Abstract
Gastrodin, the major component isolated from the rhizome of the Chinese traditional medicinal herb Gastrodia elata (“Tianma”), has a long history in the treatment of epilepsy and other neurological disorders. However, the molecular mechanisms are not clear. Here, we found that gastrodin ameliorated pentylenetetrazole (PTZ)-induced epileptic seizures with improvement of the electroencephalographic pattern in mice. Further studies demonstrated that gastrodin decreased the levels of the pro-inflammatory cytokines interleukin-1β and tumor necrosis factor-α while increasing interleukin-10, an anti-inflammatory cytokine in the brain. Furthermore, gastrodin attenuated the PTZ-induced microglial activation along with inhibition of mitogen-activated protein kinases, cAMP response element binding protein, and NF-κB. Our data suggest that gastrodin attenuates seizures by modulating the mitogen-activated protein kinase-associated inflammatory responses.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0084-z) contains supplementary material, which is available to authorized users.
Keywords: Gastrodin, Epilepsy, IL-1β, TNF-α, IL-10, MAPK, CREB, NF-κB
Introduction
Epilepsy is one of the most common chronic brain disorders, characterized by frequently repeated seizures [1] as well as emotional and cognitive dysfunctions [2, 3]. Around 50 million people worldwide suffer from epilepsy [4]. It is well known that transient aberrant hyper-synchronous electrical activity of brain networks caused by an imbalance of excitation and inhibition is the major cause [5]. Epilepsy seriously affects the quality of life and imposes a huge social and financial burden [6]. A role of inflammation in its development has been reported [7, 8]—multiple pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) and interleukin-1 beta (IL-1β) are increased at the mRNA and protein levels in both patients and rat models of epilepsy [9–14].
A lower seizure threshold accompanies the over-expression of TNF-α in transgenic mice [15]. A single nucleotide polymorphism of IL-1β has been associated with temporal lobe epilepsy [16], and knock-down of IL-1β signaling not only delays the occurrence of stage 5 seizures and prevents seizure generalization, but also increases the threshold for after-discharge induction [17]. Meanwhile, some anti-inflammatory cytokines such as IL-10 decrease in the mouse brain after convulsive seizures are elicited [18, 19]. Clinical data have shown that the level of IL-10 is significantly decreased in patients with incurable epilepsy [20]. These data suggest that inflammatory factors play key roles in epileptogenesis. Hence, regulating the associated inflammatory factors might be a therapeutic strategy for epilepsy.
Gastrodin is a traditional Chinese herbal medicine which has long been used for the treatment of neurological disorders and has been shown to be effective for neurodegenerative diseases [21–24], pain [25, 26], and mental illness [27]. It has been reported that gastrodin protects cultured hippocampal neurons from Aβ-induced neurotoxicity by regulating the extracellular signal-regulated kinase 1/2 (ERK1/2)–Nrf2 pathway [28]. Gastrodin also inhibits the apoptosis of dopaminergic neurons in a model of Parkinson’s disease [21]. Furthermore, the neuroprotective effects of gastrodin have been confirmed in the rat model of transient middle cerebral arterial occlusion and oxygen/glucose deprivation as well as in rat hippocampal neurons with glutamate-induced injury [29]. However, the effect of gastrodin on seizures and the underlying mechanisms are not clear.
In this study, we induced seizures in mice by intraperitoneal injection of pentylenetetrazole (PTZ) [30, 31] and studied the effects of gastrodin on the induced seizure behaviors. We also investigated the mechanisms underlying these effects.
Materials and Methods
Antibodies and Chemicals
Gastrodin and PTZ from Sigma-Aldrich (St. Louis, MO) were dissolved in distilled water. Mouse monoclonal antibodies against IL-1β, TNF-α, ERK 1/2, phospho-ERK1/2, c-Jun N-terminal kinase (JNK), phospho-JNK, p38 MAPK (p38), and phospho-p38 were from Cell Signaling Technology (Beverly, MA). Rabbit polyclonal antibodies against IL-10, phospho-IκB-α, phospho-CREB, CD11b, and β-actin were from Abcam (Cambridge Science Park, Cambridge, UK). All antibodies were diluted at 1:1000 for Western blotting, and at 1:200 for immunofluorescence.
Animals
Healthy adult male C57BL/6 mice were purchased from the Experimental Animal Center of Tongji Medical College, Huazhong University of Science and Technology. All were housed with food and water accessible ad libitum. All animal experiments were performed according to the ‘Policies on the Use of Animals and Humans in Neuroscience Research’ revised and approved by the Society for Neuroscience in 1995. Mice were kept under a 12-h light/dark cycle with lights on from 07:00 to 19:00 and for further studies the mice were sacrificed after isoflurane inhalation anesthesia, (2%–3% for induction and 1.5%–2% for maintenance).
PTZ Model
The protocol for acute PTZ used the method of Dhir [32]. The mice were kindled by intraperitoneal (i.p.) injection of PTZ (90 mg/kg, 0.1 mL/100 g body weight). During 2–30 min after the injection, mice that showed 3 consecutive seizures reaching Stage 4 or above, meeting the criteria of the model, were used as the model mice. Up to 24 h after injection, the model mice were randomly divided to receive no further treatment (Ctrl group), administration of the same volume (0.1 mL/100 g body weight, i.p.) of normal saline (EP-NS group), 50 mg/kg gastrodin (EP-GS 50 mg/kg group), 100 mg/kg gastrodin (EP-GS 100 mg/kg group), or 200 mg/kg gastrodin (EP-GS 200 mg/kg group). After another 2 h, PTZ (35 mg/kg) was injected into mice in the EP-NS group and the groups with the 3 doses of GS to re-induce seizures.
The seizure stages were evaluated as follows [32, 33]: Stage 0, no reaction; Stage 0.5, atypical behavior; Stage 1, generalized myoclonic jerks; Stage 2, atypical minimal seizures; Stage 3, minimal seizures without loss of rearing reflex; Stage 4, generalized seizures without tonic phase; and Stage 5, generalized tonic-clonic seizures.
Behavioral Test
We measured the latency from PTZ injection to myoclonic jerks, the first observable behavioral response, and the latency from PTZ injection to generalized tonic-clonic seizures. The behavioral changes were recorded for 30 min (n = 15). The seizure score was evaluated as follows [32, 33]: Score 0, no behavioral seizure; Score 1, myoclonic jerks; Score 2, Straub’s tail; Score 3, clonus. After the behavioral test, the mice were humanely decapitated under inhalation anesthesia for Western blotting and immunofluorescence.
Stereotaxic Surgery
PTZ model mice (n = 3–5) underwent stereotaxic surgery 2 weeks before the electroencephalogram (EEG) test. Electrodes made from polyimide-insulated stainless-steel wires (outside diameter, 100 or 75 μm; resistance, 0.87–1.50 Ω/cm) were implanted into the cortex. Surgical procedures were as described by Wu et al. [34]. Briefly, the isoflurane-inhalation anesthetized mouse was placed on a stereotaxic frame. After skin incision and exposure of the skull, three small holes (diameter >0.5 mm) were made with an automatic micromanipulator drill and a mini-drill bit. Two electrodes were placed into the temporal cortex (bregma, –1.1 mm; lateral, 1.8 mm; depth, 1.1 mm). Then the third electrode, the reference electrode, was placed into the contralateral cortex at a proper position near the parietal cortical recording site. After these electrodes were located at the desired sites, they were fixed and covered with acrylic. After the acrylic dried, mice were released from the stereotaxic frame and returned to the home cage.
EEG Recording
All mice fully recovered from the surgery and showed no neurological deficits. EEGs were recorded via amplifiers and extended head-stages (Model-300, AM Systems Inc., Carlsborg, WA). The head-stage was connected to the electrode via wires. EEG signals were recorded in the frequency range 0.01–1,000 Hz and amplified 1000–2000 times before digitization. EEG was monitored for 12 h. Data acquisition, storage, and analyses used Pclamp software (Molecular Devices, San Francisco, CA).
Western Blotting
The cortex was removed and samples were homogenized at a ratio of 9.0 mL buffer/1.0 g tissue in a buffer containing (in mmol/L): 10 Tris-HCl (pH 7.6), 50 NaF, 1 Na3VO4, 1 EDTA, 1 benzamidine, 1 phenylmethylsulfonylfluoride, and a mixture of protease inhibitors (2 mg/L each of aprotinin, leupeptin, and pepstatin A). The tissue homogenate was added to one-third volume of 4× sample buffer containing 200 mmol/L Tris-HCl (pH 7.6), 8% sodium dodecyl sulfate, and 40% glycerol, boiled in a water bath for 10 min, and then sonicated for 5 s. The lysate was centrifuged at 12,000 g for 15 min at 25 °C. The supernatant was stored at −80 °C for Western blotting analysis.
Immunofluorescence
For immunofluorescence, mice were deeply anesthetized with isoflurane inhalation and sacrificed by intracardiac injection of 0.9% NaCl at 37 °C followed by fixative (4% paraformaldehyde in 0.1 mol/L PB, pH 7.4, at 4 °C). The brain was removed, post-fixed in the same fixative overnight, and then cut into sections on a vibratome (S100, TPI; Leica, Nussloch, Germany). Thirty-micrometer sections were cut in the coronal plane near the positions of the electrodes, immersed in 0.1 mol/L PB (pH 7.4), washed three times for 10 min each in the same buffer, and treated with 0.3% hydrogen peroxide-0.5% Triton-X 100-PBS for 20 min at room temperature to quench endogenous peroxidases and to rupture membranes. Subsequently, sections were incubated in 3% normal goat serum in PBS for 30 min followed by incubation with primary antibodies against IL-1β and CD11b overnight at 4 °C. Finally, Alexa Fluor546-conjugated and Alexa Fluor488-conjugated secondary antibodies (1:200, Invitrogen, Rockville, MD) were used for immunofluorescence. The images were visualized under a laser confocal microscope (LSM510; Carl Zeiss, Inc., Thornwood, NY).
Statistical Analysis
Data are presented as mean ± SD, and were analyzed with SPSS software (Version 16.0; SPSS Inc., Chicago, IL). One-way ANOVA followed by the LSD post hoc test was used to determine statistical significance. Null hypotheses were rejected at the 0.05 level.
Results
Gastrodin Ameliorates the Intensity and Increases the Latency of Seizures as well as Improving the EEG Pattern
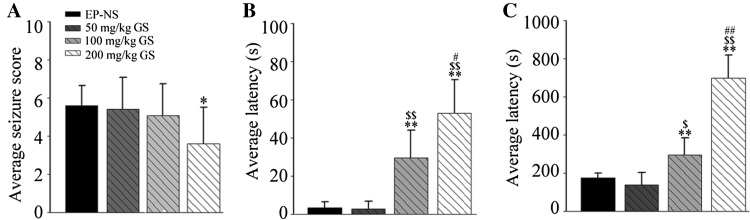
For ethological testing, we first established the acute seizure model in mice by i.p. injection of PTZ. Then we tested the effect of gastrodin on seizures using rating scales [32]. We found that 200 mg/kg gastrodin significantly reduced the intensity of seizures compared with the EP-NS group (Fig. 1A). Meanwhile, both the 100 mg/kg and 200 mg/kg EP-GS groups showed significantly longer latencies to reach the myoclonic jerk (Fig. 1B) and generalized tonic-clonic seizure stages (Fig. 1C) than the EP-NS and 50 mg/kg EP-GS groups. These data suggested that PTZ efficiently induces seizures and gastrodin alleviates them.
Fig. 1.
Gastrodin reduced the seizure score and increased the latency to seizures. A Average seizure scores (n = 15; *P < 0.05 vs EP-NS group). B, C Average latency to myoclonic jerks (B) and generalized tonic-clonic seizures (C) (n = 15; **P < 0.01 vs EP-NS group; $ P < 0.05, $$ P < 0.01 vs 50 mg/kg EP-GS group; # P < 0.05, ## P < 0.01 vs 100 mg/kg EP-GS group).
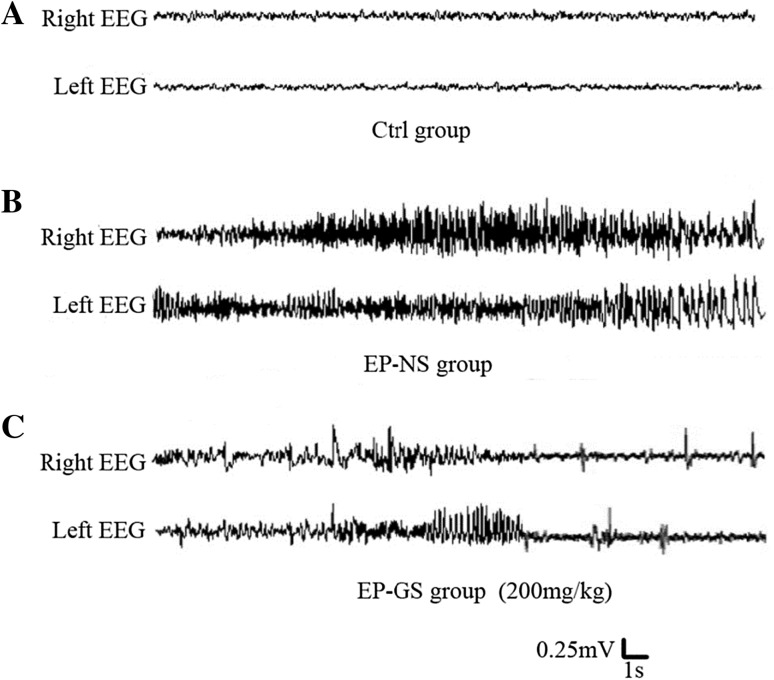
To further verify whether the increased latency and the decreased intensity in the EP-GS groups were due to reduced seizures, we examined the EEG in freely-moving PTZ model mice. The EP-NS group displayed ictal polyspike activity accompanied by stereotypical seizure behaviors with a typical duration (Fig. 2B). In the EP-GS 200 mg/kg group, the frequent generalized spike-and-wave discharges became infrequent and progressively stabilized at an average of 5–10 discharges/h. The frequency within a discharge ranged from 2.3 to 15 Hz, most discharges occurring at 5–6 Hz (Fig. 2C). Unlike the ictal polyspike activity in the EP-NS group, the spike-and-wave discharges in the EP-GS 200 mg/kg group were much shorter in duration (4–5 s) and did not involve a behavioral component. Such brief spike-and-wave discharges did not occur in the control group (Fig. 2A). These data showed that gastrodin attenuates PTZ-induced seizures by inhibiting the abnormal synchronous discharges.
Fig. 2.
Representative EEG recordings. A Normal EEG from an awake mouse with saline injection. B EEG pattern from a model mouse during a persistent ictal episode showing polyspike discharges in both hemispheres. C Gradually stabilizing EEG pattern from a model mouse given gastrodin (200 mg/kg) followed by injection of PTZ (35 mg/kg), which induced a re-seizure.
Gastrodin Modulates Inflammatory Responses and Inhibits IL-1β Expression in CD11b+ Microglia in PTZ Model Mice
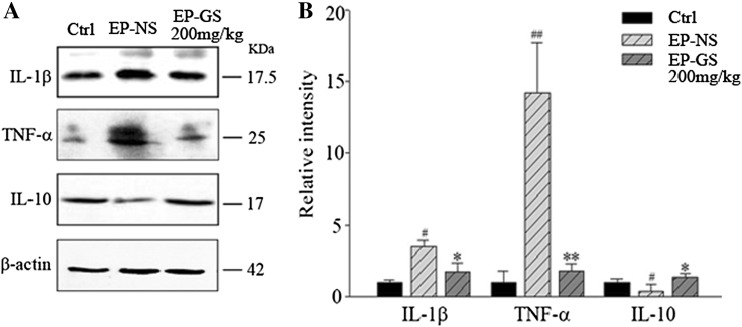
Pro-inflammatory and anti-inflammatory cytokines have been implicated in experimental seizure models and in clinical cases of epilepsy [35]. To explore the underlying protective mechanisms of gastrodin, we investigated whether it could modulate the expression of pro-inflammatory (IL-1β and TNF-α) and anti-inflammatory (IL-10) cytokines in the model mice. The protein levels of IL-1β and TNF-α were higher while IL-10 was lower in the EP-NS group than in the control group. However, the protein levels of IL-1β and TNF-α were lower, while the level of IL-10 was higher in the EP-GS group than in the EP-NS group (Fig. 3A, B). These data suggested that gastrodin alleviates PTZ-induced neuroinflammation in the brain.
Fig. 3.
Gastrodin inhibited the expression of interleukin (IL)-1β and tumor necrosis factor α (TNF-α), and increased the expression of IL-10 in the cortex of the pentylenetetrazole mouse model. A Western blots and B quantitative analysis of bands in A (n = 3; # P < 0.05, ## P < 0.01 vs Ctrl; * P < 0.05, ** P < 0.01 vs EP-NS).
As IL-1β is primarily synthesized by activated microglia [36, 37], glia-mediated inflammation may play a role in the pathogenesis of seizures [38]. We found CD11b+ microglia cells in the temporal lobe. Double immunofluorescence staining confirmed that IL-1β co-stained with activated CD11b+ microglia in the EP-NS group, while the number of IL-1β-positive cells noticeably decreased in the EP-GS group (Fig. 4). These data suggested that gastrodin reduces IL-1β in the microglia of the PTZ model mice.
Fig. 4.
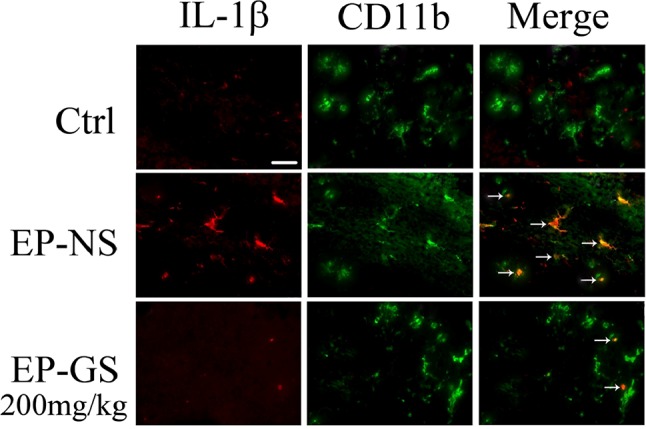
Gastrodin inhibited IL-1β expression in CD11b+ microglia in the temporal lobe of PTZ model mice. In the EP-NS group, IL-1β was found exclusively in activated CD11b+ microglia in cortex (arrows). IL-1β expression was remarkably decreased in the EP-GS group, and a few IL-1β+ cells did not overlap with CD11b+ cells (arrows). Scale bar, 20 µm.
Gastrodin Inhibits MAP Kinase Phosphatase-1 (MKP-1)/Mitogen-Activated Protein Kinase (MAPK) Signaling in PTZ Model Mice
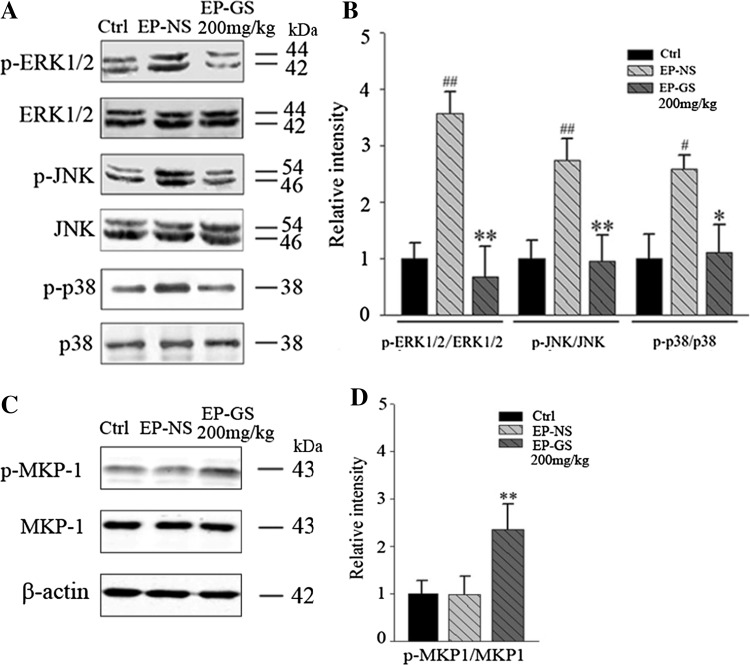
Since inflammatory responses involve the activation of MAPK [39], we examined the alterations of MAPK signaling (ERK1/2, JNK, and p38) by Western blot. We found that the phosphorylation of ERK1/2, JNK, and p38 was higher in the EP-NS group than in the control group, and gastrodin treatment reversed this effect (Fig. 5A, B). No difference was detected in the total protein levels of ERK1/2, JNK, and p38 among the three groups (Fig. 5A, B). These data suggested that gastrodin attenuates the PTZ-induced activation of the MAPK signaling pathway in the brain.
Fig. 5.
Gastrodin inhibited MAP kinase phosphatase-1 (MKP-1)/mitogen-activated protein kinase signaling in PTZ model mice. A and C, Western blots; B and D, quantitative analysis (n = 3; # P < 0.05, # P < 0.01 vs Ctrl; * P < 0.05, ** P < 0.01 vs EP-NS group).
MKP-1 can dephosphorylate MAPK to inhibit the activity of ERK, JNK, and p38 in the stress reaction [39, 40], and thus participates in the regulation of the inflammatory reaction, so we also measured MKP-1. The results showed that gastrodin treatment increased the phosphorylation level of MKP-1 compared with the EP-NS group (Fig. 5C, D), suggesting the involvement of MKP-1 activation in the gastrodin-induced inhibition of MAPK.
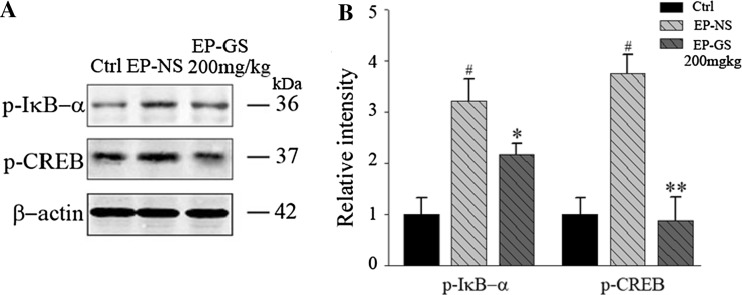
Gastrodin Suppresses PTZ-Induced Phosphorylation of IκB-α and CREB
Inflammatory signals induce CREB phosphorylation [41, 42], which further inactivates NF-κB and thus inhibits the inflammatory response [43], while phosphorylation-dependent IκB-α degradation activates NF-κB. Therefore, we measured the phosphorylation levels of CREB and IκB-α and found that PTZ treatment remarkably increased these levels, while gastrodin attenuated them (Fig. 6). These data suggested the involvement of IκB-α and CREB in PTZ-induced seizures and the anti-inflammatory effects of gastrodin.
Fig. 6.
Gastrodin suppressed the PTZ-induced phosphorylation of IκB-α and CREB, as revealed by Western blotting (n = 3; # P < 0.05 vs Ctrl group; * P < 0.05, ** P < 0.01 vs EP-NS group).
Gastrodin Alone Did Not Influence the Expression of IL-1β, p-p38, p-MKP-1, p-IκB-α, and p-CREB in Normal Mice
We further investigated whether gastrodin had an effect on the above key pro-inflammatory factors and kinases. The results showed that gastrodin did not change the expression of IL-1β and the phosphorylation levels of p38, MKP-1, IκB-α, and CREB (Fig. S1). These data suggested that gastrodin plays a role only in the stress state.
Discussion
Epilepsy is a group of central nervous system diseases with characteristic seizures, repeatability and muscle rigidity [44, 45]. Abnormal synchronous discharge of neurons and its spread lead to the occurrence and development of epilepsy [46]. Gastrodin, a constituent of the traditional Chinese herb “Tianma”, has a long history as an anti-epilepsy drug. PTZ-induced seizures in mice provide a powerful animal model in which to search for new antiepileptic drugs [47, 48].
Clinical effectiveness is fundamental for anti-epilepsy drugs, including preventing seizures and reducing their frequency and intensity. In the present study, we demonstrated that gastrodin improved seizures by reducing their intensity and extending the latency of myoclonic jerks and/or generalized tonic-clonic seizures in a dose-dependent manner. EEG provides the highest precision in estimating the location and boundaries of an epileptogenic zone [49]. Meanwhile, electrophysiological analysis revealed that the persistent polyspike discharge induced by PTZ was converted to incidental polyspike discharge by gastrodin. Our data exhibited a positive action of gastrodin on both the behavioral presentation and electrophysiology of epilepsy.
Epilepsy is closely associated with an imbalance between pro-inflammatory and anti-inflammatory responses [50–53]. Here, we found that gastrodin inhibited the expression of IL-1β and TNF-α, with a simultaneous increase of IL-10. IL-1β and TNF-α are pro-inflammatory cytokines released by activated microglia during brain inflammation, while IL-10, an anti-inflammatory cytokine, suppresses the functions of T lymphocytes and mononuclear cells against many pro-inflammatory cytokines [54–56]. In the brain, a number of stimuli such as PTZ, Aβ, and traumatic injury stimulate the production of IL-1β and TNF-α [57–59]. Excessive pro-inflammatory cytokines from activated microglia have a toxic effect on neurons, while IL-10 modulates the ischemia-induced Ca2+ flow into neurons [60], reduces Aβ plaque formation, and ameliorates the cognitive behavior in amyloid β precursor protein transgenic mice [61]. Our data suggested that gastrodin attenuates PTZ-induced seizures by modulating microglia-mediated inflammatory responses.
Dysregulation of glial functions may cause seizures or promote epilepsy [53]. Due to the lack of evident electrical excitability and synapses, glial cells exhibit electrophysiological ‘silence’ in the brain. Recently, accumulating evidence has shown that activated glia release cytokines, which induce transcriptional and post-transcriptional signaling in the glial cell itself and in nearby cells. For example, microglia release IL-1β [62]. From our results, double-labeling of IL-1β and CD11b revealed that the great majority of IL-1β-immunoreactive cells are microglia, as identified by CD11b immunoreactivity in the temporal lobe from the seizure groups. Conversely, CD11b-positive microglia had decreased IL-1β immunoreactivity in the EP-GS group. These data suggested that gastrodin modulates the functions of microglia.
Inflammation, ischemia, apoptosis, and other pathological mechanisms are associated with regulation of the MAPK signaling pathway [63–65]. MAPK, consisting of three major subgroups, ERK1/2, JNK, and p38, plays a key role in transducing various extracellular signals to the nucleus and regulating cell growth and differentiation. The expression of pro-inflammatory and anti-inflammatory cytokines is under the control of various transcription factors, which are also regulated by the MAPK signaling pathway [66]. Our results showed that gastrodin inhibited the MAPK signaling pathway by reducing ERK1/2, JNK, and p38 phosphorylation in vivo. This implied that gastrodin enhances ERK1/2, JNK, and p38 dephosphorylation. MKP-1, another member of the MAPK family, is capable of dephosphorylating and inactivating various members of this family [66, 67]. It is known that MKP-1-deficiency enhances the phosphorylation of p38 and JNK [40]. We found that the phosphorylation level of MKP-1 increased in mice given gastrodin. These data suggested that gastrodin blocks the MAPK signal pathway via enhancing MKP-1 phosphorylation.
NF-κB and CREB play important roles in the transcriptional regulation of inflammatory mediators [68–71]. CREB is the physiological substrate of MAPK and stress-activated protein kinase-1, which is activated by ERK1/2 and p38 MAPK-mediated signaling in response to various stimuli. We found that CREB activation was markedly stimulated by PTZ, and this activation was significantly inhibited by treatment with gastrodin. NF-κB is maintained in the cytoplasm by binding to IκB-α. Activation of NF-κB via phosphorylation of its endogenous inhibitor IκB-α results in the release and nuclear translocation of active NF-κB [64]. These data further support the role of inflammatory responses in the action of gastrodin. Clinical anti-inflammatory treatment can control seizures that are resistant to conventional antiepileptic drugs in some syndromes [72, 73]. Our data suggested that gastrodin is a promising candidate for treating epilepsy.
Taken together, we found here that gastrodin attenuates PTZ-induced seizures via mechanisms involving the modulation of MAPK-associated inflammatory responses in microglia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0084-z) contains supplementary material, which is available to authorized users.
Contributor Information
Hua Wang, Email: wanghua6909@163.com.
Min Qu, Email: minqu2977@163.com.
References
- 1.Chang BS, Lowenstein DH. Epilepsy. N Engl J Med. 2003;349:1257–1266. doi: 10.1056/NEJMra022308. [DOI] [PubMed] [Google Scholar]
- 2.Realmuto S, Zummo L, Cerami C, Agro L, Dodich A, Canessa N, et al. Social cognition dysfunctions in patients with epilepsy: Evidence from patients with temporal lobe and idiopathic generalized epilepsies. Epilepsy Behav. 2015;47:98–103. doi: 10.1016/j.yebeh.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Austin JK, Dunn DW. Progressive behavioral changes in children with epilepsy. Prog Brain Res. 2002;135:419–427. doi: 10.1016/S0079-6123(02)35039-8. [DOI] [PubMed] [Google Scholar]
- 4.Thurman DJ, Beghi E, Begley CE, Berg AT, Buchhalter JR, Ding D, et al. Standards for epidemiologic studies and surveillance of epilepsy. Epilepsia. 2011;52(Suppl 7):2–26. doi: 10.1111/j.1528-1167.2011.03121.x. [DOI] [PubMed] [Google Scholar]
- 5.Ozuna J. Seizure disorders and epilepsy. Lippincotts Prim Care Pract. 2000;4:608–618. [PubMed] [Google Scholar]
- 6.Amir M, Roziner I, Knoll A, Neufeld MY. Self-efficacy and social support as mediators in the relation between disease severity and quality of life in patients with epilepsy. Epilepsia. 1999;40:216–224. doi: 10.1111/j.1528-1157.1999.tb02078.x. [DOI] [PubMed] [Google Scholar]
- 7.Uludag IF, Duksal T, Tiftikcioglu BI, Zorlu Y, Ozkaya F, Kirkali G. IL-1beta, IL-6 and IL1Ra levels in temporal lobe epilepsy. Seizure. 2015;26:22–25. doi: 10.1016/j.seizure.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Z, Peng J, Yang L, Kong H, Yin F. Interleukin-1beta plays a role in the pathogenesis of mesial temporal lobe epilepsy through the PI3K/Akt/mTOR signaling pathway in hippocampal neurons. J Neuroimmunol. 2015;282:110–117. doi: 10.1016/j.jneuroim.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 9.De Simoni MG, Perego C, Ravizza T, Moneta D, Conti M, Marchesi F, et al. Inflammatory cytokines and related genes are induced in the rat hippocampus by limbic status epilepticus. Eur J Neurosci. 2000;12:2623–2633. doi: 10.1046/j.1460-9568.2000.00140.x. [DOI] [PubMed] [Google Scholar]
- 10.Minami M, Kuraishi Y, Satoh M. Effects of kainic acid on messenger RNA levels of IL-1 beta, IL-6, TNF alpha and LIF in the rat brain. Biochem Biophys Res Commun. 1991;176:593–598. doi: 10.1016/S0006-291X(05)80225-6. [DOI] [PubMed] [Google Scholar]
- 11.Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)1beta, IL-1alpha, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. 2000;47:571–574. doi: 10.1002/1531-8249(200005)47:5<571::AID-ANA3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 12.Rijkers K, Majoie HJ, Hoogland G, Kenis G, De Baets M, Vles JS. The role of interleukin-1 in seizures and epilepsy: a critical review. Exp Neurol. 2009;216:258–271. doi: 10.1016/j.expneurol.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Bauer S, Nowak M, Norwood B, Tackenberg B, Rosenow F, et al. Cytokines and epilepsy. Seizure. 2011;20:249–256. doi: 10.1016/j.seizure.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Feng B, Chen Z. Generation of febrile seizures and subsequent epileptogenesis. Neurosci Bull. 2016;32:481–492. doi: 10.1007/s12264-016-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Probert L, Akassoglou K, Kassiotis G, Pasparakis M, Alexopoulou L, Kollias G. TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J Neuroimmunol. 1997;72:137–141. doi: 10.1016/S0165-5728(96)00184-1. [DOI] [PubMed] [Google Scholar]
- 16.Kauffman MA, Moron DG, Consalvo D, Bello R, Kochen S. Association study between interleukin 1 beta gene and epileptic disorders: a HuGe review and meta-analysis. Genet Med. 2008;10:83–88. doi: 10.1097/GIM.0b013e318161317c. [DOI] [PubMed] [Google Scholar]
- 17.Ravizza T, Noe F, Zardoni D, Vaghi V, Sifringer M, Vezzani A. Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol Dis. 2008;31:327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 18.Rao RS, Prakash A, Medhi B. Role of different cytokines and seizure susceptibility: a new dimension towards epilepsy research. Indian J Exp Biol. 2009;47:625–634. [PubMed] [Google Scholar]
- 19.Majoie HJ, Rijkers K, Berfelo MW, Hulsman JA, Myint A, Schwarz M, et al. Vagus nerve stimulation in refractory epilepsy: effects on pro- and anti-inflammatory cytokines in peripheral blood. Neuroimmunomodulation. 2011;18:52–56. doi: 10.1159/000315530. [DOI] [PubMed] [Google Scholar]
- 20.Choi J, Min HJ, Shin JS. Increased levels of HMGB1 and pro-inflammatory cytokines in children with febrile seizures. J Neuroinflammation. 2011;8:135. doi: 10.1186/1742-2094-8-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxin-induced Parkinson’s disease model. Evid Based Complement Alternat Med. 2013;2013:514095. doi: 10.1155/2013/514095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang XL, Xing GH, Hong B, Li XM, Zou Y, Zhang XJ, et al. Gastrodin prevents motor deficits and oxidative stress in the MPTP mouse model of Parkinson’s disease: involvement of ERK1/2-Nrf2 signaling pathway. Life Sci. 2014;114:77–85. doi: 10.1016/j.lfs.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Chen PZ, Jiang HH, Wen B, Ren SC, Chen Y, Ji WG, et al. Gastrodin suppresses the amyloid beta-induced increase of spontaneous discharge in the entorhinal cortex of rats. Neural Plast. 2014;2014:320937. doi: 10.1155/2014/320937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu Y, Li C, Shen W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology. 2014;34:370–377. doi: 10.1111/neup.12115. [DOI] [PubMed] [Google Scholar]
- 25.Qiu F, Liu TT, Qu ZW, Qiu CY, Yang Z, Hu WP. Gastrodin inhibits the activity of acid-sensing ion channels in rat primary sensory neurons. Eur J Pharmacol. 2014;731:50–57. doi: 10.1016/j.ejphar.2014.02.044. [DOI] [PubMed] [Google Scholar]
- 26.Sun W, Miao B, Wang XC, Duan JH, Ye X, Han WJ, et al. Gastrodin inhibits allodynia and hyperalgesia in painful diabetic neuropathy rats by decreasing excitability of nociceptive primary sensory neurons. PLoS One. 2012;7:e39647. doi: 10.1371/journal.pone.0039647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng Z, Wang H, Zhang R, Chen Y, Xue F, Nie H, et al. Gastrodin ameliorates anxiety-like behaviors and inhibits IL-1beta level and p38 MAPK phosphorylation of hippocampus in the rat model of posttraumatic stress disorder. Physiol Res. 2013;62:537–545. doi: 10.33549/physiolres.932507. [DOI] [PubMed] [Google Scholar]
- 28.Zhao X, Zou Y, Xu H, Fan L, Guo H, Li X, et al. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012;1482:13–21. doi: 10.1016/j.brainres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006;72:1359–1365. doi: 10.1055/s-2006-951709. [DOI] [PubMed] [Google Scholar]
- 30.De Deyn PP, D’Hooge R, Marescau B, Pei YQ. Chemical models of epilepsy with some reference to their applicability in the development of anticonvulsants. Epilepsy Res. 1992;12:87–110. doi: 10.1016/0920-1211(92)90030-W. [DOI] [PubMed] [Google Scholar]
- 31.Rubio C, Rubio-Osornio M, Retana-Marquez S, Veronica Custodio ML, Paz C. In vivo experimental models of epilepsy. Cent Nerv Syst Agents Med Chem. 2010;10:298–309. doi: 10.2174/187152410793429746. [DOI] [PubMed] [Google Scholar]
- 32.Dhir A. Pentylenetetrazol (PTZ) kindling model of epilepsy. Curr Protoc Neurosci 2012, Chapter 9: Unit9.37. doi: 10.1002/0471142301.ns0937s58. [DOI] [PubMed]
- 33.Velisek L, Kusa R, Kulovana M, Mares P. Excitatory amino acid antagonists and pentylenetetrazol-induced seizures during ontogenesis. I. The effects of 2-amino-7-phosphonoheptanoate. Life Sci. 1990;46:1349–1357. doi: 10.1016/0024-3205(90)90334-N. [DOI] [PubMed] [Google Scholar]
- 34.Wu C, Wais M, Sheppy E, del Campo M, Zhang L. A glue-based, screw-free method for implantation of intra-cranial electrodes in young mice. J Neurosci Methods. 2008;171:126–131. doi: 10.1016/j.jneumeth.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis N, Auvin S. Inflammation and epilepsy in the developing brain: clinical and experimental evidence. CNS Neurosci Ther. 2015;21:141–151. doi: 10.1111/cns.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clausen BH, Lambertsen KL, Meldgaard M, Finsen B. A quantitative in situ hybridization and polymerase chain reaction study of microglial-macrophage expression of interleukin-1beta mRNA following permanent middle cerebral artery occlusion in mice. Neuroscience. 2005;132:879–892. doi: 10.1016/j.neuroscience.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Davies CA, Loddick SA, Toulmond S, Stroemer RP, Hunt J, Rothwell NJ. The progression and topographic distribution of interleukin-1beta expression after permanent middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab. 1999;19:87–98. doi: 10.1097/00004647-199901000-00010. [DOI] [PubMed] [Google Scholar]
- 38.Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 2013;36:174–184. doi: 10.1016/j.tins.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Kim EK, Choi EJ. Compromised MAPK signaling in human diseases: an update. Arch Toxicol. 2015;89:867–882. doi: 10.1007/s00204-015-1472-2. [DOI] [PubMed] [Google Scholar]
- 40.Ye J, Ding M, Zhang X, Rojanasakul Y, Shi X. On the role of hydroxyl radical and the effect of tetrandrine on nuclear factor–kappaB activation by phorbol 12-myristate 13-acetate. Ann Clin Lab Sci. 2000;30:65–71. [PubMed] [Google Scholar]
- 41.Ravnskjaer K, Madiraju A, Montminy M. Role of the cAMP pathway in glucose and lipid metabolism. Handb Exp Pharmacol. 2016;233:29–49. doi: 10.1007/164_2015_32. [DOI] [PubMed] [Google Scholar]
- 42.Peng S, Yang X, Liu GJ, Zhang XQ, Wang GL, Sun HY. From the camp pathway to search the ketamine-related learning and memory. Eur Rev Med Pharmacol Sci. 2015;19:161–164. [PubMed] [Google Scholar]
- 43.McKay LI, Cidlowski JA. CBP (CREB binding protein) integrates NF-kappaB (nuclear factor-kappaB) and glucocorticoid receptor physical interactions and antagonism. Mol Endocrinol. 2000;14:1222–1234. doi: 10.1210/mend.14.8.0506. [DOI] [PubMed] [Google Scholar]
- 44.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 45.Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- 46.Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med 2015, 5. doi:10.1101/cshperspect.a022426. [DOI] [PMC free article] [PubMed]
- 47.Bialer M, White HS. Key factors in the discovery and development of new antiepileptic drugs. Nat Rev Drug Discov. 2010;9:68–82. doi: 10.1038/nrd2997. [DOI] [PubMed] [Google Scholar]
- 48.Loscher W. Critical review of current animal models of seizures and epilepsy used in the discovery and development of new antiepileptic drugs. Seizure. 2011;20:359–368. doi: 10.1016/j.seizure.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Jin B, So NK, Wang S. Advances of intracranial electroencephalography in localizing the epileptogenic zone. Neurosci Bull. 2016;32:493–500. doi: 10.1007/s12264-016-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friedman A, Dingledine R. Molecular cascades that mediate the influence of inflammation on epilepsy. Epilepsia. 2011;52(Suppl 3):33–39. doi: 10.1111/j.1528-1167.2011.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Legido A, Katsetos CD. Experimental studies in epilepsy: immunologic and inflammatory mechanisms. Semin Pediatr Neurol. 2014;21:197–206. doi: 10.1016/j.spen.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 52.de Lanerolle NC, Lee TS, Spencer DD. Astrocytes and epilepsy. Neurotherapeutics. 2010;7:424–438. doi: 10.1016/j.nurt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Zhang H, Ma H, Lu B, Li Y, Li J. Inhibitory effect of dietary n-3 polyunsaturated fatty acids to intestinal IL-15 expression is associated with reduction of TCRalphabeta+CD8alpha+CD8beta-intestinal intraepithelial lymphocytes. J Nutr Biochem. 2008;19:475–481. doi: 10.1016/j.jnutbio.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 55.Hale LP, Gottfried MR, Swidsinski A. Piroxicam treatment of IL-10-deficient mice enhances colonic epithelial apoptosis and mucosal exposure to intestinal bacteria. Inflamm Bowel Dis. 2005;11:1060–1069. doi: 10.1097/01.MIB.0000187582.90423.bc. [DOI] [PubMed] [Google Scholar]
- 56.Sydora BC, Macfarlane SM, Walker JW, Dmytrash AL, Churchill TA, Doyle J, et al. Epithelial barrier disruption allows nondisease-causing bacteria to initiate and sustain IBD in the IL-10 gene-deficient mouse. Inflamm Bowel Dis. 2007;13:947–954. doi: 10.1002/ibd.20155. [DOI] [PubMed] [Google Scholar]
- 57.Nam KN, Park YM, Jung HJ, Lee JY, Min BD, Park SU, et al. Anti-inflammatory effects of crocin and crocetin in rat brain microglial cells. Eur J Pharmacol. 2010;648:110–116. doi: 10.1016/j.ejphar.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 58.Szczepanik AM, Ringheim GE. IL-10 and glucocorticoids inhibit Abeta(1-42)- and lipopolysaccharide-induced pro-inflammatory cytokine and chemokine induction in the central nervous system. J Alzheimers Dis. 2003;5:105–117. doi: 10.3233/JAD-2003-5205. [DOI] [PubMed] [Google Scholar]
- 59.Plata-Salaman CR, Ilyin SE, Turrin NP, Gayle D, Flynn MC, Romanovitch AE, et al. Kindling modulates the IL-1beta system, TNF-alpha, TGF-beta1, and neuropeptide mRNAs in specific brain regions. Brain Res Mol Brain Res. 2000;75:248–258. doi: 10.1016/S0169-328X(99)00306-X. [DOI] [PubMed] [Google Scholar]
- 60.Tukhovskaya EA, Turovsky EA, Turovskaya MV, Levin SG, Murashev AN, Zinchenko VP, et al. Anti-inflammatory cytokine interleukin-10 increases resistance to brain ischemia through modulation of ischemia-induced intracellular Ca2+ response. Neurosci Lett. 2014;571:55–60. doi: 10.1016/j.neulet.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 61.Chakrabarty P, Li A, Ceballos-Diaz C, Eddy JA, Funk CC, Moore B, et al. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85:519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ravizza T, Gagliardi B, Noe F, Boer K, Aronica E, Vezzani A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: evidence from experimental models and human temporal lobe epilepsy. Neurobiol Dis. 2008;29:142–160. doi: 10.1016/j.nbd.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 63.Campbell J, Ciesielski CJ, Hunt AE, Horwood NJ, Beech JT, Hayes LA, et al. A novel mechanism for TNF-alpha regulation by p38 MAPK: involvement of NF-kappa B with implications for therapy in rheumatoid arthritis. J Immunol. 2004;173:6928–6937. doi: 10.4049/jimmunol.173.11.6928. [DOI] [PubMed] [Google Scholar]
- 64.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67:668–673. doi: 10.1095/biolreprod67.2.668. [DOI] [PubMed] [Google Scholar]
- 65.Zingarelli B, Yang Z, Hake PW, Denenberg A, Wong HR. Absence of endogenous interleukin 10 enhances early stress response during post-ischaemic injury in mice intestine. Gut. 2001;48:610–622. doi: 10.1136/gut.48.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chanteux H, Guisset AC, Pilette C, Sibille Y. LPS induces IL-10 production by human alveolar macrophages via MAPKinases- and Sp1-dependent mechanisms. Respir Res. 2007;8:71. doi: 10.1186/1465-9921-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang BC, Lim KJ, Suh MH, Park JG, Suh SI. Dexamethasone suppresses interleukin-1beta-induced human beta-defensin 2 mRNA expression: involvement of p38 MAPK, JNK, MKP-1, and NF-kappaB transcriptional factor in A549 cells. FEMS Immunol Med Microbiol. 2007;51:171–184. doi: 10.1111/j.1574-695X.2007.00293.x. [DOI] [PubMed] [Google Scholar]
- 68.Vaillancourt F, Morquette B, Shi Q, Fahmi H, Lavigne P, Di Battista JA, et al. Differential regulation of cyclooxygenase-2 and inducible nitric oxide synthase by 4-hydroxynonenal in human osteoarthritic chondrocytes through ATF-2/CREB-1 transactivation and concomitant inhibition of NF-kappaB signaling cascade. J Cell Biochem. 2007;100:1217–1231. doi: 10.1002/jcb.21110. [DOI] [PubMed] [Google Scholar]
- 69.Yabe T, Sanagi T, Schwartz JP, Yamada H. Pigment epithelium-derived factor induces pro-inflammatory genes in neonatal astrocytes through activation of NF-kappa B and CREB. Glia. 2005;50:223–234. doi: 10.1002/glia.20171. [DOI] [PubMed] [Google Scholar]
- 70.Vitor CE, Figueiredo CP, Hara DB, Bento AF, Mazzuco TL, Calixto JB. Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, alpha- and beta-amyrin, in a mouse model of colitis. Br J Pharmacol. 2009;157:1034–1044. doi: 10.1111/j.1476-5381.2009.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Spooren A, Kooijman R, Lintermans B, Van Craenenbroeck K, Vermeulen L, Haegeman G, et al. Cooperation of NFkappaB and CREB to induce synergistic IL-6 expression in astrocytes. Cell Signal. 2010;22:871–881. doi: 10.1016/j.cellsig.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 72.Najjar S, Pearlman D, Miller DC, Devinsky O. Refractory epilepsy associated with microglial activation. Neurologist. 2011;17:249–254. doi: 10.1097/NRL.0b013e31822aad04. [DOI] [PubMed] [Google Scholar]
- 73.Najjar S, Bernbaum M, Lai G, Devinsky O. Immunology and epilepsy. Rev Neurol Dis. 2008;5:109–116. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.