Abstract
Accumulating evidence has suggested resveratrol as a promising drug candidate for the treatment of epilepsy. To validate this, we tested the protective effect of resveratrol on a kainic acid (KA)-induced epilepsy model in rats and investigated the underlying mechanism. We found that acute resveratrol application partially inhibited evoked epileptiform discharges in the hippocampal CA1 region. During acute, silent and chronic phases of epilepsy, the expression of hippocampal kainate glutamate receptor (GluK2) and the GABAA receptor alpha1 subunit (GABAAR-alpha1) was up-regulated and down-regulated, respectively. Resveratrol reversed these effects and induced an antiepileptic effect. Furthermore, in the chronic phase, resveratrol treatment inhibited the KA-induced increased glutamate/GABA ratio in the hippocampus. The antiepileptic effects of resveratrol may be partially attributed to the reduction of glutamate-induced excitotoxicity and the enhancement in GABAergic inhibition.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0097-2) contains supplementary material, which is available to authorized users.
Keywords: Resveratrol, Epilepsy, Hippocampus, Glutamate, GABA
Introduction
Epilepsy is a serious neurological disorder marked by recurrent seizures, affecting ~50 million people worldwide [1]. Temporal lobe epilepsy (TLE) is the major form of acquired epilepsy in adults, involving seizures that often arise in the mesial temporal lobe of the hippocampus [2]. Several animal models have been developed to explore the epileptogenic mechanisms, and kainic acid (KA, also known as kainate) is widely used to induce animal models to investigate TLE [3, 4]. Despite these studies, the causes of epilepsy are still not fully understood, and few neuroprotectants have been successfully translated from the laboratory to clinical practice.
Resveratrol (3, 4, 5-trihydroxy-trans-stilbene) is a natural product found in grape skin, cranberries, peanuts, and other plants. Red wine is considered to be its most important dietary source [5]. Pharmacological studies have revealed that it influences a variety of biological processes, including antioxidant [6], anti-inflammatory [7], and anti-ageing pathways [8]. In the central nervous system, resveratrol has been shown to have neuroprotective effects against cerebral stroke [9] and KA-induced epilepsy [10]. We previously showed that chronic resveratrol treatment effectively protects the brain against focal cerebral injury [11], KA-induced seizures and neurotoxicity [12, 13], and down-regulates hippocampal KA receptor expression in rats [13]. In this study, we further evaluated its protective effects against a KA-induced model of TLE.
Glutamate (Glu) is a major excitatory transmitter in the brain, and KA can cause prolonged depolarization and even neuronal death via Glu receptors [14]. Presynaptic KA receptors regulate Glu or γ-aminobutyric acid (GABA) release [15], indicating their important role in the etiology of epilepsy. Epilepsy is associated with an excitatory/inhibitory imbalance in the underlying neuronal network [16]. For this reason, we speculated that down-regulation of inhibitory synaptic inputs to neurons precipitates in epileptic activity. KA-induced epileptogenesis is mediated partially by GluK2-containing postsynaptic KA receptors located on mossy-fiber synapses distributed extensively on CA3 pyramidal cells [17]. Epileptogenesis has also been correlated with profound changes in GABAA receptor subunits, predominantly the alpha-1 subunit [18].
In the present study, we developed a TLE model by injecting KA into the core of the right hippocampal CA3 region to evaluate the epileptogenesis and progression of TLE and analyze the mechanism underlying the antiepileptic effects of resveratrol. The effects of resveratrol on the expression of GluK2 and GABA receptor alpha-1 (GABAR-α1) in various phases of TLE and its influence on the ratio of Glu to GABA in the hippocampus of KA-induced rats were investigated.
Material and Methods
Animals
All animal studies were conducted at the Animal Facility at Anhui Medical University approved by the Association for Assessment and Accreditation of Laboratory Animal Care International. Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (8th Edn). All experimental protocols were approved by the Animal Care and Use Committee of Anhui Medical University. All efforts were made to minimize animal suffering and reduce the number of animals used. Male Wistar rats (200–250 g) were housed in a temperature-controlled (25 °C) room under a 12:12 light/dark cycle with lights on at 07:00. The animals had free access to food and water.
Rat Model of Temporal Lobe Epilepsy
Rats were anesthetized with a mixture of 70% nitrous oxide, 30% oxygen, and 1.5% halothane for surgery. A bolus of 2.5 μL KA (0.4 μg/μL) was injected slowly (during ~15 min) into the CA3 region of the right hippocampus (4.0 mm posterior to bregma, 4.4 mm lateral to midline, 3.8 mm below dura), after which the animal was held in place for 10 min. The KA-induced seizures were rated according to Racine’s standard classification: 0, normal; 1, stereotyped mounting, eye-blinking, and/or mild facial clonus; 2, head-nodding and/or several episodes of facial clonus; 3, myoclonic jerks in forelimbs; 4, clonic convulsions in forelimbs with rearing; and 5, generalized clonic convulsions associated with loss of balance [19]. Only rats that reached class 4 or above were used as successful models in the subsequent experiments, and were divided into three groups: the control, KA, and KA + Res (resveratrol) groups. Controls received an equivalent volume of saline injected into the same site. All rats in the chronic experiments were divided according to the various phases of TLE: the acute (3 days after initial status epilepticus), silent (14 days after initial status epilepticus), and chronic phases (60 days after initial status epilepticus). The KA + Res group received resveratrol intragastrically at 15 mg/kg. During the acute phase, resveratrol was administered once per day for three days after the onset of seizures. During the silent and chronic phases, resveratrol dissolved in 40% propylene glycol was administered for 10 consecutive days after initial seizure onset. The control and KA-treated rats received vehicle (40% propylene glycol in saline).
Behavioral Monitoring
After KA injection, rats were allowed free access to standard dry rat diet and tap water and were maintained under standard laboratory conditions (23 ± 1 °C) with a natural light/dark cycle. Spontaneous seizures were monitored on a video-capture system for 8 h/day, 5 days/week.
Electrophysiological Analysis
Extracellular recording was conducted two weeks after the induction of epilepsy. Immediately after deep anesthesia with ethyl ether, the brain was harvested and placed into ice-cold artificial cerebrospinal fluid (ACSF) for ~2 min. The ACSF (in mmol/L: NaCl 128, KCl 1.7, KH2PO4 1.24, MgSO4 1.3, CaCl2 2.4, NaHCO3 26.0, and glucose 10.0) was oxygenated with a mixture of 95% O2 and 5% CO2 at a pH adjusted to 7.4. Coronal slices (400 μm thick) were cut on a manual vibratome (Microslicer DTK 1500, Dousaka EM Co., Kyoto, Japan) and immediately transferred to a submerged holding chamber containing ACSF at room temperature for at least 1 h for recovery. A single slice was transferred to a recording chamber mounted on a vibration isolation table, continuously perfused with oxygenated ACSF at 1–2 mL/min at ~32°C, and viewed through an inverted microscope (IMT-2, Olympus, Tokyo, Japan). Orthodromic stimuli were delivered by a bipolar tungsten electrode placed in the stratum radiatum near the border between CA1 and CA2, targeting the Schaffer collateral/commissural pathway. Population spikes (PSs) were recorded in the CA1 pyramidal cell layer with a 3–8 MΩ resistance glass microelectrode filled with 2 mol/L NaCl solution.
Western Blot Analysis
Rats were sacrificed with an overdose of chloral hydrate, and the brain immediately harvested. The hippocampus on both sides were removed and stored at –80 °C until use. Total protein was isolated from the whole hippocampus of each rat and its concentration determined using a BCA Protein Assay kit (Pierce, Rochford, IL). After separation on a 10% resolving gel, proteins were transferred to a BioTrace PVDF membrane. The membrane was blocked with 5% nonfat dry milk in PBS for 1 h at room temperature followed by incubation with rabbit anti-KA receptor GluK2 (1:1000) or rabbit anti-GABAAR-α1 (1:1000) primary antibody diluted in blocking buffer. After three washes with PBS-0.05% Tween 20 (PBS-T), membranes were incubated with the secondary antibody and HRP-conjuncted antibody for GAPDH for 90 min at room temperature. Protein bands were visualized using an ECL Detection Kit (Amersham Biosciences, Piscataway, NJ). Membranes were washed with 100 mmol/L 2-mercaptoethanol, 2% SDS, and 62.5 mmol/L Tris-HCl (pH 6.7) at 50°C for 30 min and re-incubated with an antibody targeting a separate protein. All blots were digitized and quantified using NIH ImageJ software (National Institutes of Health, Bethesda, MD).
High-Performance Liquid Chromatography (HPLC)
Rats were deeply anesthetized with chloral hydrate (350 mg/kg, i.p.) and decapitated. The right hippocampus was immediately dissected on ice, homogenized in 1 mL saline (25,000 rpm for 10 s) and then centrifuged at 4°C (12,000 rpm for 20 min) to obtain the supernatant (1 mL). Acetonitrile (3 mL) was added to the supernatant to remove protein by centrifuging at 4 °C (12,000 rpm for 10 min). An amino-acid standard solution or the diluted sample was evaluated using pre-column derivatization with phenyl isothiocyanate solution before chromatographic analysis.
The concentrations of Glu and GABA in the hippocampus were determined by calculating the peak areas and standard curves. Microdialysates were separated by HPLC (Agilent 1100 LC/MSD) using a Venusil AA column (250 mm long, 4.6 mm in diameter, and 5 µm pore size) and analyzed with an ultraviolet detector (wavelength, 254 nm). The mobile phase contained 50 nmol/L sodium acetate (pH 5.5), deionized water (HPLC grade), methanol, and 5% (v/v) tetrahydrofuran at a flow rate of 1.0 mL/min. The column temperature was set at 40°C and a linear, graded protocol in solvent B (methyl cyanide) was carried out as follows: 0% to 10% solvent for 20 min, followed by 10% to 18% for 10 min, 18% to 30%, and then 100% for the final 10 min.
Reagents
Resveratrol, KA, bicuculline, and protease inhibitor cocktail were from Sigma (St. Louis, MO). Rabbit anti-GluK2 affinity-purified polyclonal antibody and rabbit anti-GABAAR-α1 affinity-purified polyclonal antibody were from Chemicon (Temecula, CA). Other chemicals of special grade were from Wako (Osaka, Japan).
Data Analysis
All data were analyzed with SPSS 16.0 (IBM Inc., Chicago, IL) and are expressed as mean ± SEM. Differences in PS amplitude between groups were analyzed using Student’s t-test. Differences among groups were analyzed using two-way ANOVA followed by LSD multiple comparison if significance was found. A P value <0.05 was considered statistically significant.
Results
Effect of Resveratrol on KA-Induced Epilepsy Behavior
The numbers of rats reaching at least class 4 were 0/8 in the control, 7/8 in the KA, and 6/8 in the KA + Res groups. There was no difference in the rate of seizures between the KA and KA + Res groups in the acute phase (87.5 ± 3.7% vs 75.0 ± 3.2%, P > 0.05). No rats developed spontaneous seizures in any of the three groups in the silent phase. However, the rate of spontaneous seizures was markedly lower in the KA + Res group than in the KA group in the chronic phase (87.5 ± 3.7% vs 12.5 ± 2.3%, P < 0.05).
Resveratrol Did Not Change the Population Spike Amplitude in Hippocampal CA1 of Control Rats
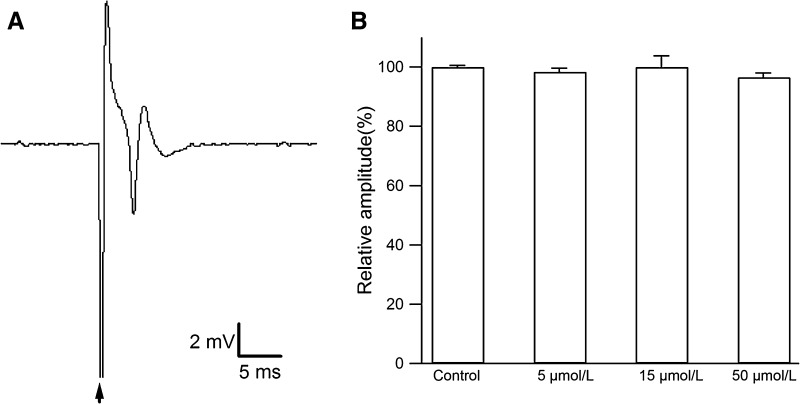
Extracellular field PSs were recorded in the stratum pyramidale during Shaffer collateral-CA1 synaptic transmission. In the control group, a single PS was recorded in the pyramidal cell layer of the CA1 region (Fig. 1A). To test the effect of various concentrations on PS amplitude, we perfused 5, 15, and 50 μmol/L resveratrol directly onto hippocampal slices from the control group for 20 min. The amplitude of the PS was not affected by these concentrations (Fig. 1B; original PSs in Fig. S1). These results showed that resveratrol itself has no effect on PS amplitude at the concentrations used.
Fig. 1.
Resveratrol has no effect on the amplitude of population spikes in the control group. A A single PS in the hippocampal CA1 pyramidal cell layer of a control rat (arrow, stimulus artifact). B Acute application of resveratrol (5, 15, and 50 μmol/L) to hippocampal slices from the control group did not alter the PS amplitude (8 slices from 3 rats, P >0.05).
Effect of Resveratrol on Epileptiform Activity in Rat Hippocampal Slices
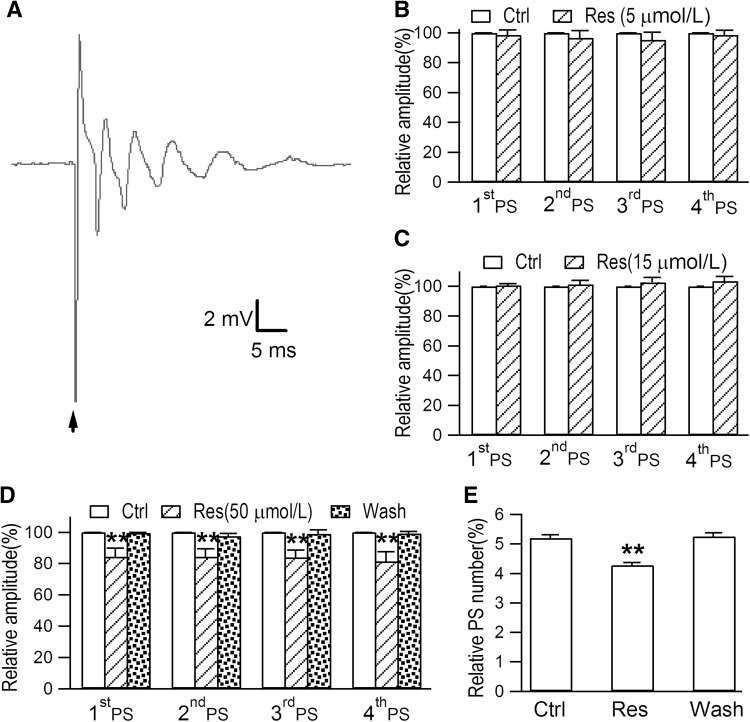
Since the hippocampus is susceptible to epileptiform activity [20], we evaluated the effect of epilepsy on PSs in the CA1 region. In slices from rats with KA-induced TLE, a stimulus applied to the pyramidal cell layer evoked epileptiform discharges that consisted of 3–6 PSs (Fig. 2A). This indicated that KA injection into the hippocampus can evoke epileptiform activity. Li et al. demonstrated that resveratrol inhibits neuronal discharges in the rat hippocampal CA1 area in a dose-dependent manner [21]. Similarly, we found that perfusion with 5 and 15 μmol/L resveratrol for 20 min did not affect the amplitudes of the first 4 PS peaks and the total number of PS peaks (Fig. 2B and C). However, perfusion with 50 μmol/L resveratrol for 20 min reversibly decreased the amplitudes of the first 4 PS peaks and the total number of PS peaks (Fig. 2D and E; original PSs in Fig. S2). This suggests that resveratrol at 50 μmol/L partially inhibits the epileptiform discharges evoked in this region.
Fig. 2.
Epileptiform discharges are evoked in the hippocampal CA1 region of rat models of TLE induced by KA. A Typical multiple peaks of PS epileptiform discharges recorded in a hippocampal slice from a rat with KA-induced TLE. B After acute perfusion with resveratrol (Res; 5 μmol/L) for 20 min, the amplitudes of the first 4 PS peaks and the total number of PS peaks were not affected (6 slices from 4 rats, P >0.05). C The amplitudes of the first 4 PS peaks and the total number of PS peaks were not affected by perfusion with 15 μmol/L Res for 20 min (6 slices from 4 rats, P >0.05). D After perfusion with 50 μmol/L Res for 20 min, the amplitudes of the first 4 PS peaks were clearly decreased (6 slices from 5 rats, **P <0.01). E When slices from the control group were perfused with 50 μmol/L Res for 20 min, the amplitudes of the PS peaks were clearly decreased (6 slices from 5 rats, **P <0.01).
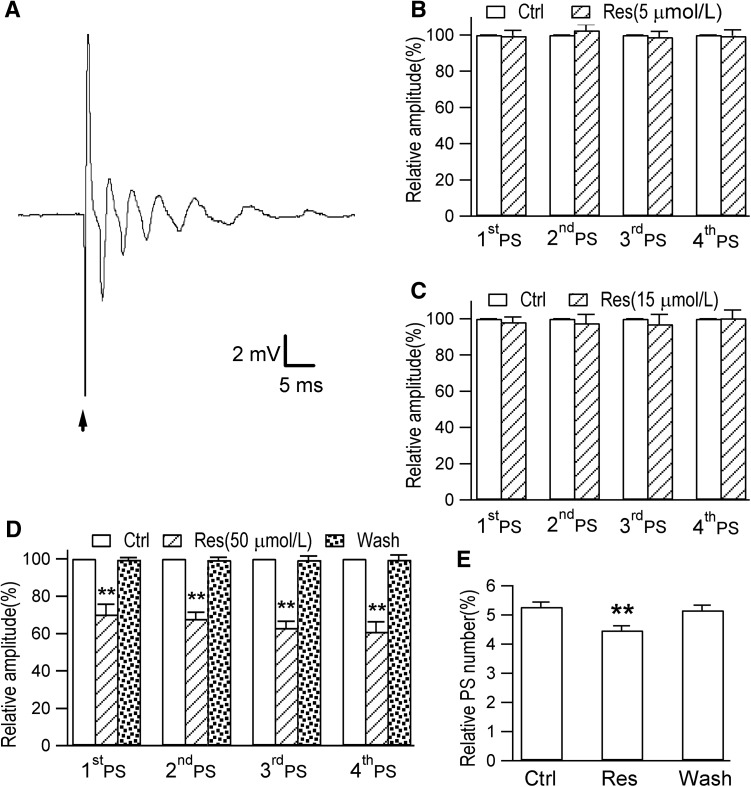
The total number of PSs during epileptiform discharges is correlated with the intensity of the test stimulus [22], while a conditioning stimulus typically fails to activate the GABAAR-mediated inhibitory pathway [23]. Here, we found that a repetitive pattern of PS epileptiform discharges was induced by perfusing ACSF with bicuculline (30 μmol/L), a GABAAR antagonist, into control hippocampal slices (Fig. 3A). These results showed that under normal physiological conditions, GABAergic inhibition may contribute to inhibiting excitatory neurotransmission and therefore plays a role in combating epileptogenesis. The amplitudes of the first 4 PS peaks and the total number of PS peaks were not significantly affected by perfusion with ACSF with 5 or 15 μmol/L resveratrol for 20 min (Fig. 3B and C). However, 50 μmol/L resveratrol perfused for 20 min decreased the amplitudes of the first 4 PS peaks and the total number of PS peaks (Fig. 3D and E; original population spikes in Fig. S3).
Fig. 3.
Epileptiform discharges are evoked by bicuculline (Bic) in the rat hippocampal CA1 region. A A repetitive pattern of PS epileptiform discharges induced by perfusing Bic (30 μmol/L) for 20 min onto hippocampal slices from the control group. B, C Acute exposure to 5 μmol/L or 15 μmol/L resveratrol (Res) for 20 min in the slices from the control group did not affect the amplitudes of the first 4 PS peaks and the total number of PS peaks (6 slices from 5 rats, P >0.05). D After perfusion with 50 μmol/L Res for 20 min, the amplitudes of the first 4 PS peaks were clearly decreased (8 slices from 5 rats, **P <0.01). E The 20-min application of 50 μmol/L Res induced a decrease in the total number of PS peaks and recovered quickly after washout (8 slices from 5 rats, **P <0.01).
Effects of Resveratrol on the Expression of GluK2 and GABA Receptor-Alpha 1 in Different Phases of Epilepsy
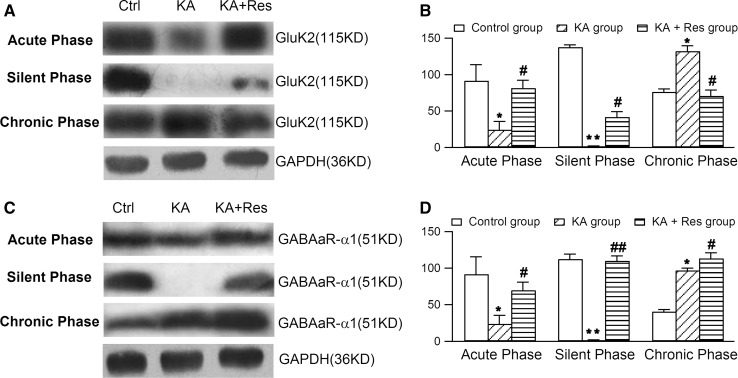
Gao et al. reported that resveratrol reversibly inhibits excitatory synaptic transmission via suppression of Glu-induced current in CA1 pyramidal neurons [24]. In the acute phase, we found that the expression of GluK2 and GABAAR-α1 in hippocampal neurons were lower in the KA group than in controls. Furthermore, compared with the KA group, resveratrol treatment up-regulated the expression of GluK2 and GABAAR-α1 (Fig. 4), indicating that it restores the expression of GluK2 and GABAAR-α1 in this phase of KA-induced epilepsy.
Fig. 4.
Effects of resveratrol (Res) on the expression of Glu-6 and GABAA receptor-α1 in different phases of epilepsy with Western blot analysis. A Representative western blots showing that Res modulated the expression of GluR6 in the hippocampus in the acute, silent, and chronic phases. B Densitometric analysis showing that GluR6 expression was reduced in the acute phase, barely detectable in the silent phase, and markedly increased in the chronic phase in the KA group. Resveratrol treatment up-regulated GluR6 expression in the acute and silent phases, but down-regulated it in the chronic phase. C Western blotting analysis of the effects of Res on the expression of GABAR-α1 in the hippocampus in the acute, silent, and chronic phases. D Densitometric analysis showing that GABAAR-α1 expression was significantly reduced in the acute phase, barely detectable in the silent phase, and markedly increased in the chronic phase in the KA group. Res treatment up-regulated GABAAR-α1 expression in the acute, silent, and chronic phases. *P <0.05, **P <0.01 vs control group; # P <0.05, ## P <0.01 vs KA group (n = 8).
In the silent phase, hippocampal GluK2 and GABAAR-α1 were barely detectable in the KA group. However, resveratrol increased the expression of GluK2 and GABAAR-α1 compared with the KA group. In the chronic phase, expression of GluK2 and GABAAR-α1 was significantly higher in the KA group than in the control group. GABAAR-α1 was found to be higher while GluK2 was lower in the KA + Res group than in the KA group (Fig. 4).
Effect of Resveratrol on Amino-Acid Levels in Different Phases of Epilepsy
The results of HPLC showed that in the acute phase, the Glu/GABA ratio in the hippocampus was higher in the KA and KA + Res groups than in the control group. Resveratrol did not inhibit the epilepsy-induced elevation in the Glu/GABA ratio. In the silent phase, there was no significant difference in the Glu/GABA ratio among the three groups. In the chronic phase, the ratio was higher in the KA group than in the control group, and lower in the KA + Res group than in the KA group (Table 1). These results suggest that resveratrol prevents the imbalance between excitatory and inhibitory amino-acid neurotransmitters in the rat hippocampus during KA-induced seizures. The neuroprotective effect of resveratrol indicates that it is capable of blocking the excitotoxic effects of glutamate in the hippocampus in the chronic phase of KA-induced epilepsy.
Table 1.
Glu/GABA Ratio in the hippocampus during different phases of epilepsy
| Group | Acute phase | Silent phase | Chronic phase |
|---|---|---|---|
| Control | 8.78 ± 1.42 | 8.12 ± 0.61 | 8.10 ± 3.63 |
| KA | 15.67 ± 1.65* | 7.14 ± 0.73 | 18.24 ± 1.00* |
| KA + Res | 12.66 ± 0.82* | 8.63 ± 1.75 | 9.44 ± 2.49# |
Values are mean ± SEM, n = 8; *P <0.05 vs control group; # P <0.05 vs KA group.
Discussion
Epilepsy is one of the most common neurological disorders. As described previously, epileptic seizures may result from an imbalance between excitatory and inhibitory neurotransmitters [25]. Other studies have demonstrated that the hippocampal concentrations of amino-acids are affected by epilepsy [26, 27]. We confirmed these findings, indicating that the GABAA and Glu receptors play important roles in the development of seizures in a model of TLE. Resveratrol demonstrated neuroprotective properties against KA-induced epilepsy. Furthermore, our data showed that the antiepileptic effects of resveratrol in the hippocampus may be associated with maintenance of the Glu/GABA ratio.
The hippocampus is vulnerable to epilepsy-induced injury, especially in the CA1 region [20]. One of our previous studies revealed that resveratrol markedly protects against KA-induced neuronal death in both the CA1 and CA3 regions in a TLE model [13]. Glial proliferation is involved in the pathogenesis of the human hippocampus in TLE, and resveratrol treatment suppresses the KA-induced activation of astrocytes and microglial cells [28]. In the present study, we found that a test stimulus was able to evoke bursts of repetitive PSs in the CA1 region in KA-induced TLE model rats, and acute perfusion with 50 μmol/L resveratrol reduced these epileptiform discharges. From these data, we speculated that resveratrol inhibits the electrical activity of hippocampal pyramidal neurons. We used bicuculline to block a large portion of GABAAR-mediated inhibition in hippocampal pyramidal cells. Acute perfusion with 50 μmol/L resveratrol reduced the bicuculline-evoked epileptiform activity in the CA1 region. Similarly, Wan et al. demonstrated that cyclothiazide-induced epileptiform activity in CA1 neurons is suppressed by the activation of extrasynaptic GABAARs [29]. Together, these results further suggest that the GABAAR plays a prominent role in seizure generation, and resveratrol inhibits the electrical activity of hippocampal pyramidal neurons by enhancing GABAergic synaptic inhibition.
Feedback regulation is common in information processing by neuronal circuits [30, 31]. KA receptors play a key role in the regulation of synaptic network activity [32, 33]. Liu et al. showed that the activation of KA-type receptors leads to anxiety-related behaviors and the upregulation of glutamatergic synapses [34]. In our study, resveratrol alone had no apparent effect on postsynaptic Glu receptors (Fig. 1). Compared to normal rats, chronic resveratrol treatment causes no observable change in rat CA1 pyramidal neurons and does not alter the expression of Bcl-2 and Bax [11]. Much evidence suggests that the neuronal damage induced by KA in the hippocampal region is followed by neuronal hyperexcitability due to the lack of a tonic GABAAR effect in the acute phase [35, 36]. It has been reported that resveratrol increases Glu uptake [37]. Consistent with these results, we found that GluK2 and GABAAR-α1 expression decreased and the Glu/GABA ratio increased in the hippocampus during the acute phase of KA-induced epilepsy. Resveratrol increased the expression of both GluK2 and GABAAR-α1 without affecting the imbalance between excitatory and inhibitory amino-acids in the hippocampus. The inability of resveratrol to inhibit epileptic seizures in the acute phase may be due to increased KA receptor density in the hippocampus [38].
The silent phase is regarded as a characteristic interval in the time-dependent epileptogenic processes. Several studies have shown that GABAergic activity is suppressed during this period [39, 40]. Our results showed that the expression of GluK2 and GABAAR-α1 in the hippocampus was almost undetectable during the silent phase in the KA group and that resveratrol increased the expression of GABAAR-α1. This effect might be partly due to resveratrol causing an increased accumulation of GABA in an attempt to control brain excitability [41]. We speculate that there may be a temporal balance of the Glu/GABA ratio in the silent phase. In the chronic phase, the expression of GluK2 and GABAAR-α1 was significantly increased in the KA group, and resveratrol down-regulated GluK2 and increased the expression of GABAAR-α1. Meanwhile, resveratrol significantly decreased the Glu/GABA ratio in the hippocampus. This evidence supports our hypothesis that the antiepileptic effects of resveratrol are closely correlated with its suppression of Glu excitation and enhancement of the inhibitory effect of GABA, yet the detailed mechanism of this process remains unclear. Further investigations are needed to better understand the precise role of resveratrol in the up-regulation of GluK2 and GABAAR-α1 compared to the KA group.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81571293 and 30900420), and the National Century Excellent Talents in University of China (NCET-06-0557). We thank Drs. Vebra Cooper and Gongliang Zhang for the proofreading.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0097-2) contains supplementary material, which is available to authorized users.
References
- 1.Fisher RS, van Emde Boas W, Blume W, Elger C, Genton P, Lee P, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE) Epilepsia. 2005;46:470–472. doi: 10.1111/j.0013-9580.2005.66104.x. [DOI] [PubMed] [Google Scholar]
- 2.Rwlin P, Kahane P. The hidden causes of surgery-resistant temporal lobe epilepsy: extratemporal or temporal plus? Curr Opin Neurol. 2005;18:125–127. doi: 10.1097/01.wco.0000162852.22026.6f. [DOI] [PubMed] [Google Scholar]
- 3.Inostroza M, Brotons-Mas JR, Laurent F, Cid E, de la Prida LM. Specific impairment of “what-where-when” episodic-like memory in experimental models of temporal lobe epilepsy. J Neurosci. 2013;33:17749–17762. doi: 10.1523/JNEUROSCI.0957-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sobayo T, Mogul DJ. Rapid onset of a kainate-induced mirror focus in rat hippocampus is mediated by contralateral AMPA receptors. Epilepsy Res. 2013;106:35–46. doi: 10.1016/j.eplepsyres.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 6.Ge JF, Peng L, Cheng JQ, Pan CX, Tang J, Chen FH, et al. Antidepressant- like effect of resveratrol: involvement of antioxidant effect and peripheral regulation on HPA axis. Pharmacol Biochem Behav. 2013;114–115:64–69. doi: 10.1016/j.pbb.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Li XM, Zhou MT, Wang XM, Ji MH, Zhou ZQ, Yang JJ. Resveratrol pretreatment attenuates the isoflurane-induced cognitive impairment through its anti- inflammation and -apoptosis actions in aged mice. J Mol Neurosci. 2014;52:286–293. doi: 10.1007/s12031-013-0141-2. [DOI] [PubMed] [Google Scholar]
- 8.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 9.Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, et al. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia–reperfusion in mice. Life Sci. 2006;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Kim IK, Song W, Lee J, Park S. The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem Res. 2013;38:117–122. doi: 10.1007/s11064-012-0897-8. [DOI] [PubMed] [Google Scholar]
- 11.Li Z, Pang L, Fang F, Zhang G, Zhang J, Xie M, et al. Resveratrol attenuates brain damage in a rat model of focal cerebral ischemia via up-regulation of hippocampal Bcl-2. Brain Res. 2012;1450:116–124. doi: 10.1016/j.brainres.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Xu Q, Qian RB, Yu F, Yu L, Kong DH, et al. Temporal lobe epilepsy animal model established by stereotaxic microinjection of kainic acid. Neural Regeneration Research. 2008;3:436–440. [Google Scholar]
- 13.Wu Z, Xu Q, Zhang L, Kong D, Ma R, Wang L. Protective effect of resveratrol against kainate-induced temporal lobe epilepsy in rats. Neurochem Res. 2009;34:1393–1400. doi: 10.1007/s11064-009-9920-0. [DOI] [PubMed] [Google Scholar]
- 14.Hasegawa T, Takano F, Takata T, Niiyama M, Ohta T. Bioactive monoterpene glycosides conjugated with gallic acid from the leaves of Eucalyptus globules. Phytochemistry. 2008;69:747–753. doi: 10.1016/j.phytochem.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Rodríguez-Moreno A, Sihra TS. Presynaptic kainate receptor facilitation of glutamate release involves protein kinase A in the rat hippocampus. J Physiol. 2004;557(Pt3):733–745. doi: 10.1113/jphysiol.2004.065029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badawy RA, Harvey AS, Macdonell RA. Cortical hyperexcitability and epileptogenesis: Understanding the mechanisms of epilepsy-part 1. J Clin Neurosci. 2009;16:355–365. doi: 10.1016/j.jocn.2008.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Mulle C, Salier A, Pérez-Otaño I, Dickinson-Anson H, Castillo PE, Bureau I, et al. Altered synaptic physiology and reduced susceptibility to kainate- induced seizures in GluK2- deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- 18.Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, et al. Brooks-Kayal, Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation.3.Mechanisms. Electroencephalogr Clin Neurophysiol. 1972;32:295–299. doi: 10.1016/0013-4694(72)90178-2. [DOI] [PubMed] [Google Scholar]
- 20.Sloviter RS, Zappone CA, Harvey BD, Bumanglag AV, Bender RA, Frotscher M. “Dormant basket cell” hypothesis revisited: relative vulnerabilities of dentate gyrus mossy cells and inhibitory interneurons after hippocampal status epilepticus in the rat. J Comp Neurol. 2003;459:44–76. doi: 10.1002/cne.10630. [DOI] [PubMed] [Google Scholar]
- 21.Li M, Wang QS, Chen Y, Wang ZM, Liu Z, Guo SM. Resveratrol inhibits neuronal discharges in rat hippocampal CA1 area. Acta Physiol Sin. 2005;57:355–360. [PubMed] [Google Scholar]
- 22.Turner DA, Wheal HV. Excitatory synaptic potentials in kainic acid- denervated rat CA1 pyramidal neurons. J Neurosci. 1991;11:2786–2794. doi: 10.1523/JNEUROSCI.11-09-02786.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng ZY, Zheng XJ, Tian C, Wang Y, Xing HY. Changes of paired-pulse evoked responses during the development of epileptic activity in the hippocampus. J Zhejiang Univ Sci B. 2011;12:704–711. doi: 10.1631/jzus.B1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 25.Szyndler J, Maciejak P, Turzyńska D, Sobolewska A, Lehner M, Taracha E, et al. Changes in the concentration of amino acids in the hippocampus of pentylenetetrazole-kindled rats. Neurosci Lett. 2008;439:245–249. doi: 10.1016/j.neulet.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Kanamori K, Ross BD. Electrographic seizures are significantly reduced by in vivo inhibition of neuronal uptake of extracellular glutamine in rat hippocampus. Epilepsy Res. 2013;107:20–36. doi: 10.1016/j.eplepsyres.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hasegawa D, Matsuki N, Fujita M, Ono K, Orima H. Kinetics of glutamate and gamma-aminobutyric acid in cerebrospinal fluid in a canine model of complex partial status epilepticus induced by kainic acid. J Vet Med Sci. 2004;66:1555–1559. doi: 10.1292/jvms.66.1555. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, Yu S, Simonyi A, Rottinghaus G, Sun GY, Sun AY. Resveratrol protects against neurotoxicity induced by kainic acid. Neurochem Res. 2004;29:2105–2112. doi: 10.1007/s11064-004-6883-z. [DOI] [PubMed] [Google Scholar]
- 29.Wan L, Liu X, Wu Z, Ren W, Kong S, Dargham RA, et al. Activation of extrasynaptic GABA(A) receptors inhibits cyclothiazide-induced epileptiform activity in hippocampal CA1 neurons. Neurosci Bull. 2014;30:866–876. doi: 10.1007/s12264-014-1466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herman JP, McKlveen JM, Solomon MB, Carvalho-Netto E, Myers B. Neural regulation of the stress response: glucocorticoid feedback mechanisms. Braz J Med Biol Res. 2012;45:292–298. doi: 10.1590/S0100-879X2012007500041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu W, Wang T, Wang X, Han J. Ih channels control feedback regulation from amacrine cells to photoreceptors. PloS Biol. 2015;13:e1002115. doi: 10.1371/journal.pbio.1002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crépel V, Mulle C. Physiopathology of kainate receptors in epilepsy. Curr Opin Pharmacol. 2015;20:83–88. doi: 10.1016/j.coph.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 34.Liu B, Feng J, Wang JH. Protein kinase C is essential for Kainate-induced anxiety-related behavior and glutamatergic synapse upregulation in prelimbic cortex. CNS Neurosci Ther. 2014;20:982–990. doi: 10.1111/cns.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman LK, Goldstein B, Rafiuddin A, Roblejo P, Friedman S. Lack of resveratrol neuroprotection in developing rats treated with kainic acid. Neuroscience. 2013;230:39–49. doi: 10.1016/j.neuroscience.2012.10.063. [DOI] [PubMed] [Google Scholar]
- 36.Mazzuferi M, Palma E, Martinello K, Maiolino F, Roseti C, Fucile S, et al. Enhancement of GABA(A)-current run-down in the hippocampus occurs at the first spontaneous seizure in a model of temporal lobe epilepsy. Proc Natl Acad Sci U S A. 2010;107:3180–3185. doi: 10.1073/pnas.0914710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Almeida LM, Piñeiro CC, Leite MC, Brolese G, Tramontina F, Feoli AM, et al. Resveratrol increases glutamate uptake, glutathione content, and S100B secretion in cortical astrocyte cultures. Cell Mol Neurobiol. 2007;27:661–668. doi: 10.1007/s10571-007-9152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monaghan DT, Cotman CW. The distribution of [3H]kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 1982;252:91–100. doi: 10.1016/0006-8993(82)90981-7. [DOI] [PubMed] [Google Scholar]
- 39.van Rootselaar AF, van der Salm SM, Bour LJ, Edwards MJ, Brown P, Aronica E, et al. Decreased cortical inhibition and yet cerebellar pathology in ‘familial cortical myoclonic tremor with epilepsy’. Mov Disord. 2007;22:2378–2385. doi: 10.1002/mds.21738. [DOI] [PubMed] [Google Scholar]
- 40.González MI. The possible role of GABAA receptors and gephyrin in epileptogenesis. Front Cell Neurosci. 2013;7:113. doi: 10.3389/fncel.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li C, Yan Z, Yang J, Chen H, Li H, Jiang Y, et al. Neuroprotective effects of resveratrol on ischemic injury mediated by modulating the release of neurotransmitter and neuromodulator in rats. Neurochem Int. 2010;56:495–500. doi: 10.1016/j.neuint.2009.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.