Trans-neuronal viral tracing is becoming one of the most important methods for mapping neuronal circuit connections, but an anterograde trans-synaptic tracer compatible with functional assays is still lacking.
Adeno-associated virus (AAV) is replication-defective, and its production of offspring needs the help of adenovirus (AV) [1] or herpesvirus co-infection [2]. The replication deficiency, non-pathogenicity, easy manipulability, and broad tissue tropism make AAV a widely-used gene-transfer vector for expressing target genes.
The viral particle of AAV has no envelope. The nucleocapsid contains a single-stranded DNA genome of 4.7 kb, consisting of two open reading frames encoding replication (Rep) and capsid (Cap) proteins, flanked by two 145-base inverted terminal repeats, which allow for synthesis of the complementary DNA strand [3]. The Rep and Cap genes are essential for the production of AAV particles, but are not necessary for gene expression in cis [4]. Thus, when AAV is used as a gene-transfer vector, the transgene cassette with its own promoter is inserted between the two inverted terminal repeats, and the replaced Rep and Cap genes are complementarily supplied in trans together with other required AV genes (E4, E2a, and VA) for viral particle production. The resulting AAV is a replication-deficient pseudovirus that expresses target gene(s) in host cells but is incapable of replicating and spreading [4, 5].
Many AAV serotypes and the corresponding AAV vectors display neuronal tropism; these include AAVs 1, 2, 4, 5, 8, and 9. Besides introducing target genes into the central nervous system for gene therapy or target-gene expression for functional studies, AAV is also used for probing neuronal connectivity and dissecting circuit functions, including wide use in optogenetics and chemogenetics. AAV expressing fluorescent proteins has been used most often to visualize the physical axonal localization between two brain regions rather than the actual functional connection. But recent studies have suggested that AAV1 is also transported anterogradely down the axon [6, 7]. The investigation by Zingg et al. showed that AAV can be used for anterograde transsynaptic tagging [8].
AAV entry via endocytosis and its axonal transport have been documented [6, 7]. However, the trans-synaptic transmission of AAV has been neglected by most researchers. By injecting AAV expressing Cre recombinase (Cre) into the central nervous system of Ai14 transgenic mice (Cre-dependent tdTomato reporter), Zingg et al. demonstrated that AAV1 and AAV9 trans-neuronally transduced the postsynaptic neuron, in which AAV-expressed Cre led to tdTomato expression and thus visualization of the cell [8]. This anterograde trans-synaptic tagging is an important improvement of the AAV applications, and complements the current viral tracers for analyzing neuronal circuits.
The following applications of AAV were demonstrated in the paper by Zingg et al.: (1) Directly tagging downstream targets. Administering AAV1-Cre alone into Ai14 reporter mice can label the direct downstream targets. (2) Labeling the input-defined subpopulation. Sequentially injecting AAV1-Cre and AAV1-FLEX-GFP into the upstream and target brain regions can label a subpopulation of neurons in the target region which receives a direct projection from the upstream region. (3) Input-defined cell-type-specific labeling. Combined injection of AAV1-DIO-Flp (upstream region) and AAVDJ-fDIO-YFP (target region) in transgenic mice expressing Cre in a specific neuron type (Vglut2-Cre or GAD2-Cre), can label the cell type-specific neurons in a nucleus innervated by the given upstream region. (4) Compatibility with functional assays. With injection of AAV1-Cre into the upstream region and AAV1-DIO-ChR2-EYFP into the target region, it is possible to dissect the function of the cell group in the target region restrictedly receiving inputs from the upstream region by using optogenetic stimulation. The work of Zingg et al. exploited a novel application of AAV as an anterograde trans-synaptic tagging tool.
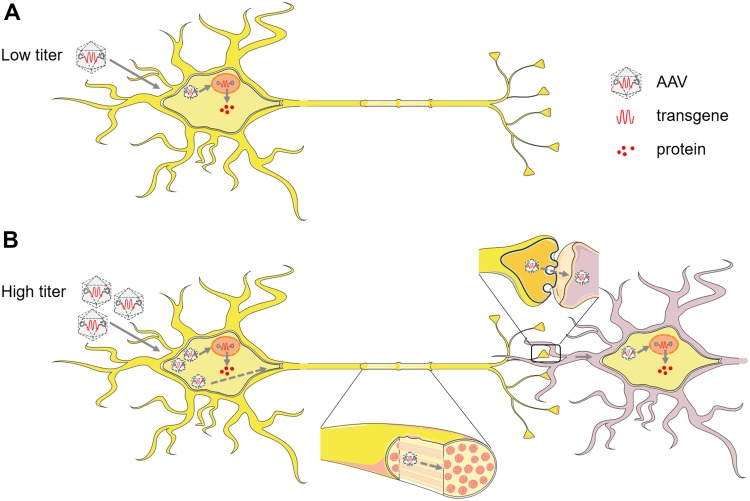
There are still limitations of AAV trans-synaptic tagging. (1) Terminal invasion. AAV1 retrogradely enters the presynaptic cells via axon terminal invasion, which limits its application in reciprocally-connected pathways. (2) Requirement for signal amplification. Either reporter mice or extra viral tools with flexed reporters are required for amplification of the fluorescence signal in the neurons trans-synaptically infected by AAV. (3) Requirement for high virus titer. Trans-neuronal AAV infection depends on a high initial virus titer (>1013 genome copies/mL), which probably results in an overload and spillover of virus particles (Fig. 1). (4) Relatively low efficiency. The replication-defective virus cannot amplify itself, and thus the efficiency of trans-neuronal tagging is low. The requirement for a high virus titer and the low efficiency of trans-neuronal tagging make the labeling reproducibility a considerable concern.
Fig. 1.
Potential mechanism of AAV trans-neuronal transduction. (A) Low titer of AAV. After entering the neurons by endocytosis, AAV particles are transported towards the nucleus through the cytoskeleton, and internalized into the nucleus, where they release the single-stranded DNA genome, which is converted to double-stranded DNA. Then the target transgene is expressed to synthesize its encoded protein. (B) High titer of AAV. Most of the AAV particles entering the cell follow the process described in A (solid lines). But the overloaded AAV capsids cannot all be uncoated and express the target gene in the initially-infected neurons. Thus, the uncoated virus is transported anterogradely along the axons to the terminal and released from the synapse, then re-enter the adjacent postsynaptic neurons, and expresses the target transgene at lower incidence (dashed lines).
In summary, this study proposed a promising novel application of AAV as a potential anterograde tracer, which may greatly facilitate mapping direct output maps and input-defined subpopulations as well as revealing their functions.
References
- 1.Atchison RW, Casto BC, Hammon WM. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 2.Stutika C, Huser D, Weger S, Rutz N, Hessler M, Heilbronn R. Definition of herpes simplex virus helper functions for the replication of adeno-associated virus type 5. J Gen Virol. 2015;96:840–850. doi: 10.1099/vir.0.000034. [DOI] [PubMed] [Google Scholar]
- 3.Hermonat PL, Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector - transduction of neomycin resistance into mammalian tissue-culture cells. Proc Natl Acad Sci U S A. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akkapaiboon P, Mori Y, Sadaoka T, Yonemoto S, Yamanishi K. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J Virol. 2004;78:7969–7983. doi: 10.1128/JVI.78.15.7969-7983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses - normal integration does not require viral gene-expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH. Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther. 2014;25:705–720. doi: 10.1089/hum.2013.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castle MJ, Perlson E, Holzbaur ELF, Wolfe JH. Long-distance axonal transport of AAV9 is driven by dynein and kinesin-2 and is trafficked in a highly motile Rab7-positive compartment. Mol Ther. 2014;22:554–566. doi: 10.1038/mt.2013.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. AAV-mediated anterograde transsynaptic tagging: mapping corticocollicular input-defined neural pathways for defense behaviors. Neuron. 2016;93:33–47. doi: 10.1016/j.neuron.2016.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]