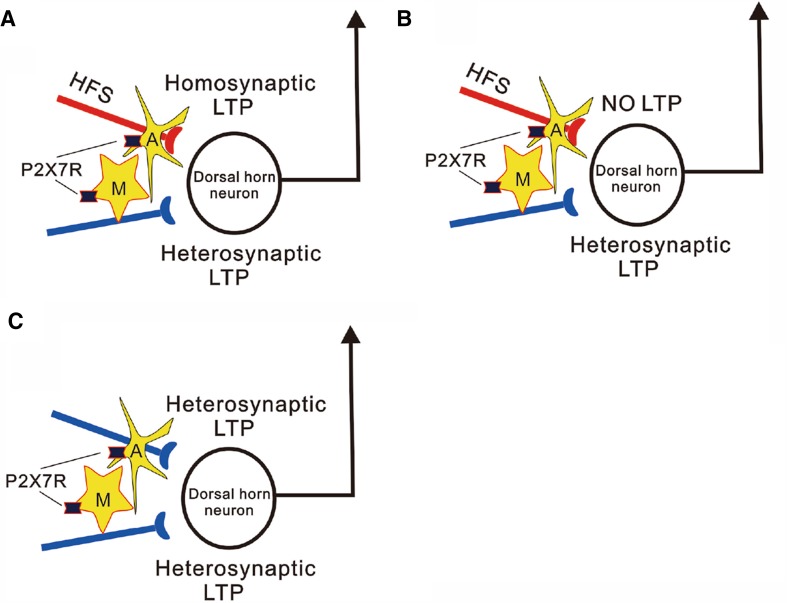
Long-term potentiation (LTP) at synapses between primary afferents and spinal dorsal horn neurons induced by noxious electrical stimulation or injury of peripheral nerve is considered to underlie chronic pain [1]. The mechanisms of the spinal LTP have been intensively investigated, since it was discovered in 1995 [2]. In recent years, spinal application of ATP [3], brain-derived neurotrophic factor (BDNF) [4] and opioid [5] has been shown to induce spinal LTP at C-fiber synapses in the absence of conditioning activation of primary afferents. This is contrary to the general belief that coincident pre- and postsynaptic activity is needed for LTP induction. Recently, Sandkühler and his co-workers reported in Science that combined activation of microglia and astrocytes by P2X7 receptor agonist BzATP induces LTP at synapses between afferent C-fibers and spinal lamina I neurons in the absence of presynaptic activation, which is termed gliogenic LTP [6] (Fig. 1C). To determine the relationship between the gliogenic LTP and high frequency stimulation (HFS)-induced LTP, they used transverse lumbar spinal cord slices with long dorsal roots which were separated into halves. Twenty two lamina I neurons that received independent monosynaptic C-fiber inputs from each dorsal root half were recorded. Homosynaptic LTP is recorded in 12 neurons, among them 6 neurons also show heterosynaptic LTP (Fig. 1A). Interestingly, heterosynaptic LTP is also induced in 5 neurons in which HFS fails to induce homosynaptic LTP (Fig. 1B). The data demonstrate that homo- and heterosynaptic LTP can be induced independently of each other in dorsal horn neurons. Importantly, they found that both homo- and heterosynaptic LTP depend on glial activation via activation of P2X7 receptors, postsynaptic NMDA receptor and D-serine (a co-agonist of NMDA receptor) released from astrocytes. Accordingly, HFS-induced LTP may manifest as homo- and/or heterosynaptic LTP and both of them depend on the glial activation. Importantly, the gliogenic LTP can be transferred between individuals, i.e., application of spinal superfusate collected from lumbar segments of animals, in which the spinal LTP has been induced by HFS, onto spinal dorsal dorsum is able to induce LTP in naïve animals. The transferable LTP is prevented by blocking TNF-α, D-serine signaling and NMDA receptors but not by blocking glial activation in recipient animals. Accordingly, the spinal LTP is a result from the accumulated bioactive substances released by glial cells called gliotransmitters in activated sites, which may travel long distances via the cerebrospinal fluid and induce LTP in remote sites. The gliogenic LTP may underlie forms of widespread pain hypersensitivity, such as secondary hyperalgesia, pain amplification in uninjured sites.
Fig. 1.
Homo- and heterosynaptic LTP in spinal dorsal horn induced by glial activation. A HFS of one pathway (red) induces LTP not only in the synapse that is activated by conditioning stimulation but also in the synapses that is not directly activated by the conditioning stimulation in a dorsal horn neurons. The former is termed homosynaptic LTP, while the later heterosynaptic LTP. B The heterosynaptic LTP can be induced in the absence of homosynaptic LTP. Both homo- and heterosynaptic LTP is depend on activation of spinal glial cells. C Combined activation of microglia and astrocytes by application of P2X7 receptor agonist BzATP induces LTP in the absence of HFS. A: Astrocyte; M: microglial cell
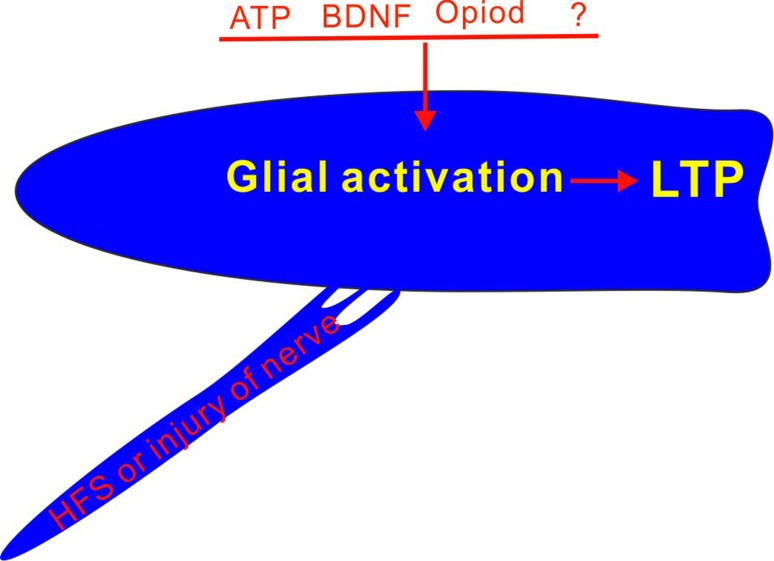
The authors believe that the gliogenic LTP is a new form of paracrine synaptic plasticity. As any factor that activates glial cells, including stimulation or injury of peripheral nerve, spinal application of ATP, BDNF or opioid is able to induce the spinal LTP, the activation of glial cells and the subsequent release of gliotransmitters may be a common mechanism of the spinal LTP. HFS is only one approach to activating glial cells, and therefore the HFS-induced LTP is probably a special case (Fig. 2).
Fig. 2.
Activation of glial cells is a common mechanisms of the spinal LTP.
Previously, the heterosynaptic LTP has been repeatedly demonstrated in spinal dorsal horn. HFS or injury of the tibial nerve induces LTP at C-fiber synapses innervated by the sural nerve [7]. HFS of C-fibers in the sciatic nerve also induces LTP at A-fiber synapses in the same nerve [8, 9]. P2X7 receptors [10], TNF-α [11], interleukine-1 beta (IL-1β) [12] and D-serine [9] have been shown critical for the spinal LTP. The novelty of the new work is that the gliotransmitters released in animals that receive HFS can induce LTP in naïve animals.
As proposed by authors, if gliogenic LTP also exists in other brain regions, it could be of relevance not only for pain but also for other disorders such as cognitive deficits, fear and stress disorders. It has been demonstrated that glial activation and the resultant increased release of gliotransmitters, such as TNF-α and IL-1β, promote LTP in spinal dorsal horn and pain sensitivity, but inhibit LTP in hippocampus and related memory (see [1] for a review). The mechanism underlying the CNS region-dependent synaptic plasticity is largely unknown. To clarify this issue, following questions should be answered. The types and amount of gliomitters released by glial cells in different regions should be determined in physiological and pathological conditions, as glial cells affect neuronal connectivity mainly by release of gliomitters. The effects of individual gliomitters and/or different combinations of them on synaptic plasticity should be uncovered.
References
- 1.Liu XG, Zhou LJ. Long-term potentiation at spinal C-fiber synapses: a target for pathological pain. Curr Pharm Des. 2015;21:895–905. doi: 10.2174/1381612820666141027115949. [DOI] [PubMed] [Google Scholar]
- 2.Liu XG, Sandkuhler J. Long-term potentiation of C-fiber-evoked potentials in the rat spinal dorsal horn is prevented by spinal N-methyl-d-aspartic acid receptor blockage. Neurosci Lett. 1995;191:43–46. doi: 10.1016/0304-3940(95)11553-0. [DOI] [PubMed] [Google Scholar]
- 3.Gong QJ, Li YY, Xin WJ, Zang Y, Ren WJ, Wei XH, et al. ATP induces long-term potentiation of C-fiber-evoked field potentials in spinal dorsal horn: The roles of P2X(4) receptors and p38 MAPK in microglia. Glia. 2009;57:583–591. doi: 10.1002/glia.20786. [DOI] [PubMed] [Google Scholar]
- 4.Zhou LJ, Yang T, Wei XA, Liu Y, Xin WJ, Chen YA, et al. Brain-derived neurotrophic factor contributes to spinal long-term potentiation and mechanical hypersensitivity by activation of spinal microglia in rat. Brain Behav Immun. 2011;25:322–334. doi: 10.1016/j.bbi.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Drdla R, Gassner M, Gingl E, Sandkuhler J. Induction of synaptic long-term potentiation after opioid withdrawal. Science. 2009;325:207–210. doi: 10.1126/science.1171759. [DOI] [PubMed] [Google Scholar]
- 6.Kronschläger MT, Drdla-Schutting R, Gassner M, Honsek SD, Teuchmann HL, Sandkühler J. Gliogenic LTP spreads widely in nociceptive pathways. Science. 2016;354:1144–1148. doi: 10.1126/science.aah5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou LJ, Ren WJ, Zhong Y, Yang T, Wei XH, Xin WJ, et al. Limited BDNF contributes to the failure of injury to skin afferents to produce a neuropathic pain condition. Pain. 2010;148:148–157. doi: 10.1016/j.pain.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 8.Gong QJ, Li YY, Xin WJ, Wei XH, Cui Y, Wang J, et al. Differential effects of adenosine A1 receptor on pain-related behavior in normal and nerve-injured rats. Brain Res. 2010;1361:23–30. doi: 10.1016/j.brainres.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 9.Ying B, Lu N, Zhang YQ, Zhao ZQ. Involvement of spinal glia in tetanically sciatic stimulation-induced bilateral mechanical allodynia in rats. Biochem Biophys Res Commun. 2006;340:1264–1272. doi: 10.1016/j.bbrc.2005.12.139. [DOI] [PubMed] [Google Scholar]
- 10.Chu YX, Zhang Y, Zhang YQ, Zhao ZQ. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain Behav Immun. 2010;24:1176–1189. doi: 10.1016/j.bbi.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Liu YL, Zhou LJ, Hu NW, Xu JT, Wu CY, Zhang T, et al. Tumor necrosis factor-alpha induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with nerve injury: the role of NF-kappa B, JNK and p38 MAPK. Neuropharmacology. 2007;52:708–715. doi: 10.1016/j.neuropharm.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Zhong Y, Zhou LJ, Ren WJ, Xin WJ, Li YY, Zhang T, et al. Interleukin-1beta induces long-term potentiation of C-fiber evoked field potentials in spinal dorsal horn in rats with neuropathic pain. Open Pain J. 2009;2:18–23. doi: 10.2174/1876386300902010018. [DOI] [Google Scholar]