Abstract
The hypobaric hypoxic environment in high-altitude areas often aggravates the severity of inflammation and induces brain injury as a consequence. However, the critical genes regulating this process remain largely unknown. The phosphatase wild-type p53-induced phosphatase 1 (WIP1) plays important roles in various physiological and pathological processes, including the regulation of inflammation in normoxia, but its functions in hypoxic inflammation-induced brain injury remain unclear. Here, we established a mouse model of this type of injury and found that WIP1 deficiency augmented the release of inflammatory cytokines in the peripheral circulation and brain tissue, increased the numbers of activated microglia/macrophages in the brain, aggravated cerebral histological lesions, and exacerbated the impairment of motor and cognitive abilities. Collectively, these results provide the first in vivo evidence that WIP1 is a critical neuroprotector against hypoxic inflammation-induced brain injury.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0095-9) contains supplementary material, which is available to authorized users.
Keywords: Hypobaric hypoxia, Inflammation, Brain injury, WIP1 phosphatase, Lipopolysaccharide
Introduction
For decades, human activity has significantly increased in high-altitude areas, and research on high-altitude sickness has attracted increasing attention. The inflammatory responses that occur at high altitude are often aggravated by the hypobaric hypoxic environment, and this hypoxic inflammation later results in injury to vital organs such as the brain and lung, including histological lesions and function impairments [1, 2]. However, little is known about the critical genes that regulate this process. Moreover, some commonly used experiment animals (e.g. mice) are not very sensitive to hypoxia.
Wild-type p53-induced phosphatase 1 (WIP1, also called PPM1D), is a serine/threonine phosphatase belonging to the type 2C phosphatase family. Previous studies in humans and genetically-engineered mouse models have shown that WIP1 plays critical roles in various physiological and pathological processes, including the regulation of inflammatory processes [3–7]. WIP1 has been shown to alter cellular signal transduction to inhibit the over-activation of inflammation in normoxia by dephosphorylating several key regulators, such as p-p38 and the p-65 subunit of NF-κB [6]. A recent study revealed that WIP1 is involved in neuroinflammation induced by the endotoxin lipopolysaccharide (LPS) under normoxic conditions, but this study was performed in cultured cells and wild-type mice and lacked direct in vivo evidence due to the absence of a knockout mouse model [8]. The pathological roles of WIP1 in systemic inflammation-induced brain injury under hypobaric hypoxia remain unknown.
In this study, we developed a simple and effective method using LPS plus a high-altitude simulation chamber to mimic hypoxic inflammation-induced brain injury in a mouse model which proved to be superior to hypobaric hypoxia alone. We then investigated the role of WIP1 phosphatase in brain injury induced by high-altitude hypoxic inflammation.
Materials and Methods
Animals
The WIP1-knockout (KO) mouse strain (129 Sv-C57BL/6 background) was a gift from Dr. Zhi-Cheng Xiao (Department of Anatomy and Developmental Biology, Monash University, Clayton, Australia), and was originally created by Dr. L.A. Donehower (Department of Molecular Virology and Microbiology, Baylor College of Medicine, Houston, TX) [9, 10]. In this study, WIP1 KO mice were crossed with C57BL/6 to generate WIP1 KO mice with C57BL/6 background. Male wild-type (WT) and WIP1-KO mice (22–25 g) at 8 weeks of age were used. The mice were maintained under a 14 h light/10 h dark cycle at 22 °C with free access to food and water. The animal protocol was approved by the Institutional Animal Care and Use Committee of Institute of Basic Medical Sciences.
Exposure to Acute Hypobaric Hypoxia and LPS
To mimic the high-altitude hypobaric hypoxic environment, the mice were placed in a decompression chamber (Guizhou Fenglei Co., LTD, China, model DYC-DWI) that reached conditions corresponding to 6000 m altitude (350 mmHg, 9.6% O2) within 10 min, and they were exposed to hypobaric hypoxia for 10 h with free access to food and water. Mice in the control group were maintained outside the chamber under normobaric normoxia. To induce inflammation, LPS (Sigma, St. Louis, MO) was dissolved in 0.9% saline and intraperitoneally (i.p.) injected into mice at 3 mg/kg. Mice in the control group were injected with the same volume of 0.9% saline.
Inflammatory Cytokine Measurement
Fresh blood samples from the mice were allowed to stand for 2 h at room temperature, and then centrifuged at 800 g at 4 °C for 20 min. The upper layer of serum was collected for enzyme-linked immunosorbent assay (ELISA). For tissue lysate preparation, mouse brain tissue was homogenized with Cell Lysis Buffer 2 (R&D systems, MN) on ice for 30 min, and then the homogenate was centrifuged at 20000 g for 30 min. The supernatant was aliquoted and stored for further experiments. The levels of TNF-α, IL-1β, IL-6, and IL-10 in serum and brain tissue homogenates were determined with the corresponding mouse ELISA kits (R&D systems) according to the manufacturer’s instructions.
Hematoxylin and Eosin Staining
Brain tissues of 6–10 mice from each experimental group were fixed in 10% neutral buffered formalin for 48 h, and then embedded in paraffin and routinely sectioned at 3–5 μm. After xylene de-waxing and graded ethanol de-benzolization, the sections were stained with eosin and hematoxylin under microscope (Olympus, Japan).
Iba-1 Immunohistochemistry
Brain sections were boiled in citrate buffer (0.01 mol/L, pH 6.0) for 10 min, and then endogenous peroxidase was quenched with 0.3% (v/v) H2O2 in 60% (v/v) methanol for 30 min. Nonspecific adsorption was minimized by incubation with 2% (v/v) normal goat serum in PBS for 20 min. The sections were then incubated with Iba-1 antibody (WAKO, Japan) overnight at 4 °C, washed in PBS, incubated with secondary antibody, and stained with a DAB peroxidase substrate kit (Boster, China). The sections were then counterstained with hematoxylin.
Locomotor Activity Test
Locomotor activity was measured in a monitoring chamber (400 mm × 400 mm × 300 mm; L × W × H) (Jiliang, China). The mice were placed in the test chamber and the spontaneous activity was recorded for 1 h. Data were collected using AniLab Loc software version 2.2 (AniLab, China).
Novel Object Recognition Test
The experimental device for measuring novel object recognition was 60 cm long, 60 cm wide, and 40 cm high. The test period was three days, and objects A and B were constructed from two different materials and colors. On the first day, there was no object in the recognition apparatus, and mice were allowed 20 min for adaptation. On the second day, there were two A objects in the apparatus, and the mice were allowed 6 min to familiarize themselves with the environment. On the third day, one of the A objects was replaced with a B object, and the time spent investigating the B object was recorded on a video tracking system. The recognition index (RI) which evaluates the ability to recognize a novel object, was calculated using the formula RI = Tn/(Tn + Tf), where Tn represents the total time of touching the novel B object with the nose, and Tf represents the total time of touching the A object with the nose.
Statistics
Data are presented as mean ± SEM, and were analyzed with Student’s t test unless otherwise indicated. P < 0.05 was considered to be statistically significant.
Results
WIP1 Depletion Enhances the Release of Inflammatory Cytokines Caused by Hypoxic Inflammation
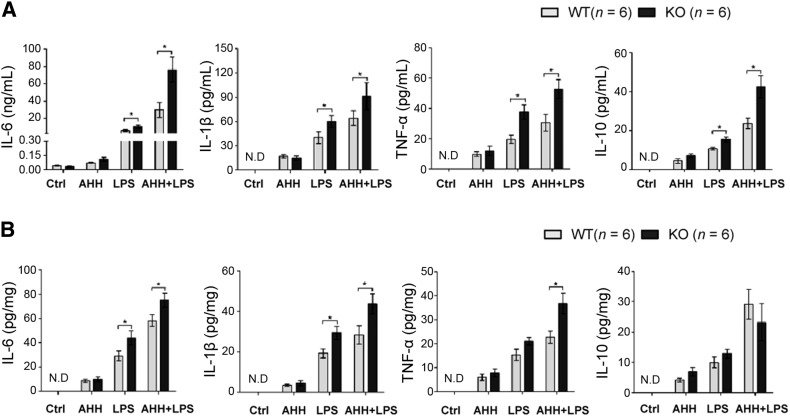
To mimic the inflammation that occurs in a high-altitude environment, we used the acute hypobaric hypoxia (AHH) method in combination with injection of a common inducer of inflammation, the endotoxin LPS. We also established cohorts with AHH or LPS treatment alone. ELISA assays were used to initially determine several critical inflammatory cytokines in the peripheral circulation and brain tissue, pro-inflammatory IL-6, IL-1β, and TNF-α as well as anti-inflammatory IL-10, to validate inflammation induction and further assess the impact of WIP1 depletion on cytokine release. The results revealed that in both the peripheral circulation and brain tissue, the highest levels of inflammatory cytokines appeared in the cohort with high-altitude hypoxic inflammation (AHH + LPS) (Fig. 1), which was consistent with the clinical fact that a high-altitude environment exacerbates the inflammatory response [1, 2]. These results suggested that AHH + LPS is more effective and simulates the clinical situation better than AHH or LPS alone in terms of mimicking hypoxic inflammation. WIP1-KO mice displayed significantly higher levels of most of the cytokines in peripheral circulation and brain tissue than WT mice after AHH + LPS (Fig. 1), which suggested that WIP1 loss augments the inflammatory response that occurs in the high-altitude environment.
Fig. 1.
WIP1 loss augments inflammatory cytokine release in both of the circulation and brain tissue of mice after hypoxic inflammation. A, B The pro-inflammatory cytokines IL-6, IL-1β, and TNF-α, and the anti-inflammatory cytokine IL-10 were measured in the peripheral circulation (A) and brain tissue (B) from WT and WIP1-KO mice after acute hypobaric hypoxia (AHH), LPS, or AHH + LPS (mean ± SEM, *P < 0.05).
WIP1 Depletion Increases the Numbers of Activated Microglia/Macrophages in Brain Tissue Following Hypoxic Inflammation
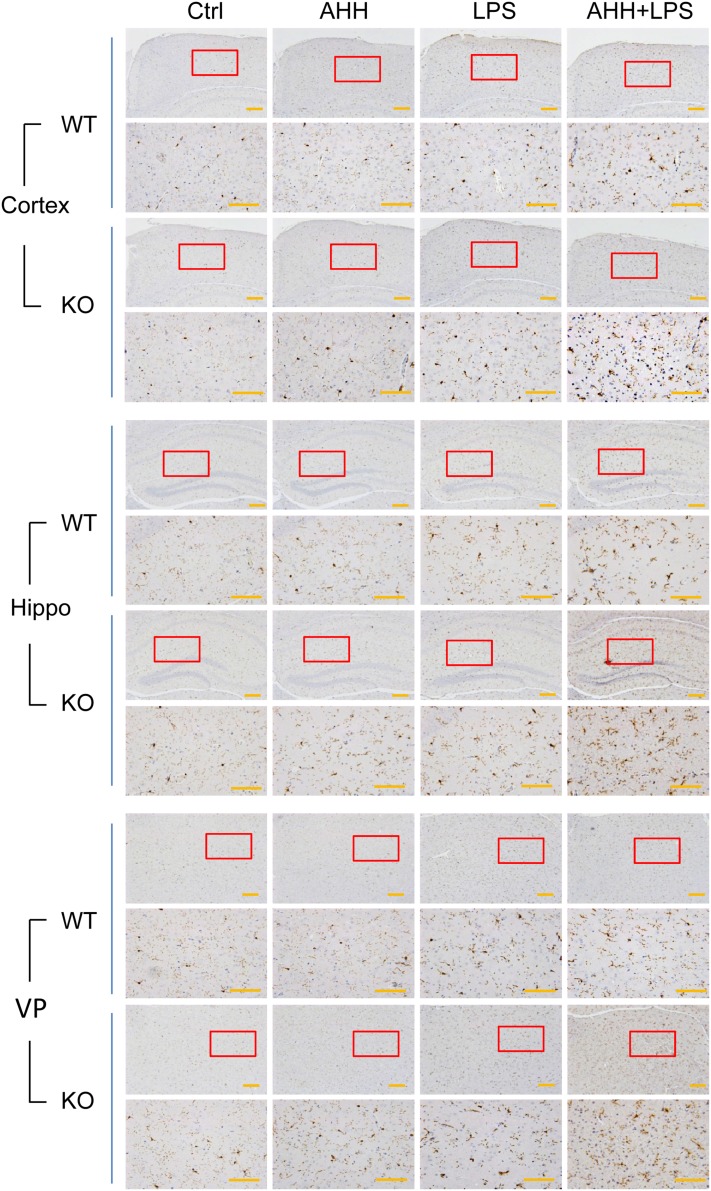
Blood monocytes and tissue macrophages constitute the main population of mononuclear phagocytes [11]. Microglia are specialized macrophages that reside in the brain and play critical roles in neuroimmune responses [12, 13]. Severe inflammation or hypoxic inflammation leads to disruption of the blood–brain barrier, followed by infiltration of peripheral monocyte-derived macrophages into the brain. Both microglia and macrophages can secrete various inflammatory cytokines and express the common activation marker Iba-1 [14]. Based on the finding that WIP1 loss led to augmented cytokine release in hypoxic inflammation, we further investigated the activation status of microglia/macrophages in the brain with Iba-1 immunohistochemistry and found that, compared with AHH or LPS alone, microglia/macrophage activation was more evident in the cortex and hippocampus in both WT and WIP1-KO mice after AHH + LPS exposure (Fig. 2). WIP1-KO mice demonstrated more severe activation than their WT counterparts (Fig. 2, Fig. S1). We also found that the ventroposterior nucleus of the thalamus (VP) was sensitive to hypoxic inflammation, as microglial/macrophage activation was also seen in the VP after AHH + LPS exposure (Fig. 2). Similarly, WIP1 loss resulted in greater activation of microglia/macrophages in the VP after hypoxic inflammation (Fig. 2, Fig. S1).
Fig. 2.
WIP1 loss leads to greater numbers of activated microglia/macrophages in the mouse brain after hypoxic inflammation. Representative images of Iba-1 immunohistochemical staining of microglia/macrophages in the cortex, hippocampus (Hippo), and ventroposterior nucleus of the thalamus (VP) in WT and WIP1-KO mice (n = 6) after acute hypobaric hypoxia (AHH), LPS, and AHH + LPS. Lower panels show an enlargement of the enclosed area in the upper panels (scale bars, 200 μm).
WIP1 Depletion Worsens Histological Lesions in the Brain Caused by Hypoxic Inflammation
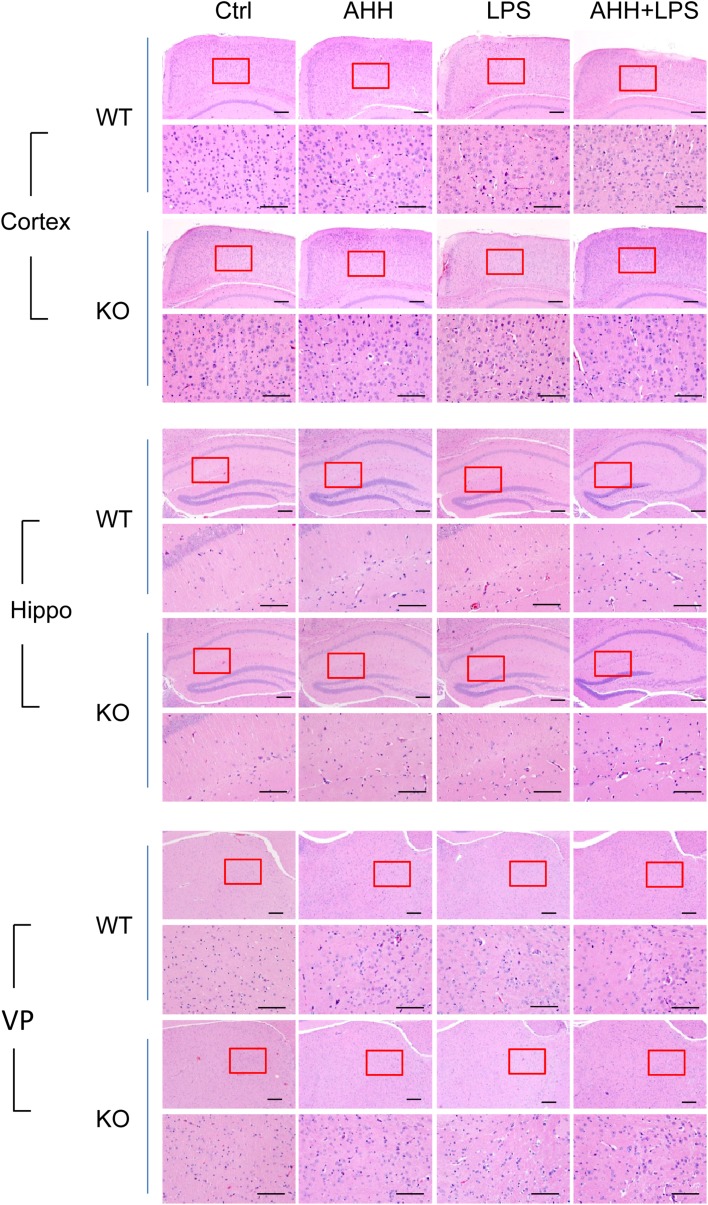
Inflammation is closely associated with cellular injury, death, and tissue lesions. Inflammatory cytokines in the peripheral circulation and interstitial fluid bind to their receptors, then initiate cellular signaling cascades, and finally affect cellular function and fate [15]. Since brain tissue is very sensitive to hypoxic inflammation and WIP1-KO mice exhibited higher inflammatory cytokine levels as well as microglia/macrophage activation after hypoxic inflammation, we evaluated the effects of WIP1 depletion on histological lesions in the brain. Although the AHH, LPS, and AHH + LPS groups showed different degrees of cell and tissue lesions in the cortex, hippocampus, and VP, including cell swelling, widened pericellular spaces, and shrunken neurons with darkly-stained pyknotic nuclei, the most severe lesions were seen in the AHH + LPS group (Fig. 3). In addition, we strikingly found that the VP was more sensitive to hypoxic inflammation, as disordered cell arrangement appeared in the VP but not in the cortex and hippocampus after AHH + LPS (Fig. 3). Moreover, the WIP1-KO mice displayed more serious histological lesions than WT mice after AHH + LPS exposure in all three regions (Fig. 3, Table S1).
Fig. 3.
WIP1 loss worsens histological lesions in brain after hypoxic inflammation. Representative images of hematoxylin and eosin staining in the cortex, hippocampus (Hippo), and ventroposterior nucleus of the thalamus (VP) of WT and WIP1-KO mice (n = 10) after acute hypobaric hypoxia (AHH), LPS, and AHH + LPS exposure. Lower panels are enlargements of the enclosed area in the upper panels (scale bars, 200 μm).
WIP1 Depletion Aggravates the Impairment of Locomotion and Cognition Caused by Hypoxic Inflammation
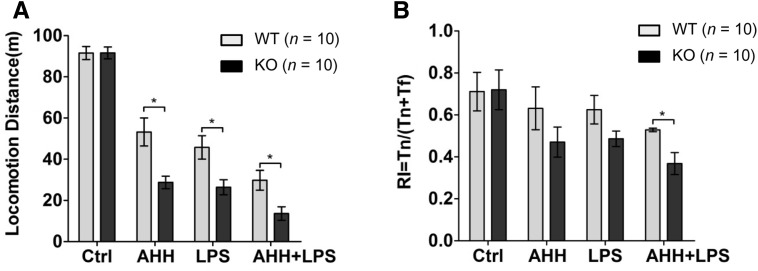
The inflammatory response and histological lesions in the brain often result in alterations of neural functions, including the impairment of motor and cognitive abilities, so we further investigated whether WIP1 loss worsened locomotion and cognition after hypoxic inflammation. The locomotion test revealed that the AHH + LPS group moved the shortest distance among the experimental cohorts (Fig. 4A), reflecting the most serious impairment of motor activity. The WIP1-KO mice moved a shorter distance than the WT mice after AHH + LPS (Fig. 4A), which suggested that WIP1 loss results in more severe impairment of motor activity. In addition, we used the novel object recognition test to evaluate cognitive ability, and the results revealed that the WIP1-KO mice spent more time recognizing a novel object than the WT mice when exposed to the AHH + LPS challenge, indicating that WIP1 deficiency leads to aggravated cognitive impairment under this condition (Fig. 4B). Collectively, these results demonstrated that, under conditions of hypoxic inflammation, WIP1 deficiency not only exacerbated the inflammatory response and brain tissue lesions, but also worsened locomotion, learning, and memory.
Fig. 4.
WIP1 loss aggravates impairment of locomotion and cognition after hypoxic inflammation. A, B Locomotor activity (A) and novel object recognition (B) were used to assess locomotion and cognition in WT and WIP1-KO mice (n = 10) after acute hypobaric hypoxia (AHH), LPS, or AHH + LPS (mean ± SEM, *P < 0.05).
Discussion
With the spread of human activity, high-altitude illness research has attracted increasing attention. However, compared with other brain disorders, there has been little research on brain injury induced by systemic inflammation in hypobaric hypoxic environments. It was necessary to establish a simple and reliable experimental animal model to simulate this pathological process. The mouse is a widely used laboratory animal and possesses many advantages, such as low cost and short generation time, and can also serve as a common host for genetic engineering modifications. Here, we developed a convenient and convincing method using LPS in combination with a decompression chamber to mimic hypoxic inflammation and the subsequent brain injury in a mouse model. Compared with LPS or hypoxia alone, this method simulated the disease symptoms better, and may help further research.
WIP1 phosphatase has been shown to suppress NF-κB signaling via dephosphorylating the p65 subunit of NF-κB, and NF-κB is well-known as a crucial regulator of inflammation [6]. However, the pathological roles of WIP1 in inflammation occurring during hypobaric hypoxia and the subsequent brain injury were unknown. Our results showed that WIP1 deletion leads to augmented IL-6, TNF-α, and IL-1β release in the peripheral circulation as well as in brain tissues after LPS plus hypoxia, consistent with previous findings that IL-6, TNF-α, and IL-1β are canonical target genes of the transcription factor NF-κB, and WIP1 is a critical negative regulator of NF-κB signaling. In addition, as for the result that the anti-inflammatory cytokine IL-10 level in brain is comparable in WT and WIP1-KO mice after AHH + LPS, we supposed that this also accounts for the fact that aggravated brain lesions appeared in WIP1-KO mice. We also noted that, unlike in the brain, the IL-10 level in WIP1-KO mouse serum was significantly higher than in the WT after AHH + LPS. Overall, these data suggest that WIP1 is involved in the selective regulation of anti- and pro-inflammatory cytokines in different tissues.
With regard to brain lesions, given that the cortex and hippocampus are critical regions for motor activity, learning, and memory, we deemed that the more serious histological lesions observed in the cortex and hippocampus of WIP1-deficient mice are closely associated with the decreased performance in locomotion and cognition tests. Besides, we unexpectedly found that the VP is another region sensitive to hypoxic inflammation, in which more serious lesions (disordered cell arrangement) were visible, and further studies are needed to address the corresponding behavioral phenotype and regulatory mechanisms behind this interesting phenomenon.
Apart from the brain injury induced by hypoxic inflammation at high altitude, an increasing number of studies have shown that hypoxia, inflammation, and microglia/macrophage activation occur in Alzheimer’s disease, Parkinson’s disease, and stroke [16–19]. Given that our data revealed that WIP1 depletion resulted in augmented inflammatory cytokine release, over-activated microglia, and cerebral histological lesions under hypoxic and inflammatory conditions, the roles of WIP1 in other severe brain diseases are worth further exploration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31401000 and 81430044), the Youth Medicine Program of the People’s Liberation Army of China (13QNP148), the National Basic Research Development Program (973 Program) of China (2012CB518200), and the Integrated Drug Discovery Technology Platform of National Science and Technology Major Projects for “Major New Drugs Innovation and Development”, China (2012ZX09J12201-005).
Footnotes
Dahu Li and Lijun Zhang have contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-016-0095-9) contains supplementary material, which is available to authorized users.
Contributor Information
Ming Fan, Email: fanmingchina@126.com.
Lingling Zhu, Email: linglingzhu@hotmail.com.
References
- 1.Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361:1967–1974. doi: 10.1016/S0140-6736(03)13591-X. [DOI] [PubMed] [Google Scholar]
- 2.Murdoch DR. Prevention and treatment of high-altitude illness in travelers. Curr Infect Dis Rep. 2004;6:43–49. doi: 10.1007/s11908-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 3.Filipponi D, Muller J, Emelyanov A, Bulavin DV. Wip1 controls global heterochromatin silencing via ATM/BRCA1-dependent DNA methylation. Cancer Cell. 2013;24:528–541. doi: 10.1016/j.ccr.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Le Guezennec X, Brichkina A, Huang YF, Kostromina E, Han W, Bulavin DV. Wip1-dependent regulation of autophagy, obesity, and atherosclerosis. Cell Metab. 2012;16:68–80. doi: 10.1016/j.cmet.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhu Y, Demidov ON, Goh AM, Virshup DM, Lane DP, Bulavin DV. Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J Clin Invest. 2014;124:3263–3273. doi: 10.1172/JCI73015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chew J, Biswas S, Shreeram S, Humaidi M, Wong ET, Dhillion MK, et al. WIP1 phosphatase is a negative regulator of NF-kappaB signalling. Nat Cell Biol. 2009;11:659–666. doi: 10.1038/ncb1873. [DOI] [PubMed] [Google Scholar]
- 7.Liu G, Hu X, Sun B, Yang T, Shi J, Zhang L, et al. Phosphatase Wip1 negatively regulates neutrophil development through p38 MAPK-STAT1. Blood. 2013;121:519–529. doi: 10.1182/blood-2012-05-432674. [DOI] [PubMed] [Google Scholar]
- 8.Tan X, Zhang J, Jin W, Li L, Xu W, Zheng H, et al. Wip1 phosphatase involved in lipopolysaccharide-induced neuroinflammation. J Mol Neurosci. 2013;51:959–966. doi: 10.1007/s12031-013-0080-y. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Nannenga B, Demidov ON, Bulavin DV, Cooney A, Brayton C, et al. Mice deficient for the wild-type p53-induced phosphatase gene (Wip1) exhibit defects in reproductive organs, immune function, and cell cycle control. Mol Cell Biol. 2002;22:1094–1105. doi: 10.1128/MCB.22.4.1094-1105.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YH, Zhang CW, Lu L, Demidov ON, Sun L, Yang L, et al. Wip1 regulates the generation of new neural cells in the adult olfactory bulb through p53-dependent cell cycle control. Stem Cells. 2009;27:1433–1442. doi: 10.1002/stem.65. [DOI] [PubMed] [Google Scholar]
- 11.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Moller T, Boddeke HW. Glial cells as drug targets: What does it take? Glia. 2016;64:1742–1754. doi: 10.1002/glia.22993. [DOI] [PubMed] [Google Scholar]
- 13.Crotti A, Ransohoff RM. Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity. 2016;44:505–515. doi: 10.1016/j.immuni.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16:249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science 2016, 352: aaf2154. [DOI] [PubMed]
- 16.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herrera AJ, Espinosa-Oliva AM, Carrillo-Jimenez A, Oliva-Martin MJ, Garcia-Revilla J, Garcia-Quintanilla A, et al. Relevance of chronic stress and the two faces of microglia in Parkinson’s disease. Front Cell Neurosci. 2015;9:312. doi: 10.3389/fncel.2015.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bou Khalil R, Khoury E, Koussa S. Linking multiple pathogenic pathways in Alzheimer’s disease. World J Psychiatry. 2016;6:208–214. doi: 10.5498/wjp.v6.i2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu S, Zhou J. Finding the ‘Guilty’ gene variant of sporadic Parkinson’s disease via CRISPR/Cas9. Neurosci Bull 2016. doi:10.1007/s12264-016-0065-2. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.