Abstract
Schizophrenia is a severe mental disorder characterized by impaired perception, delusions, thought disorder, abnormal emotion regulation, altered motor function, and impaired drive. The default mode network (DMN), since it was first proposed in 2001, has become a central research theme in neuropsychiatric disorders, including schizophrenia. In this review, first we define the DMN and describe its functional activity, functional and anatomical connectivity, heritability, and inverse correlation with the task positive network. Second, we review empirical studies of the anatomical and functional DMN, and anti-correlation between DMN and the task positive network in schizophrenia. Finally, we review preliminary evidence about the relationship between antipsychotic medications and regulation of the DMN, review the role of DMN as a treatment biomarker for this disease, and consider the DMN effects of individualized therapies for schizophrenia.
Keywords: Schizophrenia, Default mode network, Task-negative network, Task-positive network, Antipsychotics, Resting state, fMRI, DTI
Introduction
The term “default mode network” (DMN) was first coined by Raichle [1] in 2001 with regard to the “resting-state” when the brain is not actively involved in tasks demanding attention, and has rapidly become a focus of research into neuropsychiatric disorders such as schizophrenia, a severe mental disorder characterized by impaired perception, delusions, thought disorder, abnormal emotion regulation, altered motor function, and impaired drive. Schizophrenia consists of a cluster of symptoms, such as positive (hallucinations and delusions) and negative symptoms (affective flattening and apathy), and cognitive deficits (impaired working memory). Because the component brain regions of the DMN have been widely found to be abnormal in schizophrenia, and the mental processes involved in this network are relevant to this disease [2, 3], detailed characteristics of the DMN disintegration in patients with schizophrenia may shed light on its pathogenesis. Recently, increasing numbers of reports have explored the role of the DMN in schizophrenia. Here, we examine the role of a disrupted DMN in the pathogenesis of schizophrenia, linking disturbed DMN connectivity and activity to its psychopathology and cognitive deficits. Importantly, the fundamental purpose of clinical neuroimaging in schizophrenia is to translate observations of the disorder’s neurophysiology into treatment benefits for patients. Antipsychotic medication is currently the cornerstone of treatment for schizophrenia. Antipsychotic agents exert their therapeutic action through antagonism of the dopamine (DA) D2 receptor. Previous studies have suggested a potential relationship between DA signaling and DMN modulation [4–6]. We therefore further explore the effects of antipsychotics on the DMN to provide new insights into the mechanisms of action of this treatment and identify potential therapeutic targets for this disease.
The review begins by defining the DMN and describing its functional neuroanatomy, anatomical connections, and heritable basis. Second, we review the cross-sectional empirical studies of the anatomical and functional DMN in schizophrenia, and summarize the relationship between the DMN and the psychopathology of this disorder, such as positive and negative symptoms and cognitive impairments. Finally, we review preliminary findings on the association between antipsychotic treatment and modulation of the DMN, and seek their potential clinical implications for therapeutic mechanisms of action in schizophrenia.
The Default Mode Network
The DMN has become a central theme in schizophrenia and other neuropsychiatric disorders since it was addressed in a positron emission tomography (PET) study in 2001 [1]. Functional neuroimaging, such as PET and functional magnetic resonance imaging (fMRI), as well as structural neuroimaging such as diffusion tensor imaging (DTI) has been widely used to explore DMN characteristics. Here, we describe five detailed elements of the DMN: its definition, activity/deactivation, connectivity, genetic underpinnings, and anti-correlation with the task positive network (TPN).
Definition
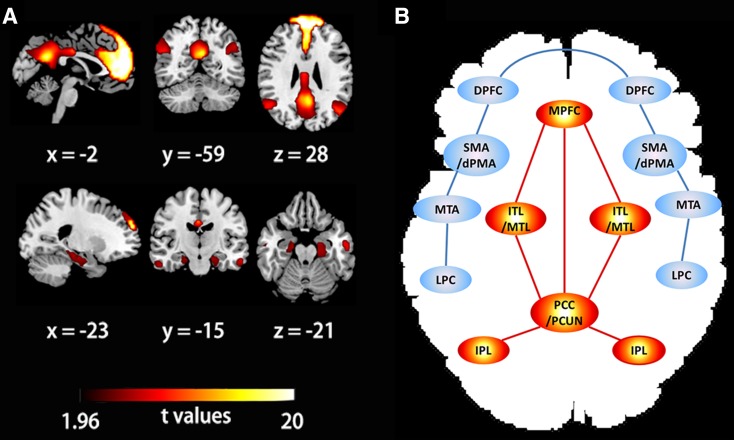
“Default mode” is a term relevant to the brain’s intrinsic functionality when it is at “rest” and this mode also continues during the performance of many tasks. “At rest” denotes a state in which one is alert and awake, but not actively engaged in tasks demanding attention. At rest, neural communication consumes 60%–80% of the brain’s energy, while during task conditions it accounts for only 0.5%–1% [7]. This cost-based comparative analysis indicates that intrinsic function during rest is at least as vital as stimulus-oriented activity during task performance in understanding the functional characteristics of the brain. The term “DMN” refers to a resting-state network that shows greater activity during the resting state than when performing tasks in a specific constellation of brain regions, including the posterior cingulate cortex and adjacent precuneus (pCC/PCUN), medial prefrontal cortex (mPFC), mesial and inferior temporal lobes (mTL/iTL), and inferior parietal lobe (iPL) [8, 9] (Fig. 1A and B). The DMN is deactivated during the initiation of a task but is active during the resting state with a high degree of connectivity across the brain areas. In PET studies, the two midline regions of the DMN, pCC and mPFC, consistently demonstrate such task-negative but rest-positive conditions across a great variety of cognitive tasks, such as working memory, a visuomotor task, a visual language task, and a mental imagery task [10, 11].
Fig. 1.
Components of the DMN and TPN. A Spatial components of the DMN. Spatial maps of the DMN were generated using independent component analysis in a group of 38 healthy volunteers, all of whom were from the control group in our previous study [65]. B Simplified illustration of the principal regions for the two anti-correlated networks, the DMN (red) and TPN (blue). Abbreviations: DMN, default mode network; TPN, task positive network; PCC/PCUN, posterior cingulate cortex and adjacent precuneus; MPFC, medial prefrontal cortex; ITL/MTL, inferior/mesial temporal lobe; IPL, inferior parietal lobe; DPFC, dorsolateral PFC; MTAs, middle temporal area; SMA, supplementary motor area; dPMA, dorsal premotor areas; LPC, lateral parietal cortex.
Activity/Deactivation within the Default Mode Network
PET provides quantitative and absolute measurements of regional oxygen consumption and blood flow when an arterial input function is obtained to determine radiotracer delivery and clearance, or relative regional activity when no arterial input function is obtained, and then the changes in brain activity can be mapped based on alterations in regional blood flow. fMRI is sensitive to blood oxygenation level, and brain activity is inferred from blood oxygen level dependent (BOLD) signal because oxygen binding to hemoglobin alters its paramagnetic properties. Only a lower amplitude of low frequency fluctuation between 0.08 and 0.1 Hz in BOLD signals is considered to represent neural activity, with higher frequencies (>0.1 Hz) reflecting respiratory and/or cardiac factors [12]. fMRI studies in healthy volunteers [13–15] have consistently demonstrated task-induced deactivation (or reduced activity) in a particular set of regions, including pCC/PCUN, mPFC, and other areas, which overlap with the DMN regions previously found by Raichle using a PET technique [1]. The DMN hypothesis is based on the observation of relative activity decreases in spatially separated areas during task performance compared with the activity during the resting state. However, direct evidence for how spatially distinct regions are connected functionally has become a vital concern in neuroimaging studies, and this is discussed below.
Connectivity within the Default Mode Network
Calculation of the temporal coherence of neuronal BOLD signals between spatially distinct regions is a common analytical approach for measuring functional connectivity-fMRI (fc-fMRI) [12]. This fc-fMRI method has been extensively used to investigate the spatial patterns of coherent BOLD fluctuations within the DMN [16]. The simplest analysis technique for assessing the temporal coherence of brain activity is to extract the BOLD signals from regions of interest (ROI, also termed seed regions) and then compute the temporal coherence coefficient between the seed region and another node (i.e. connectivity between regions A and B) [12], or between the seed region and all the remaining voxels or nodes in a network (i.e. connections of region A) [17]. To overcome the disadvantage of ROI analysis that a priori definition of the seed region is required, a popular data-driven technique named independent component analysis (ICA) [18] has been extensively used in functional connectivity analysis for the DMN. These two techniques are very common for detecting temporal coherence in BOLD time courses within the DMN, although other algorithms have also been proposed (reviewed by Fox and Raichle [12]). fc-fMRI studies can further our understanding of the functional neuroanatomy of the DMN in healthy individuals as well as in patients with neuropsychiatric disorders. In a resting-state fc-fMRI study, Greicius and colleagues [8] found that pCC shows significant functional connectivity with mPFC, inferior parietal cortex, parahippocampal gyrus, and inferior temporal cortex within the DMN in healthy volunteers, and these connections are minimally disrupted during task performance, indicating preservation of DMN functional connectivity across the rest and task conditions. Functional connectivity between pCC/PCUN and mPFC, and pCC/PCUN and mesial temporal cortex within the DMN was detected in both healthy controls and patients with mesial temporal lobe epilepsy, demonstrating the robustness of these relationships in health and disease [19].
Given the specific functional neuroanatomical organization based on the BOLD signal in the DMN, some questions arise, for example, “do the temporal correlations of BOLD signals reflect white matter tract connections?”, and “what is the relationship between anatomical and functional connectivity within the DMN?”. Studies have addressed the relationship of functional connectivity to structural connectivity such as white matter tract integrity. Combining the two common neuroimaging methods fc-fMRI and in vivo DTI tractography has directly addressed these questions. Greicius et al. [8] first explored the relationship between functional and anatomical connectivity within the DMN in healthy volunteers, and found some regions that have corresponding structural fiber pathways, such as the pCC/PCUN projection to mPFC and bilateral mTLs that has significant functional connectivity, while some significant functional connections, such as that between mPFC and mTLs, exist without known underlying direct fiber tracts [8]. Of note, fiber tracts in the DMN, such as those connecting pCC to bilateral mTLs [20], and pCC to mPFC [21, 22], are also detectable between homologous regions in nonhuman primates using effective tracers, suggesting the effectiveness of in vivo DTI tractography in tracing tracts. Subsequent neuroimaging studies [19] combining DTI tractography and fc-fMRI have confirmed a relationship between functional and anatomical connections within the DMN in agreement with Greicius et al. [8], further implying that regions that are connected directly by fiber tracts also have significant functional connections, but the inverse condition need not be true because functional connections might be linked indirectly via multisynaptic connections or more distant cortical regions.
All the above findings imply that, rather than investigating the DMN modalities separately, combining functional and anatomical measures in the same individuals can ultimately enrich our understanding of the DMN in neuropsychiatric disorders. Consistently, in a recent review, Liu et al. [23] proposed that multimodal neuroimaging analysis, identifying both the functional connections and their anatomical basis, may become a major driver for targeting disease and treatment/treatment response biomarkers with high sensitivity and specificity.
Genetic Underpinnings of the Default-Mode Network
It has been estimated that heritability for connections within the DMN is ~0.424 [24]. Heritability is defined as the portion of phenotypic variance in a population attributable to genetic variance. The heritability of DMN connectivity supports the use of DMN characteristics as intermediate phenotypes or endophenotypes of neuropsychiatric disorders. Furthermore, exploration of the genes associated with the intrinsic functional organization of the DMN could further our understanding of the molecular mechanisms underlying DMN connections. One study using both genetic and fMRI analyses links neuronal excitability genes to schizophrenia, working memory, and brain activity in a portion of the DMN (parietal cortex) [25]. Moreover, a recent study [26] published in Science further identified genes mediating the intrinsic function of the DMN. In this study, Richiardi and colleagues found that both common polymorphisms and the expression levels of 136 genes are associated with the connectivity strength of the DMN, and disease annotations for the gene list showed that the 136 genes are significantly associated with 9 neuropsychiatric diseases, including schizophrenia. What is more, the expression levels of these 136 genes were also associated with axonal connectivity in the mouse brain. The heritability of the DMN connections and its role as a biomarker in understanding the pathophysiology and treatment of schizophrenia, which has an estimated heritability of almost 80% [27], may mean that the responsible genes overlap and explain the DMN findings in schizophrenia.
Anti-correlation with the Task Positive Network
Interestingly, the DMN, a task-negative network, is temporally anti-correlated with the TPN, which is, in turn, considered to be associated with task-induced increased alertness [28]. The TPN includes dorsolateral PFC (dlPFC), bilateral middle temporal gyrus, supplementary motor areas, dorsal premotor regions, and lateral parietal cortex [29, 30] (Fig. 1B). The TPN is generally considered to be linked to externally oriented attention, whereas the DMN is associated with introspectively oriented cognitive processes such as self-referential and reflective activity. The inverse correlation of activity in the two types of networks may reflect a competition for attentional resources between external and internal orientation, competitively allocating attentional resources to ready the brain itself, or to keep the brain alert [31].
Because of the strength of the inverse correlation between the two networks, a case has been recently made for considering the TPN as a component of the DMN [9, 28]. Furthermore, an abnormal pattern of inverse correlation or imbalance between these two networks has been proposed to play an important role in the pathological mechanisms underluing some neuropsychiatric disorders [32, 33], including schizophrenia [34]. Third, a robust inverse correlation between these two networks might be functionally more important than the activity in the DMN alone [28, 35]. We therefore review both networks in schizophrenia.
Functional Activity/Deactivation and Connectivity within the DMN in Schizophrenia
Activity/Deactivation in the DMN in Schizophrenia
Many fMRI studies have demonstrated abnormal activity or deactivation within the DMN areas in patients with schizophrenia during a broad range of tasks, including the auditory oddball task [36–38], multi-source interference task [39], working memory task [40–42], selective attention task [43], and semantic repetition priming task [44]. Garrity and colleagues [36] first used fMRI to examine the auditory oddball task effects on the DMN in chronic schizophrenia patients. They found that activity in mPFC, pCC/PCUN, and left inferior and middle temporal cortex when processing the task is correlated with the severity of positive symptoms. Moreover, the abnormally increased deactivation of the PFC/anterior cingulate cortex (ACC) within the DMN in schizophrenia [36] is consistent with greater deactivation in both of the midline loci (PFC/ACC and pCC/PCUN) in the DMN in male patients with chronic schizophrenia relative to matched healthy volunteers during task performance [39, 45]. Moreover, the magnitude of PFC deactivation is correlated with patients’ task performance and emotional awareness of others [39].
However, abnormally increased deactivations of the PFC/ACC or pCC/PCUN within the DMN in these two studies [36, 39] contrast with the findings of other studies [40, 41, 44]. The n-back working memory task produces less deactivation in the PFC/ACC [40]. Decreased task-related suppression in the mPFC is correlated with working memory performance and psychopathology in schizophrenia [41]. Furthermore, Jeong et al. [44] found less deactivation in the two major DMN components (pCC/PCUN and mPFC) and less activity in the task-related areas (supramarginal and inferior frontal gyri) during the semantic priming task and vice versa during rest in chronic schizophrenia patients than in controls.
Based on the available literature, both abnormally increased and decreased DMN deactivations (or activity suppressions) have been reported in medicated patients with chronic schizophrenia (Table 1). The use of different tasks, the technical aspects of image acquisition and analysis, lack of a gold standard, underpowered studies, and extreme comparisons, as well as antipsychotic treatment effects may partly explain the current contradictory findings [46]. Therefore, further confirmation of the current results needs the evaluation of larger sample sizes of drug-naive first-episode schizophrenia patients (FESPs) as well as the examination of medication effects.
Table 1.
Summary of empirical studies on DMN activity and connectivity in schizophrenia.
| Study | Participants | Duration (mo/y) | Patient age (y) | Measure | Task/Rest | **Activity/deactivation | Connectivity | Key finding | |
|---|---|---|---|---|---|---|---|---|---|
| **Function | Anatomy | ||||||||
| Garrity 2007 [36] | 21 treated CSP, 22 C | – | 41.0 ± 10 | fMRI_3T_ICA | AOT | ↑Deactivation in PFC/ACC | Different spatial patterns in PFC, ACC, and PHG | – | *Related to PS |
| Harrison 2007 [39] | 12 treated male CSP, 14 male C | – | 32.2 ± 8 | fMRI_3T_ROI | MSI | ↑Deactivation in PFC/ACC and PCC/PCUN | – | – | *Related to CF and emotional awareness |
| Bluhm 2007 [47] | 17 treated CSP, 17 C | 117.4 ± 159.1 mo | 33.5 ± 13.8 | fc-fMRI_4T_ROI | RS | – | PCC-MPFC↓, PCC-cerebel↓, PCC-LPL↓ | – | *Related to PS and NS |
| Zhou 2007 [34] | 3 drug-naïve FESP, 15 treated CSP, 18 C | 25 ± 18 mo | 23.7 ± 4.9 | fc-fMRI_1.5T_ROI | RS | – | DMN↑; TPN↑; anti-correlation↑ | – | – |
| Calhoun 2008 [37] | 20 treated CSP, 20 C | – | 39.7 ± 10.1 | fc-fMRI3T_ICA | RS + AOT | – | – | – | HFF in CSP, LFF in C |
| Jafri 2008 [49] | 29 treated CSP, 25 C | – | 38.4 ± 11.3 | fc-fMRI_3T_ICA | RS | – | DMN↑ | – | – |
| Pomarol-Clotet 2008 [40] | 32 treated CSP, 32 C | 21.8 ± 9.1 y | 41.6 ± 8.8 | fMRI_GLM | WM | ↓ACG/MPFC deactivation; ↓DPFC activation | – | – | *Related to CF |
| Whitfield-Gabrieli 2009 [41] | 13 mostly treated patients***, 13R, 13 C | – | 22.1 ± 2.1 | fc-fMRI_3T_ROI | WM | ↓MPFC deactivation | DMN↑; TPN↑; anti-correlation↓ | – | *Related to PS and CF |
| Kim 2009a [38] | 66 treated CSP/SAP, 71 C | – | 37.4 ± 11.7 | fMRI_3T/1.5T_ICA | AOT | ↑Deactivation in DPFC | – | – | – |
| Kim 2009b [42] | 115 treated CSP, 130 C | – | 35.8 ± 11.0 | fMRI_1.5T/3T/4T_ICA | WM | ↑Deactivation in DPFC/IPL | – | – | – |
| Wolf 2009 [57] | 16 treated CSP, 16 C | 83.3 ± 57.2 mo | 34.2 ± 6.1 | fMRI_1.5T_ICA | WM | – | Decoupling of DMN and TPN | – | *Related to PS, NS, and CF |
| Mannell 2010 [43] | 16 treated CSP, 16 C | – | 40.2 ± 8.2 | fMRI_1.5T_ICA/ROI | RS + SAT | ↑Activation in PCC/PCUN | – | – | – |
| Jeong 2010 [44] | 10 male CSP, 10 male C | ~17 y | 39.7 ± 8.1 | fMRI_1.5T_ICA | SPT | ↓Deactivation in PCC/PCUN and MPFC; ↓activation in IFG | – | – | – |
| Skudlarski 2010 [55] | 27 treated CSP, 27 C | – | 38 ± 10 | fc-fMRI_3T_global network analysis + DTI | RS | – | DMN↑ | DMN↓ | – |
| Shim 2010 [48] | 19 UHRP, 20 C | – | 20.8 ± 4.1 | fc-fMRI _1.5T_ROI | RS | – | DMN↑; anti-correlation↓ | – | – |
| Sambataro 2010 [58] | 13 FESP, 6 treated CSP, 19 C | 44.1 ± 67 mo | 24.8 ± 5.7 | fc-fMRI_3T_ICA | WM | – | PCC↓, IPL↑, and PCUN↑**; vmPFC↑**** | – | – |
| Surguladze 2011 [59] | 32 treated CSP, 16 C | 15.3 ± 7.5 y | 42.6 ± 11.7 | fMRI_1.5T_ROI | FEE | ↑Deactivation in vmPFC in risperidone vs typical antipsychotics | – | – | – |
| Schneider 2011 [45] | 26 treated CSP, 13 C | 10 ± 7 y | 34 ± 7 | fMRI_1.5T_ROI | N-Back | ↑Deactivation in PCC/MPFC | – | – | – |
| Camchong 2011 [53] | 29 treated CSP, 29 C | 20.2 ± 8.9 y | 41.3 ± 9.3 | fc-fMRI_3T_ICA+DTI | RS | – | MPFC/ACG↓ | MPFC↓ | *Related to PS, NS and CF |
| Chai 2011 [56] | 16 treated CSP, 15 C | ~20 y | 41.6 ± 2.6 | fc-fMRI_3T_ROI | RS | – | Decoupling of DMN and TPN; anti-correlation↓ | – | – |
| Mingoia 2012 [50] | 25 treated CSP, 25 C | – | 30 ± 7.3 | fc-fMRI_3T_ICA | RS | – | DMN↑, DPFC↓ | *Related to NS | |
| Liemburg 2012 [52] | 25 PGI, 19 PPI, 30 C | PGI 10.5 ± 9.6 y; PPI 8.9 ± 8.2 y | PGI 33.4 ± 11.2; PPI 35.9 ± 11.9 | fc-fMRI_3T_ICA | RS | – | DMN↑ (PGI vs PPI) | *Related to self-insight | |
| Liu 2012 [29] | 6 untreated FESP, 19 treated CSP, 25 siblings, 25 C | 18.3 ± 15; 8 mo | 25.6 ± 6.8 | fc-fMRI _1.5T_ROI | RS | – | DMN↑; anti-correlation (NS) | – | – |
| Gerretsen 2014 [51] | 12 treated CSP, 8 SAP | 18.2 ± 11.9 y | 43.0 ± 12.1 | fc-fMRI_1.5T_ROI | RS | – | DMN↑ | *Related to self-insight | |
| Curcic-Blake 2015[54] | 45 treated CSP; 19 C | 15.2 ± 14.1 y | 35 ± 11 | fc-fMRI_3T_ dynamic causal modelling +DTI | SRT | – | Connectivity between vmPFC and DMN regions↑ | Tract integrity↓ | *Related to self-insight |
Abbreviations: CSP, chronic schizophrenia patients; C, controls; R, relatives; FESP, first-episode schizophrenia patients; UHRP, ultra-high risk for psychosis; SAP, schizoaffective patients; EPSP, early-phase schizophrenia patients; PGI, patients with good insight; PPI, patients with poor insight; T, Tesla; y, years; mo, months; fMRI, functional magnetic resonance imaging; DTI, diffusion tensor imaging; ICA, independent components analysis; GLM, general linear model; ROI, region of interest; TPN, task-positive network; RS, resting state; AOT, auditory oddball task; MSI, multi-source interference; SPT, semantic priming task; FEE, facial emotional expressions; SRT, self-reflection task; SAT, selective attention task; HFF, high-frequency fluctuations; LFF, low-frequency fluctuations; PS, positive symptom; NS, negative symptom; CF: cognitive function; DMN, default mode network; PCC, posterior cingulate cortex; PCUN, precuneus; IFG, inferior frontal gyri; LPL, lateral parietal lobe; IPL, inferior parietal lobe; MPFC, medial prefrontal cortex; PFC, prefrontal cortex; vmPFC, ventromedial prefrontal cortex; DPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; PHG, parahippocampal gyri; (–) not tested; (↑) for resting fMRI, increased activation; for task fMRI, increased deactivation; (↓) for resting fMRI, decreased activation; for task fMRI, reduced deactivation; (NS) not significant; (*) relationship between DMN activity/deactivation (or connectivity) and particular behavioral measures; (**) group differences of schizophrenia patients versus controls; (***) patient group includes patients in the early phase of schizophrenia, schizoaffective and schizophreniform disorder; (****) comparison of patients before and after antipsychotic agents.
Functional Connectivity in the DMN in Schizophrenia
Abnormal functional connectivity of the DMN in schizophrenia patients has been investigated during both rest [29, 34, 47–49] and task conditions [36, 41]. Using resting-state fc-fMRI with ROI analysis, Bluhm et al. [47] first reported anomalies in the temporal coherence of neuronal BOLD signals associated with the DMN in patients with schizophrenia. Specifically, patients in this study had decreased functional connectivity between the PCC seed region and other regions, including medial prefrontal, lateral parietal, and cerebellar regions, relative to controls. This report also demonstrated that positive symptoms are positively associated with functional connections between the PCC seed region and regions in auditory and attentional cortex associated with hallucinations, including bilateral temporal gyrus and premotor areas, and negative symptoms are negatively correlated with connections linking PCC to right superior temporal gyrus, ACC and premotor areas, and left inferior frontal gyrus.
In contrast to Bluhm et al. [47], using the same resting-state fc-fMRI with ROI analyses, other researchers consistently found increased functional connectivity within the DMN in schizophrenia patients (including medication-naïve and medicated chronic patients) [29, 34] and in individuals at ultra-high risk for psychosis [48] relative to healthy volunteers. Liu et al. extended these findings to unaffected siblings [29], suggesting that abnormally increased functional connectivity of the intrinsic networks may be an endophenotype of schizophrenia. Furthermore, hyper-connectivity of the DMN has also been reported using task fc-fMRI in schizophrenia patients and in the first-degree relatives of probands with schizophrenia relative to healthy controls during a working memory task [41]. Interestingly, an association between abnormally increased functional connectivity and hyperactivation in the DMN has also been reported in schizophrenia patients (including those in the early phases of schizophrenia and schizoaffective and schizophreniform disorder) and may be part of the pathophysiology of schizophrenia and underlie risk for this disorder [41]. Furthermore, lower connectivity strength in the dlPFC (center of the TPN) and temporal areas, and hyperconnectivity in PFC (center of the DMN) in the resting state [50], are simultaneously found in medicated chronic patients, and are associated with negative symptoms. This study segregates different connectivity dysfunctions in schizophrenia patients and provides further evidence that aberrant hyperconnectivity in part of the DMN is correlated with the core pathophysiology of schizophrenia. In addition, Garrity et al. [36] reported that when processing the auditory oddball task, chronic schizophrenia patients (CSPs) have significantly different spatial functional connectivity patterns in the DMN than healthy volunteers, most significantly in the PFC, ACC, and parahippocampal gyri. Moreover, chronic schizophrenia is associated with more high-frequency fluctuation and less low-frequency fluctuation in the DMN than healthy volunteers when performing the auditory oddball task [36], which is consistent with the subsequent findings of fluctuation in the DMN they reported in different CSP samples and controls [37]. Work by Gerretsen et al. [51] and Liemburg et al. [52] reported less resting-state functional connectivity in the DMN in schizophrenia with self-insight (awareness of disease) but greater functional connectivity in schizophrenia with impaired self-insight.
On the basis of recent evidence, functional hyperconnectivity within the DMN is perhaps the most common finding in comparisons of schizophrenia patients with healthy controls. It has been extensively found in different schizophrenia populations, including chronic paranoid schizophrenia, first episode schizophrenia, individuals at ultra-high risk for psychosis, and the first-degree relatives of patients with schizophrenia. Moreover, we propose that such hyperconnectivity does not reflect a specific brain state or methodological approach, because it has been reported both at rest and during a task, using a variety of analytic methods including ROI- and ICA-based fc-fMRI.
Anatomical and Functional Connectivity within the DMN in Schizophrenia
Rather than examining functional connections separately, Camchong et al. [53] first integrated DTI and fc-fMRI to fully evaluate the anatomical interactions in white matter, and the functional connectivity of regions in the gray matter within the DMN for chronic schizophrenia. Patients have decreased functional connections in the mPFC during rest, and consistently, reduced white matter integrity in the mPFC. Moreover, abnormally decreased connectivity in the mPFC of patients is correlated with positive and negative symptoms, and cognitive impairments [53].
With the hypothesis that the changes in the functional and anatomical self-reflection network (centered on the ventromedial PFC) in schizophrenia are associated with the degree of impaired self-insight, one study [54] reported that schizophrenia patients have significantly increased functional connectivity from some DMN regions, such as pCC, mPFC and iPL, to the ventromedial PFC during processing of the self-reflection task, but lower white matter integrity in anatomical connections than in healthy controls. Moreover, abnormal changes of anatomical and functional connectivity within the self-reflection network are associated with impaired self-insight in schizophrenia, suggesting that patients’ self-insight might be influenced by synaptic alterations, which induce lower fiber tract integrity and then lead to compensatory functional hyperconnectivity as a functional neural response to anatomical deficits [54]. Consistently, using global network analysis, another study [55] also reported higher resting-state functional connectivity but lower anatomical connectivity in the DMN regions in schizophrenia patients than in healthy volunteers, and therefore confirmed that anatomical connection deficits in schizophrenia may lead to functional reorganization of the DMN, resulting in functional hyperconnectivity between anatomically connected regions.
Integrating both anatomical and functional measures can provide more detailed descriptions of DMN connectivity underlying schizophrenia. The preliminary reports on the functional response of neural compensation for structural connectivity deficits [54, 55], as well as the finding of consistently fewer alterations in both the anatomical and functional connectivity within the DMN [53], imply that it would be beneficial to explore how anatomical connectivity abnormalities mediate changes in the functional connections within the DMN in schizophrenia. It is worth mentioning that none of the three studies used DTI analysis of individual white matter tracts within the DMN. Future studies investigating specific connections within the DMN are warranted for the more precise description of the white matter tracts affected and the functional components involved. Moreover, only one [53] of the above studies was conducted and driven by the hypothesis of DMN abnormalities in schizophrenia. Therefore, more studies combining fc-fMRI and DTI to specifically evaluate the functional and anatomical connections within the DMN are needed to confirm the current findings and further test the hypothesis of DMN abnormalities in the DMN.
Anti-correlated Networks
A recent review of the rationale for the TPN and DMN and preliminary findings of the two networks in schizophrenia proposes that the two anti-correlated networks are important to further understand task performance and self-referencing, and to extend current neuronal circuit models of schizophrenia [30]. Using resting-state fc-fMRI, Zhou et al. [34] first explored the anti-correlations between the TPN and DMN in paranoid patients. Their research demonstrated increased correlations within both of the networks and consistently increased anti-correlations between the two networks in paranoid patients relative to healthy individuals. The abnormally increased connectivity and anti-correlations are primarily located in the regions of the DMN that include dorsolateral MPFC, inferior temporal gyrus, and lateral parietal lobe, as well as in regions of the TPN such as dlPFC. The abnormal increases of functional connectivity within each of the two networks could be the source of aberrant sensitivity to both the self-referential and external environment; and excessive antagonism between the two networks, i.e. abnormally increased anti-correlations, would likely induce over-zealous toggling between introspective and exterospective processes in the resting brain of paranoid patients [34].
More compelling support for the involvement of anti-correlated networks in schizophrenia patients comes from Whitfield-Gabrieli et al. [41], who detected the toggling between the DMN and TPN regions and the transition from task to rest state in patients in the early phases of schizophrenia, schizoaffective or schizophreniform disorder, first-degree relatives of patients with schizophrenia, and healthy controls. However, in contrast to the study of Zhou et al. [34], this study found significantly reduced anti-correlations between the two networks in both patients and their relatives, suggesting reduced anti-correlations in the pathology and genetic risk of schizophrenia [41]. Other studies also found significantly reduced anti-correlations between the DMN and TPN in schizophrenia patients [56, 57] and individuals at ultra-high risk and prior to the onset of psychosis [48]. However, one study found no significant difference of anti-correlation between the dlPFC and other regions in the DMN in schizophrenia patients relative to healthy controls [29]. Such a disparity may result from the different analytical methods used to measure functional connectivity, for example, different seed region selection. In addition, the schizophrenic patients in all the studies of the anti-correlated networks were at different stages of the disorder, with a wide range of illness durations (Table 1). Therefore, the possibility that the inconsistent findings are influenced by the heterogeneity of schizophrenic patients should also be taken into account.
Initial Studies of the Mediating Role of the DMN as a Treatment Biomarker for Schizophrenia
It is worth mentioning that although psychotic symptom or cognitive function scales are routinely used for evaluating treatment-related changes of patients’ symptom severity, longitudinal changes in neuroimaging measures are more reliable treatment biomarkers than clinical-symptomatic measurements due to their advantage of inherent objectivity. Recently, preliminary evidence has suggested that the DMN is responsive to antipsychotic medications. For instance, during responses to both fearful and happy expressions, schizophrenia patients treated with risperidone monotherapy have more normal task-related suppression in DMN areas (temporal pole and mPFC) than patients treated with typical antipsychotic agents [59].
More compelling evidence about the mediating role of the DMN in antipsychotic medications comes from the work of Sambataro et al. [58]. With the hypothesis that olanzapine (an atypical antipsychotic agent) may affect working memory by modulating functional connectivity in the DMN, Sambataro et al. examined 19 schizophrenia patients (13 drug-naive and 6 drug-free), and 19 normal controls. Seventeen out of the 19 patients underwent the 8-week longitudinal study and received two fMRI scans at 4 and 8 weeks of olanzapine treatment. To control for repetition effects of task learning, Sambataro et al. also scanned the 19 volunteers twice at the same time interval. The task fMRI data were analyzed using ICA. The authors found that patients have weaker connectivity in pCC as well as increased connectivity in PCUN and iPL compared with healthy controls. The olanzapine treatment effect is associated with increased connectivity in ventromedial PFC. Sambataro et al. proposed that olanzapine treatment may influence functional connectivity within the DMN via modulating the dopaminergic pathway directly in the pCC/PCUN, or indirectly in the ventromedial PFC [58].
Further evidence has revealed a potential relationship between DMN regulation and DA signaling. Pharmacological studies have provided evidence that treatment with dopaminergic agonists such as apomorphine [4], levodopa [5], and modafinil [6] in healthy individuals or patients with Parkinson’s disease regulate the activity of the two midline components of the DMN, MPFC and PCC/PCUN. Furthermore, recent imaging genetic investigations have shown that gene polymorphisms of catechol-O-methy1 transferase [60] and DA receptor [61], which are associated with cortical DA levels, regulate the activity and connectivity strength of the MPFC and PCC/PCUN. All this strong evidence suggests a potential modulatory role of the DMN in antipsychotic medications, as all antipsychotic drugs bind to the DA receptor [62].
The above preliminary reports imply a potential role of DMN as a treatment biomarker for schizophrenia, which raises the possibility that changes in DMN connectivity or activation might be a surrogate measure for the development of antipsychotic agents. However, the role of the DMN in responding to antipsychotic medication still has not been extensively explored, and many issues remain unclear, for example: “what is the effect of antipsychotic treatment on both the anatomical and functional DMN during both the resting and task state?”, and “how do changes in activity or connectivity of the DMN evolve over time in schizophrenia patients, and how are these associated with the occurrence of treatment resistance, persistent social dysfunction, and recovery of social function in some patients?”. Therefore, future longer-term follow-up investigations in medication-naïve FESPs during both the resting and task states, using multi-model experiments combining both anatomical and functional DMN, offer promise to clarify these unresolved issues. Importantly, DMN characterization may be used for patient selection to different treatments as a predictor of potential benefit. In this context, there is a need to correlate baseline DMN characterization with longitudinal changes in clinical outcomes across a sample of patients overall in future studies. It is also clinically useful to use a longitudinal method to explore the relationship between treatment-related changes in DMN measures and alterations in psychotic symptoms. Finally, future studies that investigate DMN characterization using individual-level analysis are needed to further identify treatment response biomarkers, and to benefit patients at the individual level.
Conclusions
In this review, we have described the important role of disrupted activity and connectivity of the DMN in the pathological mechanism underlying schizophrenia, and drawn out the clinical implications of the DMN dysfunction for the positive and negative symptoms and cognitive impairments of schizophrenia. The reverse relationship between the DMN and TPN is also an important parameter for further understanding of vulnerabilities in external and internal perceptions in schizophrenia patients. Importantly, as there are still many inconsistences in the current findings, for example, increased vs decreased connectivity or activity within the DMN, increased vs decreased anti-correlation between the TPN and DMN, as well as inconsistent locations of the DMN abnormalities, future research may benefit from investigating large samples of drug-naïve patients with first-episode schizophrenia. More importantly, preliminary findings that activity and connectivity within the DMN are plastic and can respond to effective medications offer hope that the DMN can play a role in mediating the effects of antipsychotic medications, with longer-term potential for developing more individualized and effective therapies for schizophrenia.
Into the Future
First, studies of the DMN may prove valuable in the diagnosis of schizophrenia, and permit the exploration of more effective antipsychotic agents. Second, in view of evidence for the role of genetics in the changed activity and connectivity of the DMN in schizophrenia [25, 26], as well as the potential association between DA signaling and DMN modulation [4–6, 58], the relationship between genetic variation and antipsychotic treatment-related regulation of the DMN should be explored further in longitudinal studies with large samples of treatment-naïve FESPs. Third, the potential influence of environmental factors on the activity and connectivity of the DMN also needs consideration. Fourth, because the divergence of findings among studies could represent differences in the activity and connectivity of the DMN that might be involved in different stages of this disease, it could be important to explore the DMN throughout the course of schizophrenia to assess duration-related DMN deficits. Fifth, as brain developmental anomalies may occur long before the diagnosis of schizophrenia is made [63], future follow-up studies should systematically investigate the developmental trajectory of activity or connectivity of the DMN in individuals at risk for developing schizophrenia and compare this with normal populations. If at-risk individuals with abnormal DMN activity or connectivity are most likely to develop schizophrenia, the abnormal DMN pattern may assist in early detection and treatment. Sixth, the DMN is proposed to be an important part of the triple network model, which is implicated in the cognitive dysfunction and aberrant saliency mapping of schizophrenia and other neuropsychiatric disorders [64]. The triple network model consists of the salience network, central executive network, and DMN. Given that dysfunction in one network would influence the other two [64], future studies are needed to systematically explore the interaction of these three networks in schizophrenia.
Acknowledgements
This review was supported by the National Natural Science Foundation of China (81271484, 81471361, 30900486, and 81371480), the National Basic Research Development Program (973 Program) of China (2012CB517904), and the Nation Sponsored Study Abroad Program from China Scholarship Council (201506370095). Dr. Mann receives royalties from the Research Foundation for Mental Hygiene for commercial use of the C-SSRS.
Contributor Information
Xiao-Gang Chen, Email: chenxghn@gmail.com.
Jin-Song Tang, Email: tangjinsonghn@gmail.com.
References
- 1.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Fan L, Qiu C, Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci Bull. 2015;31:207–219. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Tang J, Chen L, Liao Y, Zhou B, He Y, et al. Reduced middle cingulate gyrus volume in late-onset schizophrenia in a Chinese Han population: a voxel-based structural MRI study. Neurosci Bull. 2015;31:626–627. doi: 10.1007/s12264-015-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagano-Saito A, Liu J, Doyon J, Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the Tower of London task. Neurosci Lett. 2009;458:1–5. doi: 10.1016/j.neulet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 5.Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage 2007, 37: 1083–1090; discussion 1097–1089. [DOI] [PubMed]
- 8.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broyd SJ, Demanuele C, Debener S, Helps SK, James CJ, Sonuga-Barke EJ. Default-mode brain dysfunction in mental disorders: a systematic review. Neurosci Biobehav Rev. 2009;33:279–296. doi: 10.1016/j.neubiorev.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54:287–298. doi: 10.1016/S0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 11.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. Common Blood Flow Changes across Visual Tasks: II. Decreases in Cerebral Cortex. J Cogn Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 12.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 13.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 14.McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR. Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage. 2006;29:1185–1191. doi: 10.1016/j.neuroimage.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thomason ME, Chang CE, Glover GH, Gabrieli JD, Greicius MD, Gotlib IH. Default-mode function and task-induced deactivation have overlapping brain substrates in children. Neuroimage. 2008;41:1493–1503. doi: 10.1016/j.neuroimage.2008.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, et al. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci U S A. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–151. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Default mode network abnormalities in mesial temporal lobe epilepsy: a study combining fMRI and DTI. Hum Brain Mapp. 2011;32:883–895. doi: 10.1002/hbm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466:48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 21.Parvizi J, Van Hoesen GW, Buckwalter J, Damasio A. Neural connections of the posteromedial cortex in the macaque. Proc Natl Acad Sci U S A. 2006;103:1563–1568. doi: 10.1073/pnas.0507729103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teipel SJ, Bokde AL, Meindl T, Amaro E, Jr, Soldner J, Reiser MF, et al. White matter microstructure underlying default mode network connectivity in the human brain. Neuroimage. 2010;49:2021–2032. doi: 10.1016/j.neuroimage.2009.10.067. [DOI] [PubMed] [Google Scholar]
- 23.Liu S, Cai W, Liu S, Zhang F, Fulham M, Feng D, et al. Multimodal neuroimaging computing: a review of the applications in neuropsychiatric disorders. Brain Inform. 2015;2:167–180. doi: 10.1007/s40708-015-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, et al. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heck A, Fastenrath M, Ackermann S, Auschra B, Bickel H, Coynel D, et al. Converging genetic and functional brain imaging evidence links neuronal excitability to working memory, psychiatric disease, and brain activity. Neuron. 2014;81:1203–1213. doi: 10.1016/j.neuron.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richiardi J, Altmann A, Milazzo AC, Chang C, Chakravarty MM, Banaschewski T, et al. BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks. Science. 2015;348:1241–1244. doi: 10.1126/science.1255905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 28.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, et al. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophr Bull. 2012;38:285–294. doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williamson P. Are anticorrelated networks in the brain relevant to schizophrenia? Schizophr Bull. 2007;33:994–1003. doi: 10.1093/schbul/sbm043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy DP, Courchesne E. The intrinsic functional organization of the brain is altered in autism. Neuroimage. 2008;39:1877–1885. doi: 10.1016/j.neuroimage.2007.10.052. [DOI] [PubMed] [Google Scholar]
- 34.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, et al. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- 37.Calhoun VD, Kiehl KA, Pearlson GD. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum Brain Mapp. 2008;29:828–838. doi: 10.1002/hbm.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim DI, Mathalon DH, Ford JM, Mannell M, Turner JA, Brown GG, et al. Auditory oddball deficits in schizophrenia: an independent component analysis of the fMRI multisite function BIRN study. Schizophr Bull. 2009;35:67–81. doi: 10.1093/schbul/sbn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison BJ, Yucel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophr Res. 2007;91:82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 40.Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychol Med. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- 41.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim DI, Manoach DS, Mathalon DH, Turner JA, Mannell M, Brown GG, et al. Dysregulation of working memory and default-mode networks in schizophrenia using independent component analysis, an fBIRN and MCIC study. Hum Brain Mapp. 2009;30:3795–3811. doi: 10.1002/hbm.20807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: A methodological comparison in patients with schizophrenia and healthy controls. Hum Brain Mapp. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jeong B, Kubicki M. Reduced task-related suppression during semantic repetition priming in schizophrenia. Psychiatry Res. 2010;181:114–120. doi: 10.1016/j.pscychresns.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider FC, Royer A, Grosselin A, Pellet J, Barral FG, Laurent B, et al. Modulation of the default mode network is task-dependant in chronic schizophrenia patients. Schizophr Res. 2011;125:110–117. doi: 10.1016/j.schres.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- 47.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, et al. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behav Brain Funct. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingoia G, Wagner G, Langbein K, Maitra R, Smesny S, Dietzek M, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138:143–149. doi: 10.1016/j.schres.2012.01.036. [DOI] [PubMed] [Google Scholar]
- 51.Gerretsen P, Menon M, Mamo DC, Fervaha G, Remington G, Pollock BG, et al. Impaired insight into illness and cognitive insight in schizophrenia spectrum disorders: resting state functional connectivity. Schizophr Res. 2014;160:43–50. doi: 10.1016/j.schres.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liemburg EJ, van der Meer L, Swart M, Curcic-Blake B, Bruggeman R, Knegtering H, et al. Reduced connectivity in the self-processing network of schizophrenia patients with poor insight. PLoS One. 2012;7:e42707. doi: 10.1371/journal.pone.0042707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Camchong J, MacDonald AW, 3rd, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curcic-Blake B, van der Meer L, Pijnenborg GH, David AS, Aleman A. Insight and psychosis: Functional and anatomical brain connectivity and self-reflection in Schizophrenia. Hum Brain Mapp. 2015;36:4859–4868. doi: 10.1002/hbm.22955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biol Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolf RC, Vasic N, Sambataro F, Hose A, Frasch K, Schmid M, et al. Temporally anticorrelated brain networks during working memory performance reveal aberrant prefrontal and hippocampal connectivity in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1464–1473. doi: 10.1016/j.pnpbp.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 58.Sambataro F, Blasi G, Fazio L, Caforio G, Taurisano P, Romano R, et al. Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912. doi: 10.1038/npp.2009.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surguladze SA, Chu EM, Marshall N, Evans A, Anilkumar AP, Timehin C, et al. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J Psychopharmacol. 2011;25:722–733. doi: 10.1177/0269881110363316. [DOI] [PubMed] [Google Scholar]
- 60.Stokes PR, Rhodes RA, Grasby PM, Mehta MA. The effects of the COMT Val108/158Met polymorphism on BOLD activation during working memory, planning, and response inhibition: a role for the posterior cingulate cortex? Neuropsychopharmacology. 2011;36:763–771. doi: 10.1038/npp.2010.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sambataro F, Fazio L, Taurisano P, Gelao B, Porcelli A, Mancini M, et al. DRD2 genotype-based variation of default mode network activity and of its relationship with striatal DAT binding. Schizophr Bull. 2013;39:206–216. doi: 10.1093/schbul/sbr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pantelis C, Yucel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophr Bull. 2005;31:672–696. doi: 10.1093/schbul/sbi034. [DOI] [PubMed] [Google Scholar]
- 64.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Zong X, Hu M, Li Z, Cao H, He Y, Liao Y, et al. N-acetylaspartate reduction in the medial prefrontal cortex following 8 weeks of risperidone treatment in first-episode drug-naive schizophrenia patients. Sci Rep. 2015;5:9109. doi: 10.1038/srep09109. [DOI] [PMC free article] [PubMed] [Google Scholar]