Dear Editor,
Childhood Disintegrative Disorder (CDD), also known as Heller’s syndrome and disintegrative psychosis, is a rare progressive neurological disorder, characterized by a late onset (>2 years of age) and regression of language, social function, and motor skills [1]. Based on 4 surveys of CDD from different countries, the pooled estimate for the prevalence of CDD is 0.17/10,000, 600 times less prevalent than autism [2].
CDD is characterized by an onset between 3 and 4 years of age after a period of apparently normal development; a severe regression with the progressive loss or marked impairment of spoken language, loss of play, loss of social skills, and loss of bowel and bladder control; a prodromal period of behavioral disruption with extreme agitation, fearfulness, and possible hallucinations, and a poor outcome with severe intellectual deterioration.
CDD was first described by Thomas Heller in 1908. However, the definition has continued evolving over the past century, perhaps because of the rarity and lack of explanation for the condition. Initially, CDD was considered strictly a medical disorder and was believed to have identifiable medical causes. However, no specific medical or neurological cause has been found to account for all occurrences of the disorder by investigators who reviewed the reported cases [3]. Should CDD be considered a distinct diagnosis? Under the proposed DSM-5 revisions, all pervasive developmental disorders, including CDD, will be subsumed under the single diagnostic category of autism spectrum disorders. The rationale for this is the similarity between these disorders, as it is now thought that their symptoms place them on a continuum with autism. Thus, CDD is also considered a low-functioning form of autistic spectrum disorder [3].
Interestingly, mucopolysaccharidosis III (MPS III) is a rare genetic disease characterized by progressive cognitive decline and severe hyperactivity, with an onset between 2 and 6 years of age. Moreover, patients are often initially misdiagnosed as autism spectrum disorders, idiopathic developmental delay, attention deficit/hyperactivity disorder, or combinations of these, placing them at risk for unnecessary testing and treatments [4, 5]. Clinically, MPS III is composed of four different subtypes, each of which is caused by a deficiency in a different enzyme in the catabolic pathway of heparan sulfate, a type of glycosaminoglycan (GAG). All four subtypes are inherited in an autosomal recessive pattern. Collectively, the reported incidence of MPS III varies between 0.28 and 4.1 per 100,000 live births [6].
Here we report the case of a sibling presenting with CDD. First, two brothers were seen by a pediatric psychiatrist at the Outpatient Clinic of the Department of Child and Adolescent Psychiatry at the Shanghai Mental Health Center. They had both gradually lost language and life skills after the age of 4 years. According to their parents, the elder brother (K) is 10 years old, the younger brother (Z) is 8 years old, and both were born in Henan Province, China. They showed almost normal pervasive development before they were 4 years old. K was able to pronounce his first words (“mama”, “papa”) at 10 months and could walk at 12 months. He was able to use idiomatic phrases to communication when he was 2 years old. Z started to speak in single words (“mama”, “papa”) at 7 months, walk at 10 months, and use idiomatic phrases after 1 year. However, after reaching 4 years of age they both quickly regressed in terms of language, cognition, and social skills and behavior. K became incontinent and nonverbal after 5 years and started to communicate by screaming and crying without appropriate eye contact or facial expressions. He lost all ability to communicate at the age of 6. The general development of the younger brother, Z, was almost the same as his brother’s. After Z was 4 years old, he experienced social and learning problems. He ceased to show initiative in communications and merely responded to simple questions without making eye contact. Z became incontinent and showed intellectual deficiency after 5 years old. At the age of 10, when visiting a child psychiatrist for the second time, Z was only able to phonate single words, fuzzily.
The medical history of Z included intermittent convulsions, difficulty sleeping involving waking up and sitting on the bed 4–10 times per night, frequent ear infections, and diarrhea. K showed facial dysmorphias and gastrointestinal symptoms. On physical examination, the patients were conscious but apathetic and not in acute distress. The neurological examination was unremarkable. Their lungs were clear. K’s heart rate was 84 beats per minute, and Z’s was 80.
Clinical scales, including the Autism Diagnostic Interview-Revised (ADI-R), the Child Autism Rating Scale (CARS), and the Autism Behavior Checklist (ABC), were used to assess the general condition of each brother. In the ADI-R, the present scores of K and Z in the domain of reciprocal social interaction were 35 and 34, respectively (cutoff = 10). At the age of 4, the scores of reciprocal social interaction were 9 (K) and 2 (Z). The present scores of communication (verbal total) were 19 (K) and 25 (Z) (cutoff = 8), but they were 11 (K) and 1 (Z) at the age of 4. In the area of repetitive behaviors and stereotyped patterns, their scores were 0 (K) and 10 (Z) as of this work, and 5 (K) and 1 (Z) at the age of 4 (cutoff = 3) (Table S1). K’s CARS score was 60 (full marks) as of this work and 57 as of 2 years ago. Z’s CARS scores were 59 as of this work and 40 as of 2 years ago, indicating severe autistic symptoms of K and Z (Table S2). The general scores of K and Z in the ABC were >67, indicating that the deterioration had continued quickly over the past 2 years (Table S3).
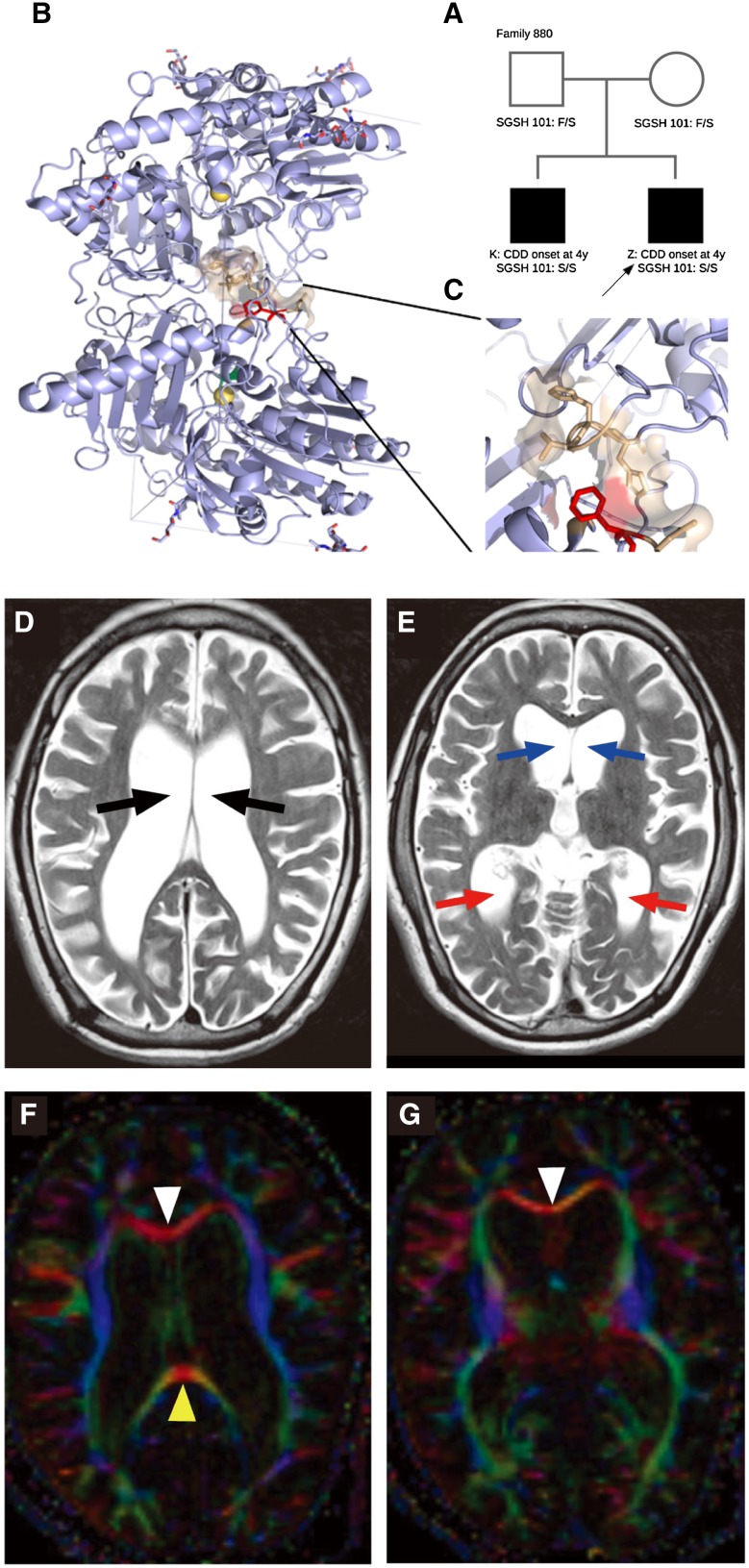
The particular family history and disease burden (Fig. 1A) strongly suggested an inherited disease with recessive, de novo X-linked or germline mutations. Therefore, whole-exome sequencing was performed on peripheral blood samples from the four-member family. One mutation, Chr17:78188885 A>G, SGSH (N-sulphoglucosamine sulphohydrolase) F101S, was found to fit the homozygous-recessive genetic model. The mutation was unique to the family. It was not found in any public database or in any of the in-house Chinese controls (12500). Both parents were heterozygous SGSH F101S carriers, and both offspring were homozygous SGSH F101S mutants (Fig. 1B). The mutation, located in the dimerization surface of the SGSH protein, may negate enzymatic function (Fig. 1C).
Fig. 1.
Genetic genogram, structure of protein SGSH, and images of the brain of patient K. A Genetic genogram. B, C Protein structure and Sanger sequencing for mutation sites in the SGSH gene. The F101 residue is shown in red, interacting residues from the other part of the enzyme dimer are shown in yellow. D Cerebral atrophy and ventriculomegaly (arrows) are shown on T2WI. E Blue and red arrows indicate anterior and posterior horns of the lateral ventricle. F, G Fractional anisotropy map showing a thin corpus callosum genu (white triangle) and splenium (yellow triangle).
To further identify abnormalities in the brain structure of the patients carrying SGSH mutations, magnetic resonance imaging (MRI) was performed on the elder brother, K, with T2WI and DTI. K’s gray matter had become thinner, with a larger sulcus in the bilateral frontal lobes and temporal lobes than in the occipital lobe. K’s supratentorial ventricles showed remarkable dilatation (Fig. 1D, E). An FA map based on DTI showed that the white matter fibers in K’s brain appeared sparse and degenerated, especially in the corpus callosum (Fig. 1F, G), while the healthy parents appeared normal under brain imaging examination (Fig. S1, S2).
Biochemical and metabolic assessments of MPS IIIA were performed to determine whether these brothers with homozygous SGSH mutations had any defects in glycol-metabolism. First, urine samples from both brothers underwent a color change by reaction with a cationic dye on treated filter paper, and excess GAG was noted in the brothers’ urine samples. In analytical electrophoresis tests, K’s sample showed bands of GAG excretion at dermatan sulfate and heparan sulfate (HS), and Z’s showed bands at chondroitin sulfate and HS areas. The results of enzyme activity assays showed that the activity of N-sulfoglucosamine sulfohydrolase had downgraded to 7.7 nmol/g/h (normal cutoff ≥119.6 nmol/g/h), but other enzymes were normal.
SGSH encodes N-sulfoglucosamine sulfohydrolase (MIM: 605270), and mutations hamper the degradation of heparan sulfate, which causes MPS IIIA, a rare autosomal-recessive inherited metabolic disease. The symptoms of MPS IIIA are caused by enzyme deficiency, preventing a necessary metabolic step in the degradation of heparan sulfate. In patients carrying loss-of-function mutations of SGSH, undegraded heparan sulfate accumulates in the central nervous system, where heparan sulfate in lysosomes leads to severe neurodegeneration [5, 7].
The SGSH mutation c.302T>C (p.F101S) has not been previously entered into the Human Gene Mutation Database (HGMD Professional 2015.12) (http://www.hgmd.cf.ac.uk/ac/index.php), which contains 118 mutations (including 91 missense/nonsense mutations) from MPS IIIA patients. Thus, the SGSH F101S mutation is considered to be a new recessive allele for MPS IIIA and CDD.
Due to its rarity, there have been few neuroimaging studies of CDD cases. The MRI data of a girl (8 years old) affected with MPS IIIA showed a thin corpus callosum in the posterior area [8]. In our patient K, the corpus callosum was thinner than usual and showed severe degeneration. In addition, the girl showed hyperintensity in the sub-cortical area and hippocampus on T2WI images and the imaging was not clear [8]. However in patients with MPS, abnormal brain structures are frequently observed under MRI and CT: Virchow-Robin perivascular spaces, white matter abnormalities, and ventriculomegaly [9].
From the viewpoint of symptomatology, the clinical features of patients K and Z corresponded to the diagnostic standards of CDD. Surprisingly, the child psychiatrist did not realize that they were affected by MPS IIIA before the results of whole-exome sequencing and biochemical and metabolic assessments became available. One study reported that when 21 children with SGSH gene mutations and enzyme deficiency were assessed using the Autism Diagnostic Observation Schedule, 13 met the criteria for autism. Among them, social and emotional abnormalities were most frequent, but repetitive behaviors and restricted interests were largely absent, which is consistent with the ADI-R results of the sibling [4]. Children affected by MPS IIIA may show autistic behaviors which lead to a misdiagnosis of ASD by psychiatrists or pediatricians [6, 10].
Although the pathogenetic mechanism of CDD is still not clear, a few reports have suggested a link between MPS IIIA and CDD/ASD [4]. According to previous reports, MPS III has an incidence of 1% among autistic patients, but the true rate might be lower [11]. A recent study screened 778 Turkish patients with ASD for inherited metabolic abnormalities by examination of urinary GAG and homocysteine levels. In that work, 300 of the patients whose metabolic and physical examinations met the study’s criteria were enrolled, and among them, one patient was diagnosed with MPS III [12].
In summary, this work describes a newly-discovered disease-causing mutation, F101S, in the SGSH gene, mutations of which impair the degradation of heparan sulfate and classically cause MPS IIIA. This case provides a possible genetic explanation for the specific symptoms of patients with CDD. It is worth noting that pediatric psychiatrists should be aware of potential metabolic diseases, particularly when a patient has a strongly-inherited tendency or specific regressive pattern. Genetic screening and biochemical tests are very effective to clarify the diagnosis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Chinese Academy of Sciences Strategic Priority Research Program, China (XDB02050400), the National Natural Science Foundation of China (91432111), and the Shanghai Second Medical University—Institute of Neuroscience Research Center for Brain Disorders, China (2015NKX005). We gratefully acknowledge the participation of the brothers with CDD and their parents, without whom this study could not have been done. We also acknowledge members of the laboratories of Drs. Qiu Z, Du Y, and Wang Z for valuable discussions.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0119-0) contains supplementary material, which is available to authorized users.
Contributor Information
Yi Zhang, Email: zy@eulertechnology.com.
Zilong Qiu, Email: zqiu@ion.ac.cn.
Ya-Song Du, Email: yasongdu@163.com.
References
- 1.Homan KJ, Mellon MW, Houlihan D, Katusic MZ. Brief report: childhood disintegrative disorder: a brief examination of eight case studies. J Autism Dev Disord. 2011;41:497–504. doi: 10.1007/s10803-010-1063-2. [DOI] [PubMed] [Google Scholar]
- 2.Fombonne E. Prevalence of childhood disintegrative disorder. Autism. 2002;6:149–157. doi: 10.1177/1362361302006002002. [DOI] [PubMed] [Google Scholar]
- 3.Hendry CN. Childhood disintegrative disorder: should it be considered a distinct diagnosis? Clin Psychol Rev. 2000;20:77–90. doi: 10.1016/S0272-7358(98)00094-4. [DOI] [PubMed] [Google Scholar]
- 4.Rumsey RK, Rudser K, Delaney K, Potegal M, Whitley CB, Shapiro E. Acquired autistic behaviors in children with mucopolysaccharidosis type IIIA. J Pediatr. 2014;164(1147–1151):e1. doi: 10.1016/j.jpeds.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wijburg FA, Wȩgrzyn G, Burton BK, Tylki-Szymańska A. Mucopolysaccharidosis type III (Sanfilippo syndrome) and misdiagnosis of idiopathic developmental delay, attention deficit/hyperactivity disorder or autism spectrum disorder. Acta Paediatr Int J Paediatr. 2013;102:462–470. doi: 10.1111/apa.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valstar MJ, Ruijter GJG, van Diggelen OP, Poorthuis BJ, Wijburg FA. Sanfilippo syndrome: A mini-review. J Inherit Metab Dis. 2008;31:240–252. doi: 10.1007/s10545-008-0838-5. [DOI] [PubMed] [Google Scholar]
- 7.Fedele AO. Sanfilippo syndrome: Causes, consequences, and treatments. Appl Clin Genet. 2015;8:269–281. doi: 10.2147/TACG.S57672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philippe A, Craus Y, Rio M, Bahi-Buisson N, Boddaert N, Malan V, et al. Case report: an unexpected link between partial deletion of the SHANK3 gene and Heller’s dementia infantilis, a rare subtype of autism spectrum disorder. BMC Psychiatry. 2015;15:256. doi: 10.1186/s12888-015-0631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calleja Gero ML, González Gutiérrez-Solana L, López Marín L, López Pino M a, Fournier Del Castillo C, Duat Rodríguez a. Neuroimaging findings in patient series with mucopolysaccharidosis. Neurologia. 2012;27:407–413. doi: 10.1016/j.nrl.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 10.de Bildt A, Sytema S, Zander E, Bölte S, Sturm H, Yirmiya N, et al. Autism diagnostic interview-revised (ADI-R) algorithms for toddlers and young preschoolers: application in a non-US sample of 1,104 children. J Autism Dev Disord. 2015;45:2076–2091. doi: 10.1007/s10803-015-2372-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritvo ER, Mason-Brothers A, Freeman BJ, Pingree C, Jenson WR, McMahon WM, et al. The UCLA-university of utah epidemiologic survey of autism: the etiologic role of rare diseases. Am J Psychiatry. 1990;147:1614–1621. doi: 10.1176/ajp.147.12.1614. [DOI] [PubMed] [Google Scholar]
- 12.Kiykim E, Zeybek CA, Zubarioglu T, Cansever S, Yalcinkaya C, Soyucen E, et al. Inherited metabolic disorders in Turkish patients with autism spectrum disorders. Autism Res. 2016;9:217–223. doi: 10.1002/aur.1507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.