Abstract
Patients with schizophrenia undergo changes in brain plasticity. In the present study, we characterized motor cortical-striatal plasticity in such patients. Compared with the potentiation following high-frequency repetitive transcranial magnetic stimulation in the control group, the patients demonstrated impaired plasticity of corticostriatal motor-evoked potentials recorded from hand muscles. Notably, the loss of cortical plasticity was correlated with impaired motor learning in a rotary pursuit task. Moreover, the loss of plasticity was correlated with the symptoms of schizophrenia. The results suggest that the progression of schizophrenia is accompanied by altered cortical plasticity and functioning.
Keywords: Schizophrenia, Cortical plasticity, Motor learning, human
Introduction
Schizophrenia is associated with changes in brain plasticity [1–4]. For instance, patients demonstrate deficits in learning motor skills and paired associative stimulation induced by transcranial magnetic stimulation (TMS) [5, 6]. The associative plasticity is thought to involve cortico-cortical or thalamo-cortical afferents to the motor cortex [5, 7], and potentially the associative striatal pathways. However, it is unknown if the plasticity in the direct cortico-striatal pathway (e.g. induced by repetitive stimulation over the motor cortex) is affected in schizophrenia.
High-frequency repetitive TMS (rTMS) or intermittent theta-burst stimulation (iTBS) over the motor cortex induces direct potentiation of the cortico-striatal pathway, as reflected by the motor-evoked potential (MEP) recorded from the upper limb (e.g. hand). The pathway is involved more in motor-striatum connectivity rather than the associative striatum (e.g. sensory-motor integration). Here, we used 10-Hz rTMS to investigate plasticity of the cortico-striatal pathway in schizophrenic patients and the correlation of this plasticity with their motor skill learning and positive/negative symptoms.
Materials and Methods
Participants
Twenty-one healthy individuals served as controls (22–60 years old, 17 male, 4 female, right-handed) and 47 schizophrenic patients (14–58 years old, 37 male, 10 female, right-handed) were recruited. None had cardiovascular disease, a history of epilepsy, or other chronic disease.
All the participants were volunteers and gave written informed consent. The study was approved by the Ethics Committees of Ningbo Kangning Hospital and Nanjing Normal University. All experimental procedures followed the guidelines for human medical research (Declaration of Helsinki).
TMS Procedures
For rTMS stimulation, high-frequency TMS pulses (10 Hz at 90% of the resting motor threshold, 5-s on, 10-s off for 10 min; 2000 pulses divided into 40 repeats at 15-s intervals) [8, 9] were applied over the left primary motor cortex with a CCY-I TMS instrument (Yiruide Co., Wuhan, China), using a “figure-of-8” coil for accurate targeting. The motor region was located using the Yiruide Cap system, and confirmed by hand muscle contraction as well as the MEP signal with a single TMS pulse. The center of the navigation cap was located at the vertex according to the instructions with the system.
MEP Procedures
MEPs were recorded from the abductor pollicis brevis muscle of the right hand using the CCY-I TMS affiliated MEP system and analyzed with CCY-I MEP software. For MEP recording, 20 MEPs (at 5-s intervals) were evoked by single-pulse TMS over the M1 region, and the peak value was averaged.
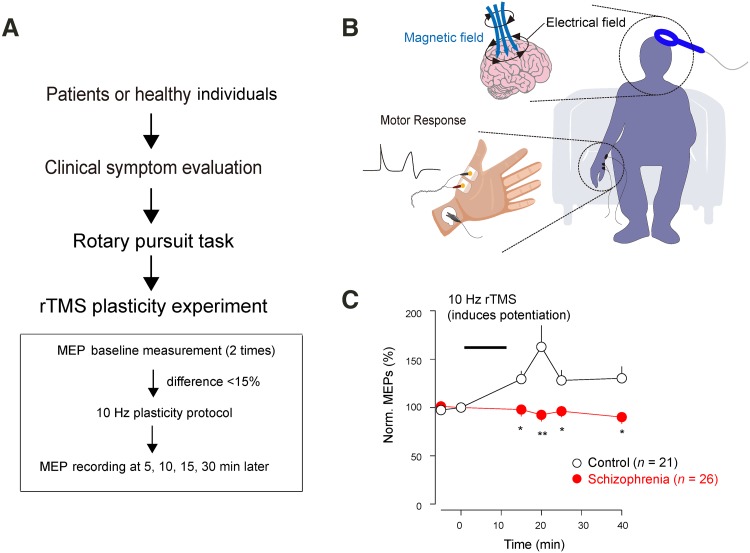
Baseline values were recorded at two points (separated by 5 min) and the plasticity protocol was applied if there was no more than a 15% difference between the two baseline values (Fig. 1). MEPs were re-measured at 5, 10, 15, and 30 min after the plasticity protocol [8].
Fig. 1.
Diminished motor-striatal plasticity in schizophrenia. A Flowchart of the whole experiment. B Design of TMS and MEP recordings. A single TMS pulse was delivered to the left motor cortex and the MEP was recorded from abductor pollicis brevis of the right hand. C 10-Hz rTMS potentiated MEPs in controls but not in the schizophrenic group.
Rotary Pursuit Task
In the rotary pursuit motor learning task [6], the participants were asked to trace a point on the rotating plate with a probe (Bei Da Qing Niao Co., Beijing, China), and the time of probe contact with the rotating point was recorded in 4 trials, each separated by 10 min. Prior to the test, each participant was familiarized with the test for <3 trials to prevent over-learning. The speed of the rotating plate was adjusted to reach a starting baseline between 10 and 25 s.
Clinical Symptom Evaluation
Positive and negative symptoms were evaluated by two experienced psychiatrists using the Scale for the Assessment of Positive Symptoms (SAPS) and the Scale for the Assessment of Negative Symptoms (SANS) scores. All schizophrenic patients were receiving drug treatment (ranging from 2 days to 30 years), including Olanzapine, Clozapine, Quetiapine, Aripiprazole, Risperidone, and Sodium Valproate. For antipsychotic dosage calculation, all drug doses were converted to Olanzapine equivalents.
Statistics
Data are presented as mean ± SEM. The inter-group differences were analyzed using the two-sample t-test and P < 0.05 was considered as statistically significant. SPSS 16.0 software (SPSS Co., Chicago, IL) was used for all analyses.
Results and Discussion
Diminished Cortical Plasticity in Schizophrenia
We recorded stable baseline MEPs (Fig. 1) and then applied the potentiation protocol (10 Hz for 10 min) to healthy individuals. Repetitive 10-Hz TMS stimulation of the motor cortex rapidly potentiated MEPs in the control group (n = 21) lasting for at least 30 min, but not in the schizophrenic group (effective n = 26) at all time-points measured (Fig. 1C). We also noted that the schizophrenic group exhibited more variation in the MEP peaks (data not shown), and 21 patients were excluded from this dataset due to failure to obtain a stable baseline.
Impaired Motor Skill Learning is Correlated with Altered Cortical Plasticity
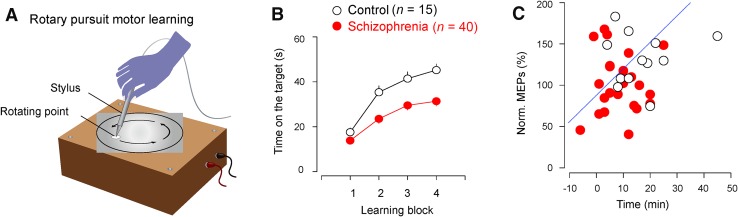
We then used a motor rotary pursuit task to create learning curves for both healthy controls and patients (Fig. 2A). We found that the schizophrenia group learned more slowly than the control group (Fig. 2B), suggesting that motor cortical functioning is impaired in schizophrenia. The motor cortical plasticity was positively correlated with the motor learning improvement at the same time points in both the control and schizophrenia groups (Fig. 2C).
Fig. 2.
Impaired motor learning in schizophrenia. A In the rotary pursuit motor learning task, participants attempted to trace a point on a rotating plate with a probe. Each block lasted for 60 s (at 10-min intervals) and the time with the probe on the target point was recorded. B The schizophrenia group performed worse during the rotary pursuit learning task (P < 0.01). C Correlation between cortical plasticity and motor learning improvement in control and patient groups (Pearson’s r = −0.366, adjusted r 2 = 0.109).
Altered Cortical Plasticity is Correlated with Clinical Symptoms
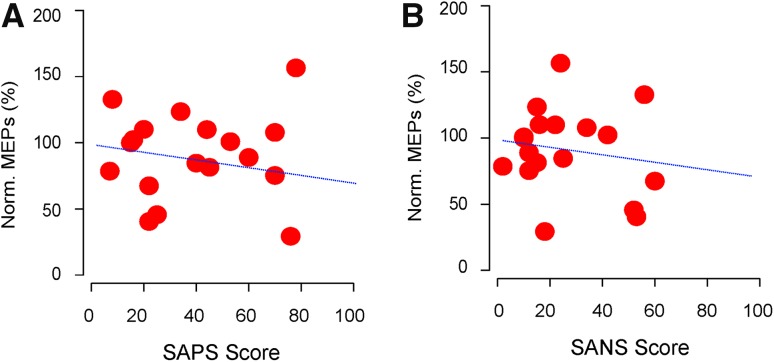
We found that the plastic changes induced by the 10-Hz protocol were negatively correlated with the SAPS scores (Fig. 3A), and even more negatively correlated with the SANS scores (Fig. 3B), suggesting that there might be some shared mechanisms underlying the impaired cortical plasticity and the symptoms.
Fig. 3.
Altered motor cortical plasticity is correlated with symptoms of schizophrenia (n = 26 patients). A Correlation between cortical plasticity and SAPS scores (Pearson’s r = 0.032, adjusted r 2 = −0.06). B Correlation between cortical plasticity and SANS scores (Pearson’s r = −0.16, adjusted r 2 = −0.03).
Impact of History and Antipsychotic Dosage on Cortical Plasticity
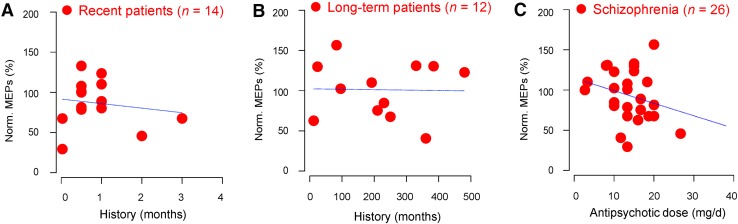
We then investigated whether disease history or antipsychotic dosage affects the cortical plasticity of schizophrenic patients. We found no correlation between cortical plasticity and disease history in both recently-admitted (Fig. 4A) and long-term schizophrenic patients (Fig. 4B). On the other hand, the equivalent antipsychotic dosage was weakly negatively correlated with cortical plasticity (Fig. 4C).
Fig. 4.
Effects of disease history and antipsychotic use on cortical plasticity. A and B No correlation between cortical plasticity and disease history in recently-admitted (A, Pearson’s r = −0.2, adjusted r 2 = −0.05) and long-term schizophrenia patients (B, Pearson’s r = −0.02, adjusted r 2 = −0.1). C Weak correlation between cortical plasticity and equivalent antipsychotic dosage (Pearson’s r = −0.257, adjusted r 2 = 0.027).
Schizophrenia is associated with altered brain plasticity [3, 4, 10, 11]. The common mechanisms are N-methyl-D-aspartate receptor deficiency [12], aberrant synaptic connections [13], disrupted inhibitory circuits [10, 14], and dysregulated dopaminergic signaling [15]. When probed with non-invasive brain stimulation, deficits have been found in the associated potentiation induced by paired associative stimulation [5], direct MEP depression induced by low-frequency rTMS or continuous TBS [16, 17], as well as plasticity induced by transcranial direct-current stimulation [18–20]. In the present study, we further report that the motor cortico-striatal pathway potentiation induced by high-frequency rTMS is impaired in schizophrenia. In addition, this is accompanied by altered motor skill learning in these patients.
The impaired plasticity was associated with clinical symptoms. One possible explanation is that motor cortex plasticity, motor learning, and positive/negative symptoms all originate from dysregulated dopaminergic signaling. This suggests that the impaired cortical plasticity and functioning is general across other cortical regions, given the widespread nature of cortical dopaminergic fibers. In future, it will be interesting to investigate this possibility using combined rTMS and EEG recording, to probe the cortical plasticity in other areas, for instance. It will also be interesting to determine whether the cortical plasticity is restored in certain patients (e.g. those with negative symptoms) receiving dopaminergic agonists.
In summary, here we present behavioral and neurophysiological evidence that schizophrenic patients have impaired motor cortex plasticity and skill learning, which might be due to disrupted dopamine signaling.
Acknowledgements
This work was supported by the Program of Medical Science of Ningbo Municipality, Zhejiang Province, China (2013A23), the Ningbo Municipal Innovation Team of Life Science and Health, Zhejiang Province, China (2015C110026), and the National Natural Science Foundation of China (81501164 and 81611130224). We thank our laboratory members for their support.
Footnotes
Dongsheng Zhou, Feng Pang, Shiyan Liu, Ying Shen, Lingjiang Liu and Zezhong Fang have contributed equally to this work.
Contributor Information
Chuang Wang, Email: wangchuang@nbu.edu.cn.
Zhenyu Hu, Email: hzy86690952@163.com.
Ti-Fei Yuan, Email: ytf0707@126.com.
References
- 1.Voineskos D, Rogasch NC, Rajji TK, Fitzgerald PB, Daskalakis ZJ. A review of evidence linking disrupted neural plasticity to schizophrenia. Can J Psychiatry. 2013;58:86–92. doi: 10.1177/070674371305800205. [DOI] [PubMed] [Google Scholar]
- 2.Hasan A, Falkai P, Wobrock T. Transcranial brain stimulation in schizophrenia: targeting cortical excitability, connectivity and plasticity. Curr Med Chem. 2013;20:405–413. [PubMed] [Google Scholar]
- 3.Frost DO, Tamminga CA, Medoff DR, Caviness V, Innocenti G, Carpenter WT. Neuroplasticity and schizophrenia. Biol Psychiatry. 2004;56:540–543. doi: 10.1016/j.biopsych.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 4.McCullumsmith RE. Evidence for schizophrenia as a disorder of neuroplasticity. Am J Psychiatry. 2015;172:312–313. doi: 10.1176/appi.ajp.2015.15010069. [DOI] [PubMed] [Google Scholar]
- 5.Frantseva MV, Fitzgerald PB, Chen R, Moller B, Daigle M, Daskalakis ZJ. Evidence for impaired long-term potentiation in schizophrenia and its relationship to motor skill learning. Cereb Cortex. 2008;18:990–996. doi: 10.1093/cercor/bhm151. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz BL, Rosse RB, Veazey C, Deutsch SI. Impaired motor skill learning in schizophrenia: implications for corticostriatal dysfunction. Biol Psychiatry. 1996;39:241–248. doi: 10.1016/0006-3223(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 7.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 8.Shen Y, Cao X, Shan C, Dai W, Yuan TF. Heroin addiction impairs human cortical plasticity. Biol Psychiatry. 2016 doi: 10.1016/j.biopsych.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Cao X, Tan T, Shan C, Wang Y, Pan J, et al. 10-Hz repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex reduces heroin cue craving in long-term addicts. Biol Psychiatry. 2016;80:e13–14. doi: 10.1016/j.biopsych.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Tang Y, Zhang T, Edelman B, Zeng B, Zhao S, Li C, et al. Prolonged cortical silent period among drug-naive subjects at ultra-high risk of psychosis. Schizophr Res. 2014;160:124–130. doi: 10.1016/j.schres.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Strube W, Bunse T, Nitsche MA, Wobrock T, Aborowa R, Misewitsch K, et al. Smoking restores impaired LTD-like plasticity in schizophrenia: a transcranial direct current stimulation study. Neuropsychopharmacology. 2015;40:822–830. doi: 10.1038/npp.2014.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahn RS, Sommer IE. The neurobiology and treatment of first-episode schizophrenia. Mol Psychiatry. 2015;20:84–97. doi: 10.1038/mp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridgman AC, Barr MS, Goodman MS, Chen R, Rajji TK, Daskalakis ZJ, et al. Deficits in GABAA receptor function and working memory in non-smokers with schizophrenia. Schizophr Res. 2016;171:125–130. doi: 10.1016/j.schres.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Remington G. Alterations of dopamine and serotonin transmission in schizophrenia. Prog Brain Res. 2008;172:117–140. doi: 10.1016/S0079-6123(08)00906-0. [DOI] [PubMed] [Google Scholar]
- 16.Fitzgerald PB, Brown TL, Marston NA, Oxley T, De Castella A, Daskalakis ZJ, et al. Reduced plastic brain responses in schizophrenia: a transcranial magnetic stimulation study. Schizophr Res. 2004;71:17–26. doi: 10.1016/j.schres.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 17.Hasan A, Brinkmann C, Strube W, Palm U, Malchow B, Rothwell JC, et al. Investigations of motor-cortex cortical plasticity following facilitatory and inhibitory transcranial theta-burst stimulation in schizophrenia: a proof-of-concept study. J Psychiatr Res. 2015;61:196–204. doi: 10.1016/j.jpsychires.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Hasan A, Nitsche MA, Rein B, Schneider-Axmann T, Guse B, Gruber O, et al. Dysfunctional long-term potentiation-like plasticity in schizophrenia revealed by transcranial direct current stimulation. Behav Brain Res. 2011;224:15–22. doi: 10.1016/j.bbr.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 19.Hasan A, Nitsche MA, Herrmann M, Schneider-Axmann T, Marshall L, Gruber O, et al. Impaired long-term depression in schizophrenia: a cathodal tDCS pilot study. Brain Stimul. 2012;5:475–483. doi: 10.1016/j.brs.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Hasan A, Bergener T, Nitsche MA, Strube W, Bunse T, Falkai P, et al. Impairments of motor-cortex responses to unilateral and bilateral direct current stimulation in schizophrenia. Front Psychiatry. 2013;4:121. doi: 10.3389/fpsyt.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]