Abstract
To date, we still lack disease-modifying therapies for Alzheimer’s disease (AD). Here, we report that long-term administration of benfotiamine improved the cognitive ability of patients with AD. Five patients with mild to moderate AD received oral benfotiamine (300 mg daily) over 18 months. All patients were examined by positron emission tomography with Pittsburgh compound B (PiB-PET) and exhibited positive imaging with β-amyloid deposition, and three received PiB-PET imaging at follow-up. The five patients exhibited cognitive improvement as assayed by the Mini-Mental Status Examination (MMSE) with an average increase of 3.2 points at month 18 of benfotiamine administration. The three patients who received follow-up PiB-PET had a 36.7% increase in the average standardized uptake value ratio in the brain compared with that in the first scan. Importantly, the MMSE scores of these three had an average increase of 3 points during the same period. Benfotiamine significantly improved the cognitive abilities of mild to moderate AD patients independently of brain amyloid accumulation. Our study provides new insight to the development of disease-modifying therapy.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0067-0) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Therapy, Benfotiamine, PiB-PET, Amyloid deposition
Introduction
Alzheimer’s disease (AD) is a devastating neurodegenerative disorder in the aging society and has severely impacted the global healthcare system. Unfortunately, there is no effective disease-modifying therapy for AD. To date, almost all clinical trials aiming to halt or delay AD progression have failed. Particularly, multiple approaches such as vaccines, antibodies, and β or γ-secretase inhibitors, have been exploited to reduce amyloid deposition in the brain but have little beneficial effect on the cognitive ability of AD patients [1–4]. Generally, the failure in cognitive improvement is attributed to the fact that these therapies were performed on patients at the dementia stage of AD, and this may be too late.
Despite these difficulties, there is an unmet need to reduce the suffering of millions of AD patients and the increasingly staggering cost of caring for them. Given that AD is a complex disorder with multiple etiologies and courses of disease progression, current strategies do not necessarily encompass all possible mechanisms that may be used to delay or halt it. Therefore, new approaches are needed to explore other possibilities for disease-modifying therapy than those commonly used in current clinical trials. For instance, the disturbance of cerebral glucose metabolism is an invariant and significant pathophysiological alteration in AD, which precedes and is closely correlated with cognitive impairment [5, 6]. Thus, factors associated with brain glucose hypometabolism may be potential therapeutic targets.
Thiamine diphosphate (TDP), the active form of thiamine, is critical for glucose metabolism because it serves as a key coenzyme of three rate-limiting enzymes of glucose catabolism (pyruvate dehydrogenase and α-ketoglutarate dehydrogenase in the Krebs cycle, and transketolase in the pentose phosphate pathway). Both the TDP level and the activity of TDP-dependent enzymes are significantly reduced in blood and brain autopsy samples from AD patients [7–9]. Our previous study showed that TDP reduction is a significant biomarker for AD diagnosis [10]. The reduction of brain glucose metabolism and its possible pathogenic indicator, TDP reduction, implicate multiple pathogenic pathways in AD, including oxidative stress [11], neuroinflammation [12], and enhanced activity of glycogen synthase kinase-3 [13] and β-secretase [14]. Therefore, we hypothesized that disruption of thiamine metabolism directly contributes to AD pathogenesis by perturbing glucose utilization and by activating multiple pathophysiological cascades in the brain [15]. Based on this hypothesis, a strategy of simultaneously correcting the abnormal thiamine metabolism while treating other pathogenic factors may be a viable approach to modify the progression of AD.
Benfotiamine is a synthetic thiamine derivative with better bioavailability than thiamine and has been shown to prevent abnormal glucose metabolism via multiple pathways [16, 17]. Our previous study demonstrated that benfotiamine improves cognitive impairment in a mouse model of AD (amyloid precursor protein/presenilin-1 (APP/PS1) transgenic mice) [18]. Here, we investigated the effects of benfotiamine on the cognitive ability and amyloid accumulation in the brain assayed by positron emission tomography with Pittsburgh compound-B (PiB-PET) in five patients with mild to moderate AD.
Materials and Methods
Study Design and Participants
This study was designed as an open and uncontrolled study with the purpose of assessing long-term cognitive improvement after benfotiamine administration. It was approved by the Committee of Medical Ethics of Zhongshan Hospital, Fudan University (No. 2009-013). All patients or their caregivers provided written informed consent.
Five patients with mild to moderate AD from the outpatient clinic of the Neurology Department at Zhongshan Hospital were recruited. They were diagnosed with AD according to the clinical criteria proposed by the National Institute of Aging-Alzheimer’s Association in 2011 [19] and PiB-PET imaging. All patients displayed an insidious onset of the disease and gradual progression of cognitive dysfunction. Based on careful inquiry into their medical history, a neurological/psychological examination, and cranial CT and/or MRI scans, these patients had no other diseases known to cause cognitive decline, e.g., cerebrovascular disease or toxic–metabolic disorder. The neuropsychological evaluations included the Mini-Mental Status Examination (MMSE), Activities of Daily Living, Clinical Dementia Rating, and Hamilton Depression Rating Scales. Blood folate, vitamin B12, free triiodothyronine, free thyroxine, and thyroid stimulating hormone levels were in the normal range for all patients.
All patients received oral benfotiamine (300 mg/day; Doctor’s Best, Irvine, CA) over 18 months. Cases I to IV continued previous donepezil administration (5 mg/day) during benfotiamine administration. Case V had only monotherapy with benfotiamine. Detailed demographic data is listed in Supplemental Table S1.
Measurement of Blood Thiamine Metabolites
TDP, thiamine monophosphate (TMP), and thiamine levels were determined using high performance liquid chromatography (HPLC), as previously described [18]. Briefly, 150 μL of whole blood anticoagulated with heparin was collected and immediately deproteinized with an equal volume of 7.4% perchloric acid. After centrifugation at 10,000 rpm at 4 °C for 6 min, the supernatant was collected and stored at −20 °C. Thiamine and its phosphate esters were derivatized into thiochromes using potassium ferricyanide and separated by gradient elusion on a C18 reversed-phase analytical column (250 × 4.6 mm2) by HPLC (Agilent 1100, Santa Clara, CA); the derivatives were detected using an excitation wavelength of 367 nm and an emission wavelength of 435 nm. Blood TDP, TMP, and thiamine levels were quantified using standard samples (Sigma-Aldrich, St. Louis, MO).
PiB-PET Imaging
Synthesis of the radioactive ligand PiB with 11C was based on the method described previously [20]. Approximately 10 mCi of radiotracer was injected through the opisthenar vein within 60 s and was flushed with 1 mL saline. Subsequently, 3D dynamic PET acquisition was performed from 0 to 60 min post-injection following an attenuation correction CT using a PET/CT scanner (Biograph 64 Truepoint, Siemens Medical Solutions, Munich, Germany). The 40- to 60-min static images were reconstructed using an iterative 3D method with a Gaussian filter of 6 mm in Full Width of Half Maximum. The pixel size was 2.0 mm and the slice thickness was 1.5 mm. The deposition/retention of 11C-PiB was calculated using the method previously described by Klunk et al. [21]. Data from all participants imaged by PiB-PET was used to calculate the in vivo amyloid plaque load represented by SUVR, which is defined as the ratio of the standardized uptake value in each region of interest (ROI) to that of the cerebellar cortex [22].
The PiB-PET examination was performed on three patients before benfotiamine administration and on two patients at month 6 of benfotiamine administration. Further, three patients received follow-up PiB-PET imaging after 18 months of benfotiamine administration. All five patients had SUVRs > 1.3 for PiB.
Results
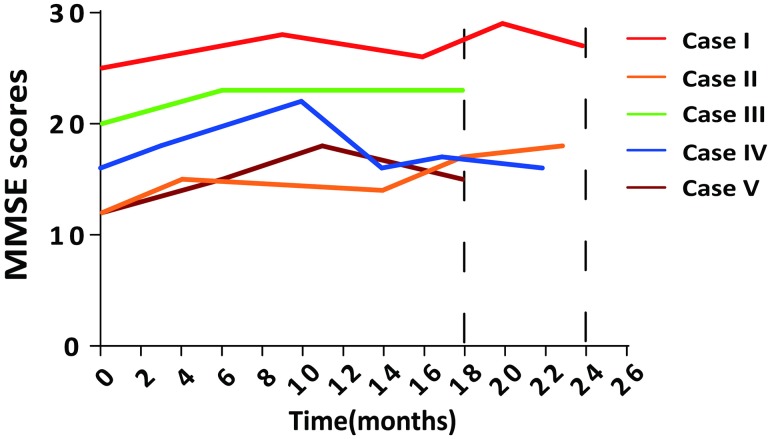
Changes in the cognitive function of all five patients were evaluated by MMSE scoring or by the Clinical Dementia Rating during the 18 months of treatment or longer. We found that the MMSE scores increased by 2 to 6 points in four patients and did not significantly change in one patient, averaging 3.2 ± 1.3 points (n = 5) at month 18 of benfotiamine administration (case I was assayed at month 20 and case IV at month 17). Three patients received follow-up examinations later than 22 months. The MMSE scores of case I increased from 25 to 27 points and of case II from 12 to 18 points during the follow-up period. In case IV, the MMSE score at month 22 did not significantly differ from that at baseline (Fig. 1; Table 1).
Fig. 1.
Benfotiamine improved cognitive function as assessed by MMSE scores.
Table 1.
Neuropsychological functions and PiB SUVRs of the five cases given benfotiamine.
| Case No. | Time (months) | Neuropsychological evaluation | PiB SUVRs | |||||
|---|---|---|---|---|---|---|---|---|
| MMSE (–/30) | CDR (–/3) | ADL (–/32) | FC | PC | TC | Average | ||
| Case I | 0 | 25 | 0.5 | 8 | 2.034 | 1.598 | 1.947 | 1.860 |
| 9 | 28 | 0.5 | 8 | |||||
| 16 | 26 | 1 | 8 | |||||
| 20 | 29 | 1 | 8 | 2.504 | 2.409 | 2.390 | 2.435 | |
| 24 | 27 | 0.5 | 8 | |||||
| Case II | 0 | 12 | 1 | 10 | 2.584 | 1.895 | 1.811 | 2.097 |
| 4 | 15 | 1 | 10 | |||||
| 14 | 14 | 1 | 16 | |||||
| 18 | 17 | 1 | 13 | 4.040 | 3.022 | 2.857 | 3.306 | |
| 23 | 18 | 1 | 10 | |||||
| Case III | 0 | 20 | 1 | 8 | ||||
| 6 | 23 | 1 | 11 | 1.498 | 1.727 | 1.314 | 1.513 | |
| 18 | 23 | 1 | 9 | |||||
| Case IV | 0 | 16 | 1 | 8 | 1.485 | 1.338 | 1.375 | 1.399 |
| 3 | 18 | 0.5 | 8 | |||||
| 10 | 22 | 1 | 9 | |||||
| 14 | 16 | 0.5 | 8 | |||||
| 17 | 17 | 1 | 8 | |||||
| 22 | 16 | 1 | 9 | |||||
| Case V | 0 | 12 | 2 | 9 | ||||
| 6 | 15 | 1 | 9 | 1.447 | 1.242 | 1.386 | 1.358 | |
| 11 | 18 | 1 | 8 | |||||
| 18 | 15 | 1 | 9 | 1.606 | 1.502 | 1.461 | 1.532 | |
MMSE Mini-Mental Status Examination, CDR Clinical Dementia Rating, ADL Activities of Daily Living, FC frontal cortex, PC parietal cortex, TC temporal cortex, SUVR ratio of the standardized uptake value.
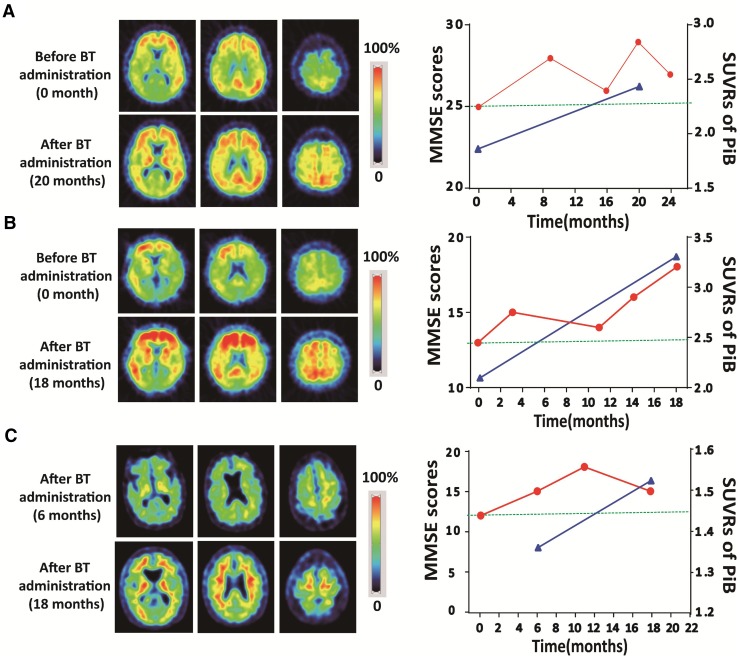
The effect of benfotiamine on amyloid deposition was also evaluated. Three cases (I, II, and V) received a follow-up PiB-PET examination. After benfotiamine administration, all three cases still manifested significantly elevated amyloid deposition in spite of the improvement in their cognitive functions. The SUVRs of the frontal, parietal, and temporal cortices increased from the first to the second PiB-PET scan. The average SUVR of the frontal, parietal, and temporal cortices at the second PiB-PET imaging was 2.42 ± 0.79, representing a 36.7% increase from that at the first scan (1.77 ± 0.39; Table 1; Fig. 2). Importantly, the MMSE scores of the three cases had an average increase of 3 points during the same period.
Fig. 2.
Benfotiamine improved cognitive function as assessed by MMSE scores independent of brain amyloid deposition in AD patients. Average PiB SUVRs representing brain amyloid loading and cognitive function assessed by MMSE scoring before and after benfotiamine administration in cases I (A) and II (B). C Case V received the first PiB-PET scan at month 6 after benfotiamine administration and a follow-up PiB-PET imaging at month 18. Amyloid accumulation in the brain increased significantly despite improved cognitive function, BT benfotiamine.
Discussion
Our study showed that AD patients with mild-to-moderate dementia manifested a long-term (over 18 months) improvement in cognitive ability after benfotiamine administration, despite the progressive exacerbation of brain amyloid accumulation as evaluated by PiB-PET scans. These results reveal two important messages: (1) the progression of brain dysfunction in the dementia stage of AD can be halted and even improved, and (2) the alteration of cognitive capability is independent of brain amyloid accumulation, which is consistent with previous results showing that the reduction of brain amyloid accumulation by vaccines, antibodies, or β- and γ-secretase inhibitors has little beneficial effect on the cognitive ability and disease progression of AD patients [1–4].
The brain has the most abundant energy consumption in the human body, and the maintenance of its function is dependent on energy metabolism. Because glucose is the main substrate of energy metabolism, the brain is vulnerable to the dyshomeostasis of glucose metabolism. A disturbance of brain glucose metabolism is one of the most important pathophysiological features, and precedes the overt symptoms of AD by decades. Furthermore, the reduction in brain glucose metabolism is significantly correlated with the degree of cognitive impairment and the progression of AD. For this reason, AD has even been considered to be “brain insulin resistance” or type III diabetes [23]. Thus, brain glucose hypometabolism and its pathogenic factors, such as altered thiamine metabolism, may be targeted for disease-modifying therapy to treat AD.
In a previous study, we demonstrated the beneficial effect of benfotiamine against cognitive impairment in a mouse model of AD. Here, we present the results of a clinical case study showing the potential of benfotiamine as a disease-modifying drug for AD. As a derivative of thiamine with better bioavailability, benfotiamine has been demonstrated to exert beneficial effects against abnormal glucose metabolism and its consequences via multiple mechanisms, including the elimination of oxidative stress [16, 17] and the inhibition of glycogen synthase kinase-3 [18], which are both considered to be major pathogenic factors that cause neurodegeneration in AD. The better bioavailability and the pharmacological effects via multiple mechanisms against abnormal glucose metabolism and its consequences may explain why benfotiamine administration but not thiamine supplementation [24] had a long-term beneficial effect on cognitive ability in AD patients.
Although the results of the current study are encouraging, there are evident caveats. First, the results need to be validated by randomized, double-blinded, placebo-controlled clinical trials. Second, whether brain glucose hypometabolism in AD patients is significantly altered and correlated with the improvement of cognitive ability after benfotiamine administration needs to be further evaluated. Third, it will be necessary in future studies to determine whether the beneficial effect on AD was due to benfotiamine alone or its combination with donepezil because four patients in our study also took 5 mg donepezil per day in addition to benfotiamine.
In summary, our results show that benfotiamine can produce a long-term improvement (over 18 months) in the cognitive ability of AD patients during continued brain amyloid accumulation. These results indicate that brain dysfunction may be independent of amyloid deposition and that the disease progression can be halted in the dementia stage of AD. Our study provides new insights into understanding the mechanism of cognitive impairment in AD and suggests a new direction to develop disease-modifying therapies for the dementia stage. Further clinical trials in this direction are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by the Key Fund for Developing New Drugs from the Ministry of Science and Technology of China (2014ZX09101005-005), the National Natural Science Foundation of China (81071019), the National Key Basic Research Program of China (2011CBA00400), the Natural Science Foundation of Shanghai Municipality, China (13JC1401500), and the Fund for Medical Emerging Cutting-Edge Technology in Shanghai of China (SHDC12012114). We thank Dr. Mu-ming Poo (Institute of Neuroscience, State Key Laboratory of Neuroscience, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) for his valuable insight and critique of the article. We also thank Dr. Alexandra H. Marshal (Department of Laboratory Medicine and Pathobiology, St. Michael’s Hospital, University of Toronto) for her careful editing of the article. We gratefully acknowledge all of the participants for their contributions to this study.
Footnotes
Xiaoli Pan and Zhichun Chen have contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0067-0) contains supplementary material, which is available to authorized users.
References
- 1.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 2.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 4.Doody RS, Raman R, Farlow M, Iwatsubo T, Vellas B, Joffe S, et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N Engl J Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 5.Edison P, Archer HA, Hinz R, Hammers A, Pavese N, Tai YF, et al. Amyloid, hypometabolism, and cognition in Alzheimer disease: an [11C]PIB and [18F]FDG PET study. Neurology. 2007;68:501–508. doi: 10.1212/01.wnl.0000244749.20056.d4. [DOI] [PubMed] [Google Scholar]
- 6.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 7.Gibson GE, Sheu KF, Blass JP, Baker A, Carlson KC, Harding B, et al. Reduced activities of thiamine-dependent enzymes in the brains and peripheral tissues of patients with Alzheimer’s disease. Arch Neurol. 1988;45:836–840. doi: 10.1001/archneur.1988.00520320022009. [DOI] [PubMed] [Google Scholar]
- 8.Mastrogiacoma F, Bettendorff L, Grisar T, Kish SJ. Brain thiamine, its phosphate esters, and its metabolizing enzymes in Alzheimer’s disease. Ann Neurol. 1996;39:585–591. doi: 10.1002/ana.410390507. [DOI] [PubMed] [Google Scholar]
- 9.Heroux M, Raghavendra Rao VL, Lavoie J, Richardson JS, Butterworth RF. Alterations of thiamine phosphorylation and of thiamine-dependent enzymes in Alzheimer’s disease. Metab Brain Dis. 1996;11:81–88. doi: 10.1007/BF02080933. [DOI] [PubMed] [Google Scholar]
- 10.Pan X, Fei G, Lu J, Jin L, Pan S, Chen Z, et al. Measurement of blood thiamine metabolites for Alzheimer’s disease diagnosis. EBioMedicine. 2016;3:155–162. doi: 10.1016/j.ebiom.2015.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, et al. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer’s mouse model. Neurobiol Aging. 2009;30:1587–1600. doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang D, Hazell AS. Microglial activation is a major contributor to neurologic dysfunction in thiamine deficiency. Biochem Biophys Res Commun. 2010;402:123–128. doi: 10.1016/j.bbrc.2010.09.128. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Sun X, Yu Z, Pan X, Gu F, Chen J, et al. Exposure to pyrithiamine increases beta-amyloid accumulation, Tau hyperphosphorylation, and glycogen synthase kinase-3 activity in the brain. Neurotox Res. 2011;19:575–583. doi: 10.1007/s12640-010-9204-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Yang G, Li W, Fan Z, Sun A, Luo J, et al. Thiamine deficiency increases beta-secretase activity and accumulation of beta-amyloid peptides. Neurobiol Aging. 2011;32:42–53. doi: 10.1016/j.neurobiolaging.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Chen Z, Zhong C. Decoding Alzheimer’s disease from perturbed cerebral glucose metabolism: implications for diagnostic and therapeutic strategies. Prog Neurobiol. 2013;108:21–43. doi: 10.1016/j.pneurobio.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Hammes HP, Du X, Edelstein D, Taguchi T, Matsumura T, Ju Q, et al. Benfotiamine blocks three major pathways of hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- 17.Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A. The multifaceted therapeutic potential of benfotiamine. Pharmacol Res. 2010;61:482–488. doi: 10.1016/j.phrs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, et al. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- 19.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 21.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 22.Ng S, Villemagne VL, Berlangieri S, Lee ST, Cherk M, Gong SJ, et al. Visual assessment versus quantitative assessment of 11C-PIB PET and 18F-FDG PET for detection of Alzheimer’s disease. J Nucl Med. 2007;48:547–552. doi: 10.2967/jnumed.106.037762. [DOI] [PubMed] [Google Scholar]
- 23.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 24.Blass JP, Gleason P, Brush D, DiPonte P, Thaler H. Thiamine and Alzheimer’s disease. A pilot study. Arch Neurol. 1988;45:833–835. doi: 10.1001/archneur.1988.00520320019008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.