Ketamine is a noncompetitive N-methyl-D-aspartate receptor (NMDAR) antagonist that has attracted widespread attention for its rapid-onset antidepressant effects, especially in individuals with treatment-resistant depression and suicidal ideation [1–3]. Compared with the traditional antidepressants that take weeks, if not months, to benefit patients and are associated with a high rate of relapse, ketamine exerts its antidepressant effects within several hours. These clinical benefits can last for 2 weeks after a single injection [1, 2, 4]. However, ketamine still has limited clinical application, mainly because of its psychotomimetic side-effects and liability of abuse. A recent paper in Nature [5] showed that the ketamine metabolite enantiomer (2R,6R)-hydroxynorketamine (HNK) has rapid and sustained antidepressant effects without the side-effects associated with ketamine, such as abuse potential. The discovery of (R)-ketamine is a landmark in the field of depression. Investigations of its mechanism of action will inspire the development of a new generation of rapid-acting antidepressants that are safer and have few dissociative side-effects [6].
Ketamine is a racemic mixture with equal proportions of (R)- and (S)-ketamine. Compared with (R)-ketamine, (S)-ketamine has been shown to have a more than four-fold greater affinity for NMDARs, greater anesthetic potency, and serious psychotomimetic side-effects [7–9]. The antidepressant efficacy of ketamine has centered on the inhibition of NMDAR-mediated glutamate neurotransmission, holding promise for future glutamate-modulating strategies. However, other NMDAR antagonists have only relatively modest antidepressant effects compared with ketamine, in both pre-clinical and clinical studies [10, 11]. Further investigations are needed to improve our understanding of ketamine’s mechanism of action.
Zanos et al. [5] concentrated on ketamine metabolites and investigated the mechanism by which it exerts rapid and sustained antidepressant effects. The authors hypothesized that if ketamine has rapid-onset effects mainly by inhibiting NMDARs, then (S)-ketamine would be predicted to be more potent than (R)-ketamine, and alternative NMDAR inhibitors would also have similar effects. In sharp contrast to this prediction, however, they found that the (R)-ketamine enantiomer has greater antidepressant efficacy in the forced swim test, the novelty-suppressed feeding test, and the learned helplessness test. These results are consistent with previous studies [12, 13], in which (R)-ketamine appeared to be a more potent, long-lasting, and safe antidepressant than (S)-ketamine. MK-801, another NMDAR antagonist, also has rapid antidepressant effects but they are not maintained [14, 15]. These findings raise doubts about the NMDAR-dependent antidepressant responses to ketamine.
Ketamine is stereo-selectively and region-specifically hydroxylated into a wide range of metabolites, including norketamine (norKET), hydroxyketamine, dehydronorketamine, and HNK [16, 17]. A previous study showed that the plasma concentrations of ketamine metabolites are correlated with the depressive, psychotic, and dissociative symptoms in patients with major depressive disorder and bipolar disorder [18]. The authors hypothesized that the active ketamine metabolites whose effects last beyond the time-frame of ketamine’s effects may contribute to the long-lasting antidepressant action. They identified (2S,6S;2R,6R)-HNK, together with norKET, as the major metabolites in both plasma and the brain of mice. Moreover, they found that the levels of (2S,6S;2R,6R)-HNK are three-fold higher in female than in male brains, which may explain the greater antidepressant effects in female mice after ketamine administration in the forced swim test.
To further investigate the putative role of (2S,6S;2R,6R)-HNK in the antidepressant responses to ketamine, the authors used deuteration at the C6 position of ketamine to reduce its rate of metabolism without altering its pharmacological and physiological properties. The deuterated ketamine failed to induce sustained antidepressant effects 24 h after administration, suggesting that (2S,6S;2R,6R)-HNK is necessary for such effects. Consistent with the greater antidepressant action of (R)-ketamine than (S)-ketamine [6, 13], (2R,6R)-HNK, which is exclusively metabolized from (R)-ketamine, has more potent, dose-dependent antidepressant actions, while (2S,6S)-HNK has antidepressant effects at higher doses.
The clinical utility of ketamine is significantly limited by its psychotomimetic side-effects and abuse potential. Zanos et al. [5] showed that (2R,6R)-HNK lacks dissociative and psychotomimetic side-effects in a wide range of tests, even in drug discrimination and self-administration paradigms that evaluate the liability of drug abuse/addiction. In contrast, both ketamine and (2S,6S)-HNK have serious abuse potential and other side-effects. (S)-ketamine but not (R)-ketamine causes deficits of parvalbumin-positive neurons in the medial prefrontal cortex and hippocampus in mice [19], and this may be responsible for the psychotic side-effects.
Using electrophysiological, electroencephalographic (EEG), and biochemical techniques, the authors further investigated possible mechanisms that underlie the rapid and sustained antidepressant effects of (2R,6R)-HNK. Ketamine is widely accepted to act mainly by inhibiting NMDARs in GABAergic interneurons, thus disinhibiting glutamatergic neurons, leading to the activation of downstream signaling and synaptic protein synthesis [20–22] (Fig. 1). Zanos et al. [5] examined the extent of (2R,6R)-HNK dependence on NMDAR activation. Surprisingly, they found that (2R,6R)-HNK induces robust increases in a variety of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (AMPAR)-dependent electrophysiological signatures, without affecting NMDAR-mediated currents in rat hippocampal slices. When the AMPAR antagonist NBQX was applied 10 min before ketamine and (2R,6R)-HNK treatment, it blocked both the acute and sustained antidepressant actions of (2R,6R)-HNK, highlighting the critical role of AMPARs in its antidepressant effects. EEG also confirmed that (2R,6R)-HNK acutely increases gamma power in vivo, similar to ketamine, and NBQX pretreatment blocks the (2R,6R)-HNK-induced increase in gamma power. These findings indicate that the antidepressant actions of (2R,6R)-HNK are dependent on AMPARs.
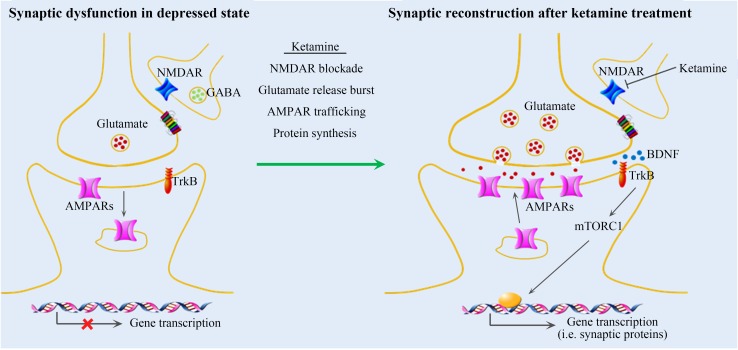
Fig. 1.
Rapid antidepressant mechanism of ketamine in medial prefrontal cortex. Repeated stress causes a malfunction of synaptic connectivity, characterized by decreases in glutamate release and AMPAR function, signal transduction, and synaptic protein synthesis, resulting in decreased number and function of spine synapses. Ketamine treatment is thought to cause disinhibition of GABAergic interneurons through blockade of NMDARs, resulting in widespread bursts of glutamate release in the medial prefrontal cortex. Glutamate release further activates AMPARs, contributing to BDNF release and the activation of a series of downstream pathways such as mTORC1 signaling, which increases synaptic protein synthesis and AMPAR trafficking. This widespread activation of signaling pathways leads to synapse recovery and regeneration, and thus remission of the depressed state.
The antidepressant effects of ketamine require the activation of several intracellular signaling pathways [15, 23, 24] and the enhancement of AMPAR-mediated synaptic plasticity [20, 25]. The authors examined the biochemical profiles of ketamine and (2R,6R)-HNK in the hippocampus and medial prefrontal cortex, two mood-related brain regions. Ketamine and (2R,6R)-HNK induced similar biochemical changes in the hippocampus, suggesting that they have overlapping pathways that mediate their antidepressant actions.
Zanos et al. [5] found that both ketamine and its metabolite (2R,6R)-HNK decrease the phosphorylation of eukaryotic translation elongation factor 2 (eEF2) and increase the expression of brain-derived neurotrophic factor (BDNF), GluA1, and GluA2 in hippocampal synaptoneurosomes 24 h after treatment. Combined with the electrophysiological and EEG data, we speculate that AMPAR-mediated maintenance of synaptic potentiation, BDNF release, and protein synthesis through eEF2 dephosphorylation underlies the sustained (24 h) antidepressive effects of ketamine metabolites (Fig. 2). However, Zanos et al. found that both ketamine and (2R,6R)-HNK have no significant effect on mTOR phosphorylation and BDNF levels at 1 h after treatment, which is not consistent with previous studies regarding the involvement of AMPAR-mediated BDNF/TrkB/mTORC1 (mammalian target of rapamycin complex 1) signaling in the rapid antidepressant effects of ketamine [20, 21, 26] (Fig. 1). Thus these conclusions should be treated with caution, needing more investigations and further verification.
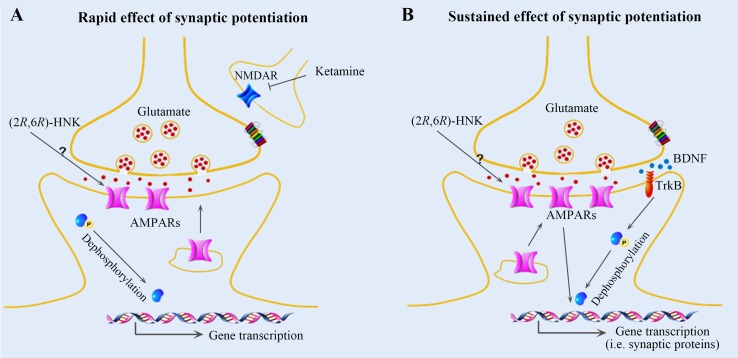
Fig. 2.
Mechanisms of rapid and sustained antidepressant actions of ketamine metabolites in the hippocampus. Racemic ketamine is metabolized into a wide range of components. (2S,6S)-HNK and (2R,6R)-HNK are the major HNK metabolites in both plasma and the brain in mice. (2R,6R)-HNK elicits rapid (A) and sustained (B) antidepressant effects, whereas (2S,6S)-HNK has no such beneficial effects and induces ketamine-like side-effects. (A) Ketamine and its metabolites may both contribute to the rapid antidepressant effects. Ketamine blocks NMDARs in GABAergic interneurons, and (2R,6R)-HNK induces glutamate release and activates AMPARs through an unknown mechanism, both of which lead to eukaryotic translation elongation factor 2 (eEF2) dephosphorylation and rapid antidepressant effects. (B) Glutamate bursts are induced by (2R,6R)-HNK through an unknown mechanism to stimulate AMPARs, resulting in BDNF release and protein synthesis through eEF2 dephosphorylation, which may be responsible for the sustained antidepressant effects of (2R,6R)-HNK.
Altogether, Zanos et al. [5] discovered that (2R,6R)-HNK, one of the main metabolites of ketamine in the brain, mimics the antidepressant effects of ketamine but has innocuous side-effects. (2R,6R)-HNK has a wide range of antidepressant-related effects in mice, and these behavioral, electrophysiological, and intracellular changes depend on AMPARs. Although the exact mechanism underlying the antidepressant action of ketamine is still debated, the findings of Zanos et al. [5] are a landmark in ketamine research. Their study extends our understanding of its antidepressant effects and will inspire the development of a new generation of antidepressants with fewer side-effects. Future investigations are needed to confirm and elucidate the mechanisms of the rapid and sustained antidepressant effects of ketamine and its metabolites and, more importantly, to develop safer antidepressants that may be substituted for ketamine.
References
- 1.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/S0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ, et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety. 2014;31:335–343. doi: 10.1002/da.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krystal JH, Sanacora G, Duman RS. Rapid-acting glutamatergic antidepressants: the path to ketamine and beyond. Biol Psychiatry. 2013;73:1133–1141. doi: 10.1016/j.biopsych.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K. R-ketamine: a rapid-onset and sustained antidepressant without risk of brain toxicity. Psychol Med. 2016;46:2449–2451. doi: 10.1017/S0033291716000969. [DOI] [PubMed] [Google Scholar]
- 7.Moaddel R, Abdrakhmanova G, Kozak J, Jozwiak K, Toll L, Jimenez L, et al. Sub-anesthetic concentrations of (R, S)-ketamine metabolites inhibit acetylcholine-evoked currents in α7 nicotinic acetylcholine receptors. Eur J Pharmacol. 2013;698:228–234. doi: 10.1016/j.ejphar.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domino EF. Taming the ketamine tiger. Anesthesiology. 2010;113:678–684. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 9.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–1193. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 10.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 11.Iadarola ND, Niciu MJ, Richards EM, Vande Voort JL, Ballard ED, Lundin NB, et al. Ketamine and other N-methyl-D-aspartate receptor antagonists in the treatment of depression: a perspective review. Ther Adv Chronic Dis. 2015;6:97–114. doi: 10.1177/2040622315579059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang JC, Li SX, Hashimoto K. R(−)-ketamine shows greater potency and longer lasting antidepressant effects than S(+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–141. doi: 10.1016/j.pbb.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5:e632. doi: 10.1038/tp.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 15.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desta Z, Moaddel R, Ogburn ET, Xu C, Ramamoorthy A, Venkata SL, et al. Stereoselective and regiospecific hydroxylation of ketamine and norketamine. Xenobiotica. 2012;42:1076–1087. doi: 10.3109/00498254.2012.685777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caslavska J, Thormann W. Stereoselective determination of drugs and metabolites in body fluids, tissues and microsomal preparations by capillary electrophoresis (2000–2010) J Chromatogr A. 2011;1218:588–601. doi: 10.1016/j.chroma.2010.08.072. [DOI] [PubMed] [Google Scholar]
- 18.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Han M, Zhang JC, Ren Q, Hashimoto K. Loss of parvalbumin-immunoreactivity in mouse brain regions after repeated intermittent administration of esketamine, but not R-ketamine. Psychiatry Res. 2016;239:281–283. doi: 10.1016/j.psychres.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22:238–249. doi: 10.1038/nm.4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med. 2015;66:509–523. doi: 10.1146/annurev-med-053013-062946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Jing L, Toledo-Salas JC, Xu L. Rapid-onset antidepressant efficacy of glutamatergic system modulators: the neural plasticity hypothesis of depression. Neurosci Bull. 2015;31:75–86. doi: 10.1007/s12264-014-1484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol 2014, 18. doi:10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed]
- 25.Koike H, Chaki S. Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res. 2014;271:111–115. doi: 10.1016/j.bbr.2014.05.065. [DOI] [PubMed] [Google Scholar]
- 26.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]