Dear Editor,
Haploid embryonic stem cells (haESCs) hold great potential for genetic screening and the analysis of recessive phenotypes. Several studies have recently reported the generation of mammalian haESCs through gamete manipulation, and evaluated the benefits of using them for studying functional genomics in different mammals [1–4]. These haESCs have been shown to give rise to three germ layers by spontaneous differentiation both in embryoid bodies in vitro and in teratomas in vivo. Injection of genetically-modified androgenetic haESCs into oocytes has led to the successful generation of transgenic mice [1, 4]. All these data suggest that haESCs, like diploid ESCs, can acquire pluripotency. However, in previous studies, terminally-differentiated cells derived from mouse haESCs, such as neurons, lost their haploidy due to spontaneous diploidization both in vivo and in vitro [1, 4, 5], and it still remains undetermined whether neural differentiation can be induced in haploid mouse cells. Here, we demonstrated the differentiation of mouse androgenetic haESCs into haploid neural stem cells (NSCs), then into haploid neurons, and illustrated the dynamics of haploidy status during the neural differentiation of haESCs in vitro.
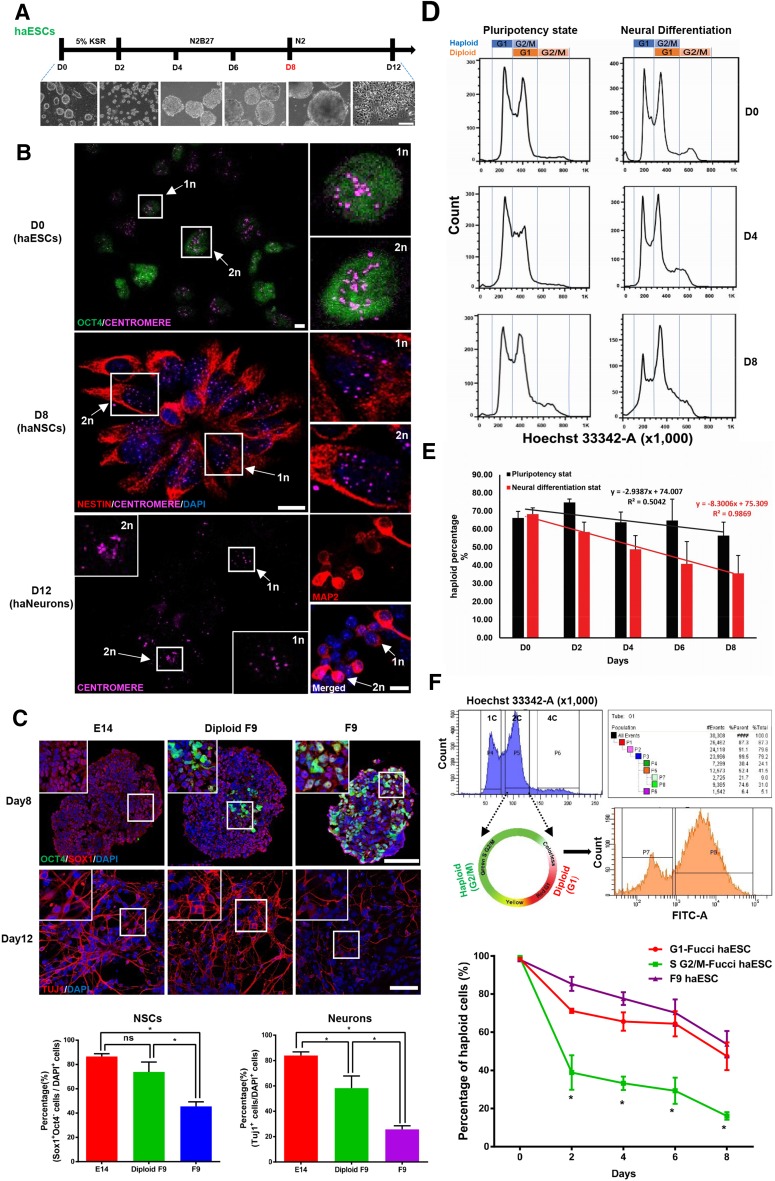
To study the neural differentiation of mouse haESCs, the previously reported serum-free, floating embryoid body approach was modified [6]. Following this approach, the attached haESCs were gradually induced into suspended neurospheres then neurons in vitro (Fig. 1A). The Oct4-GFP knock-in haESC line, F9, was used to monitor the decline of pluripotent cells during the neural differentiation process [1]. The F9 cells displayed a normal growth curve with a doubling time of ~22 h during passages (Fig. S1A). To avoid heterogeneity of the haESCs due to spontaneous diploidization, those containing a 1-copy DNA set were enriched by fluorescence-activated cell sorting (FACS), and used to initiate neural differentiation on day 0. On day 8, the neurospheres were dissociated into single cells and haploid cells were again collected by FACS. The expression levels of the NSC marker genes Pax6, Nestin, and Sox1 in haploid cells on day 8 were dramatically higher than those in haploid cells on day 0, while expression of the pluripotency genes Oct4 and Nanog was lower, indicating the generation of haploid NSCs by day 8 (Fig. S1B). It is well documented that Sox1-positive and Oct4-negative cells are putative NSCs in the mouse [7–9]. On day 8, approximately half of the haploid cells were Sox1+/Oct4− NSCs (Fig. S1C). As the ploidy in individual cells can be visualized by immunostaining for centromere protein and quantifying centromere foci, the haploidy of ESCs, NSCs, and neurons were directly revealed by co-expression of Oct4 with 1-set centromere protein in ESCs on day 0, co-expression of Nestin with 1-set centromere protein in NSCs on day 8, and co-expression of the neuronal marker Map2 with 1-set centromeres in neuronal cells on day 12 (Fig. 1B), confirming the differentiation of mouse androgenetic haESCs into haploid neurons through a haploid NSC stage, and demonstrating the maintenance of haploidy during the neural differentiation process.
Fig. 1.
Derivation of neurons from mouse haploid ESCs (haESCs). A Schematic of the method of directing neural differentiation from haESCs to neurons (upper panel), and the neural differentiation processing from attached haESCs, to suspended embryoid bodies, to neurons (lower panel; scale bar 100 μm). B Co-staining of Oct4 and centromeres in haESCs on day 0, Nestin and centromeres in neural stem cells on day 8, and Map2 and centromeres in neurons on day 12 (scale bars 10 μm). The arrows indicate the haploid cells (1n) and diploid cells (2n). C Expression of Oct4 and Sox1 in neural stem cells on day 8 and Tuj1 in neurons on day 12 derived from diploid E14 ESCs, diploidized F9 haESCs, and F9 cells (upper panels; scale bars 50 μm), and their quantification (lower panels). D FACS analysis of DNA content in haESCs in the pluripotent and neural differentiation states on days 0, 4, and 8. E Percentages of haploid cells among haESCs in the pluripotent and neural differentiation states on days 0, 2, 4, 6, and 8, and deduced diploidization tendency and rate during haESC maintenance and neural differentiation. F Enrichment of Fucci-haESCs in G1 and G2/M by FACS analysis and Fucci assay. Note that the Fucci assay is shown graphically (upper panels), and the dynamics of haploidy status is plotted as percentage of haploid cells on days 0, 2, 4, 6, and 8 during G1-Fucci haESC, G2/M-Fucci haESC, and F9 cell neural differentiation (lower panel). n = 4 independent experiments. All data are presented as the mean ± SD; *P < 0.05, two-tailed t test.
We subsequently investigated the neural differentiation potential of mouse haESCs. Immunostaining and single-cell PCR showed that about half of the Sox1- or Pax6-positive haploid cells on day 8 expressed Oct4 (Figs. S1C, S2A). Consistently, the FACS analysis revealed that two-thirds of the haploid cells on day 8 were Oct4-GFP+ (Fig. S2B), implying the persistence of pluripotency during the neural differentiation of haESCs. The neural differentiation was compared among F9, diploid E14 ESCs, and diploid F9 cells, the latter derived from spontaneously-diploidized F9 haESCs. We found that the expression levels of Oct4 and Nanog on day 8 were higher in F9 than in diploid F9 and E14 cells, while the expression of NSC genes, such as Pax6, was lower (Fig. S2C). The immunostaining of Oct4 and Sox1 in embryonic bodies on day 8 detected more Oct4+ cells among the F9-derived Sox1+ cells, and the percentage of Sox1+/Oct4− NSCs and Tuj1+ neurons was much lower in F9 cells than in diploid ESCs, showing the compromised neural differentiation of haESCs (Fig. 1C). In addition, the Oct4− haploid cells on day 8 gave rise to more Tuj1+ neurons than day-8 cells with both Oct4+ and Oct4− (Fig. S2D), suggesting that the compromised neural differentiation potential of haESCs might be due to the persistence of pluripotency.
We finally addressed the dynamics of haploidy status during the neural differentiation of mouse haESCs. F9 cells in the differentiated and undifferentiated pluripotent states were sorted by FACS, and cells with a genome containing one chromosomal copy (1c), two (2c), and four copies (4c) were isolated (Fig. 1D). Due to the spontaneous diploidization of haploid cells, we deduced that the 1c-cell population harbored G1-phase haploid cells, the 2c cells harbored G2/M-phase haploid and G1-phase diploidized cells, and 4c cells contained G2/M-phase diploidized cells (Fig. 1D). Based on the proportions of 2c- and 4c-cells in each phase of diploid F9 cells during neural differentiation by FACS analysis (Fig. S3A), a mathematical model was formulated and used to calculate the percentage of G2/M-phase haploid cells in the differentiated and undifferentiated states (Fig. S3B). Then, the dynamics of haploid cell volume during neural differentiation and in the pluripotent state were plotted according to the proportion of total haploid cells. We found that the haploidy loss of haESCs occurred at a rate of 2.9% of cells every two days during the pluripotent state, while the haploidy loss increased to 8.3% during neural differentiation, showing that the haploidy loss or diploidy acquisition occurs much faster during neural differentiation than in the pluripotent state, and the haploidy maintenance of haESCs is easier in the pluripotent state (Fig. 1E). Fucci technology labels individual G1-phase nuclei red and those in the S/G2/M phases green using anti-phase oscillating protein fluorescent probes [10]. Then cells in G1 express red fluorescence and in S/G2/M green, which can be easily separated and enriched by FACS sorting. In order to directly visualize and determine the percentage of haploid cells in the 2c-cell population, Fucci-haESCs were established by taking advantage of Fucci technology. The G2/M-phase haploid cells in 2c-cells were distinguishable by the color of fluorescence, and their percentage was obtained after FACS analysis (Fig. 1F). The Fucci-haESCs in G1 and G2/M were each collected and neural differentiation was performed. The dynamics of haploidy status of G1 Fucci-haESCs during neural differentiation was similar to that of F9 cells, while the diploidy acquisition of G2/M Fucci-haESCs was much faster (Fig. 1F), suggesting that the cell cycle is relevant to the maintenance of haploidy in mouse haESCs during neural differentiation.
In summary, we demonstrated here that mouse androgenetic haESCs retained haploidy during the neural differentiation process, and gave rise to haploid neurons. The neural differentiation potential of the haESCs was compromised due to the persistence of pluripotency. The dynamics of haploidy status of haESCs were investigated, showing that the irreversible haploidy loss of haESCs occurred more quickly during neural differentiation than in the pluripotent state, and the haploidy maintenance of haESCs was easier in the pluripotent state. It is interesting to note that the cell cycle might be involved in the maintenance of haploidy in haESCs during neural differentiation. The neural differentiation of haESCs recapitulated the neural development process in vivo, suggesting that mouse haESCs can serve as a useful platform for genetic screening during neural development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA01010201), the National Key Basic Research and Development Program of China (2015CB964500 and 2014CB964804), and the National Natural Science Foundation of China (91219303, 31430058, and 31401261). We thank Drs. Xueliang Zhu and Xiumin Yan for help in centromere immunostaining.
Footnotes
He Xu and Chunmei Yue have contributed equally to this work.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0110-9) contains supplementary material, which is available to authorized users.
Contributor Information
Jinsong Li, Email: jsli@sibcb.ac.cn.
Naihe Jing, Email: njing@sibcb.ac.cn.
References
- 1.Yang H, Shi L, Wang BA, Liang D, Zhong C, Liu W, et al. Generation of genetically modified mice by oocyte injection of androgenetic haploid embryonic stem cells. Cell. 2012;149:605–617. doi: 10.1016/j.cell.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Liu Z, Ma Y, Zhong C, Yin Q, Zhou C, et al. Generation of haploid embryonic stem cells from Macaca fascicularis monkey parthenotes. Cell Res. 2013;23:1187–1200. doi: 10.1038/cr.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagi I, Chia G, Golan-Lev T, Peretz M, Weissbein U, Sui L, et al. Derivation and differentiation of haploid human embryonic stem cells. Nature. 2016;532:107–111. doi: 10.1038/nature17408. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Shuai L, Wan H, Dong M, Wang M, Sang L, et al. Androgenetic haploid embryonic stem cells produce live transgenic mice. Nature. 2012;490:407–411. doi: 10.1038/nature11435. [DOI] [PubMed] [Google Scholar]
- 5.Elling U, Taubenschmid J, Wirnsberger G, O’Malley R, Demers SP, Vanhaelen Q, et al. Forward and reverse genetics through derivation of haploid mouse embryonic stem cells. Cell Stem Cell. 2011;9:563–574. doi: 10.1016/j.stem.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yue W, Li Y, Zhang T, Jiang M, Qian Y, Zhang M, et al. ESC-derived basal forebrain cholinergic neurons ameliorate the cognitive symptoms associated with Alzheimer’s disease in mouse models. Stem Cell Reports. 2015;5:776–790. doi: 10.1016/j.stemcr.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang K, Li L, Huang C, Shen C, Tan F, Xia C, et al. Distinct functions of BMP4 during different stages of mouse ES cell neural commitment. Development. 2010;137:2095–2105. doi: 10.1242/dev.049494. [DOI] [PubMed] [Google Scholar]
- 8.Wood HB, Episkopou V. Comparative expression of the mouse Sox1, Sox2 and Sox3 genes from pre-gastrulation to early somite stages. Mech Dev. 1999;86:197–201. doi: 10.1016/S0925-4773(99)00116-1. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Kamachi Y, Tanouchi A, Hamada H, Jing N, Kondoh H. Interplay of SOX and POU factors in regulation of the Nestin gene in neural primordial cells. Mol Cell Biol. 2004;24:8834–8846. doi: 10.1128/MCB.24.20.8834-8846.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.