.118.4.740. [DOI] [PubMed] [Google Scholar]
- 36.Iniguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolanos-Guzman CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]