Abstract
Orexin, released from the hypothalamus, has been implicated in various basic non-somatic functions including feeding, the sleep-wakefulness cycle, emotion, and cognition. However, the role of orexin in somatic motor control is still little known. Here, using whole-cell patch clamp recording and immunostaining, we investigated the effect and the underlying receptor mechanism of orexin-A on neurons in the globus pallidus internus (GPi), a critical structure in the basal ganglia and an effective target for deep brain stimulation therapy. Our results showed that orexin-A induced direct postsynaptic excitation of GPi neurons in a concentration-dependent manner. The orexin-A-induced excitation was mediated via co-activation of both OX1 and OX2 receptors. Furthermore, the immunostaining results showed that OX1 and OX2 receptors were co-localized in the same GPi neurons. These results suggest that the central orexinergic system actively modulates the motor functions of the basal ganglia via direct innervation on GPi neurons and presumably participates in somatic-non-somatic integration.
Keywords: Orexin, OX1 receptor, OX2 receptor, Globus pallidus internus, Basal ganglia
Introduction
Orexin, also known as hypocretin, is a neuropeptide synthesized only in the lateral hypothalamic and perifornical areas [1, 2]. The central orexinergic system, though originating from the hypothalamus, extensively innervates almost the whole brain, and thus participates in the regulation of many basic non-somatic functions, such as feeding, the sleep/wake cycle, and reward processes [3–6]. Intriguingly, accumulating evidence indicates that orexin may also be essential for somatic motor control. Orexin deficiency in rodents, dogs, and humans results in cataplexy, a motor deficit characterized by a sudden loss of muscle tone [4, 7]. During movement, orexinergic neurons are particularly active and the release of orexin increases [8–10]. Injection of orexin into the midbrain locomotor region in cats even induces locomotion [11]. Furthermore, endogenous orexin plays a critical role when an animal is facing a motor challenge [12]. However, the neural mechanisms underlying the role of orexin in somatic motor control are still largely unknown.
The globus pallidus (GP) is a major component of the basal ganglia, which is involved in the control of voluntary movement. It receives a large quantity of GABAergic innervation from the striatum and is divided into two parts, the globus pallidus internus (GPi) and the globus pallidus externus (GPe). Although the GPi is small and immersed in the fibers of the internal capsule, it integrates information from both direct pathway and indirect pathway involving the GPe and subthalamic nucleus [13], and thus constitutes the output of the basal ganglia [13] and holds a key position in the circuitry of the basal ganglia. It has been shown that GPi neurons exhibit increased firing rates and altered firing patterns in parkinsonian primates [14, 15]. Furthermore, the GPi is an optimal target of clinical deep brain stimulation for the effective alleviation of motor symptoms in Parkinson’s disease (PD) [16] and is a potential target of fibroblast transplantation for experimental therapy of levodopa-induced dyskinesias [17].
Recent morphological and immunostaining studies have shown that the GP, including the GPi, is densely innervated by direct hypothalamic orexinergic fibers [18]. An extracellular recording study in vivo revealed that orexin increases the activity of GP neurons in both normal and 6-hydroxydopamine-lesioned rats, and the orexin-induced increase in firing rate of pallidal neurons in parkinsonian rats is stronger than that in normal rats [19]. However, the exact effect of orexin on the two parts of the GP, which play different roles in the basal ganglia circuitry, is still unclear. Moreover, whether the effect of orexin on GP neuronal activity is a direct postsynaptic action or mediated by presynaptic elements needs to be clarified. Therefore, in this study, using whole-cell patch clamp recording and immunostaining techniques, the direct effects of orexin on the two types of GPi neuron, Types I and II, as well as the underlying receptor mechanism, were investigated. Our results showed that orexin directly excited both types of GPi neurons via postsynaptic OX1 and OX2 receptors, and this was confirmed by immunostaining data showing that both orexin receptors were expressed and co-localized on the same GPi neurons. These results suggest that the central orexinergic system actively regulates the motor functions of the basal ganglia by directly modulating GPi neurons.
Materials and Methods
Animals
The experiments were conducted on Sprague–Dawley rats of either sex (14–21 days of age), housed under controlled conditions with a lighting schedule of 12 h light and 12 h darkness at 22 ± 2 °C. Standard food and water were provided ad libitum. All animal experiments were approved by the Experimental Animal Care and Use Committee of Nanjing University and were conducted in accordance with the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication 85-23, revised 2011).
Brain Slice Preparation
Rats were anesthetized with urethane (40 mg/kg) and coronal brain slices (300 µm thick) containing the GPi were prepared with a vibroslicer (VT 1200 S, Leica, Wetzlar, Germany) according to the rat brain atlas of Paxinos and Watson [20]. The slices were incubated in artificial cerebrospinal fluid (ACSF, composition in mmol/L: 124 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1.3 MgSO4, 26 NaHCO3, 2 CaCl2, and 10 D-glucose) equilibrated with 95% O2 and 5% CO2 at 35 ± 0.5 °C for at least 1 h and then maintained at room temperature. During recording sessions, the slices were transferred to a submerged chamber and continuously superfused with ACSF oxygenated with 95% O2 and 5% CO2 at a rate of 2 mL/min at room temperature.
Whole-Cell Patch-Clamp Recordings
Whole-cell recordings were performed as we described previously [12, 21]. Briefly, GPi neurons were visualized under an Olympus BX51WI microscope (Tokyo, Japan) and then recorded with borosilicate glass pipettes (3–6 MΩ) filled with internal solution (composition in mmol/L: 140 K-methylsulfate, 7 KCl, 2 MgCl2, 10 HEPES, 0.1 EGTA, 4 Na2-ATP, and 0.4 GTP-Tris, adjusted to pH 7.25 with 1 mol/L KOH). Patch-clamp recordings were acquired with an Axopatch-700B amplifier (Axon Instruments, Sunnyvale, CA) and the signals were fed into a computer through a Digidata-1550 interface (Axon Instruments) for data capture and analysis (pClamp 10.4, Axon Instruments). Neurons were held at a membrane potential of −60 mV and characterized by injection of a rectangular voltage pulse (5 mV, 50 ms) to monitor the whole-cell membrane capacitance, series resistance, and membrane resistance. Neurons were excluded from the study if the series resistance was not stable or exceeded 20 MΩ.
Under current-clamp mode, a series of 1-s negative current injections (ranging from 0 to −420 pA in 60-pA steps) was used to identify and categorize the two types of GPi neuron (Types I and II), according to whether the recorded neuron exhibited the features of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [22, 23]. Then, we bathed the slices with orexin-A (0.03–3 µmol/L, Tocris, Bristol, UK) to assess its effect on membrane potential and whole-cell currents of the two types of GPi neuron recorded under current-clamp and voltage-clamp modes, respectively. Peri-stimulus time histograms of the neuronal discharges were generated to assess the effect of orexin-A on the firing rates of GPi neurons. Tetrodotoxin (TTX; 0.3 µmol/L, Alomone Labs, Jerusalem, Israel), NBQX (a potent antagonist of AMPA receptors; 20 µmol/L, Tocris), AP5 (a potent antagonist of NMDA receptors; 50 µmol/L, Tocris), and SR95531 (an antagonist of GABAA receptors; 50 µmol/L, Tocris) were used to determine whether the effect of orexin-A is postsynaptic. The selective OX1 receptor antagonist SB334867 (10 µmol/L, Tocris), the selective OX2 receptor antagonist TCS-OX2-29 (10 µmol/L, Tocris), and the selective OX2 receptor agonist [Ala11, D-Leu15]-orexin-B (1 µmol/L, Tocris) were used to investigate the underlying receptor mechanism. Before bath application of each orexinergic compound at known concentrations, the membrane potential or whole-cell current was recorded for at least 20 min to assure stability. Then, orexin-A or an orexin receptor agonist was added to the perfusing ACSF to stimulate the neuron for a test period of 1 min. Antagonists were given at least 15 min before examination of their antagonistic effects on responses induced by orexin-A or orexin receptor agonists. After each trial, cells were given at least 20 min for recovery and to prevent desensitization.
Immunofluorescence
The experimental procedures for immunostaining followed our previous reports [12, 21, 24, 25]. Rats (n = 5) were deeply anesthetized with pentobarbital sodium (40 mg/kg) and perfused transcardially with 100 mL normal saline, followed by 450–500 mL 4% paraformaldehyde in 0.1 mol/L phosphate buffer. Subsequently, the brain was removed, trimmed, postfixed in the same fixative for 12 h at 4 °C, and then cryoprotected with 20% and 30% sucrose successively for 24 h. Frozen coronal sections (25 µm thick) containing the GPi were obtained using a freezing microtome (CM 3050S, Leica) and mounted on gelatin-coated slides. The slices were rinsed in phosphate-buffered saline (PBS) containing 0.2% Triton X-100 (PBST; Sigma, St. Louis, MO) and then incubated in 10% normal bovine serum (Millipore, Bedford, MA) in PBST for 30 min. Sections were incubated overnight at 4 °C with primary antibodies against the OX1 receptor (a chicken anti-OX1 receptor polyclonal antibody, 1:200; Acris, Herford, Germany) and the OX2 receptor (a rabbit anti-OX2 receptor polyclonal antibody, 1:100; Abcam, Cambridge, UK). After a complete wash in PBS, sections were incubated in a mixture of Alexa 488-conjugated goat anti-chicken (1:2000; Invitrogen, Carlsbad, CA) and Alexa 594-conjugated goat anti-rabbit (1:2000; Invitrogen) for 2 h at room temperature in the dark. The slides were washed and mounted in UltraCruz mounting medium (Santa Cruz Biotechnology, Santa Cruz, CA). All micrographs were captured with an inverted laser scanning confocal microscope (SP8, Leica).
Statistical Analysis
All data are presented as mean ± SEM. Student’s t test was used for analysis. P < 0.05 was considered statistically significant.
Results
Orexin Excites Both Types of GPi Neuron
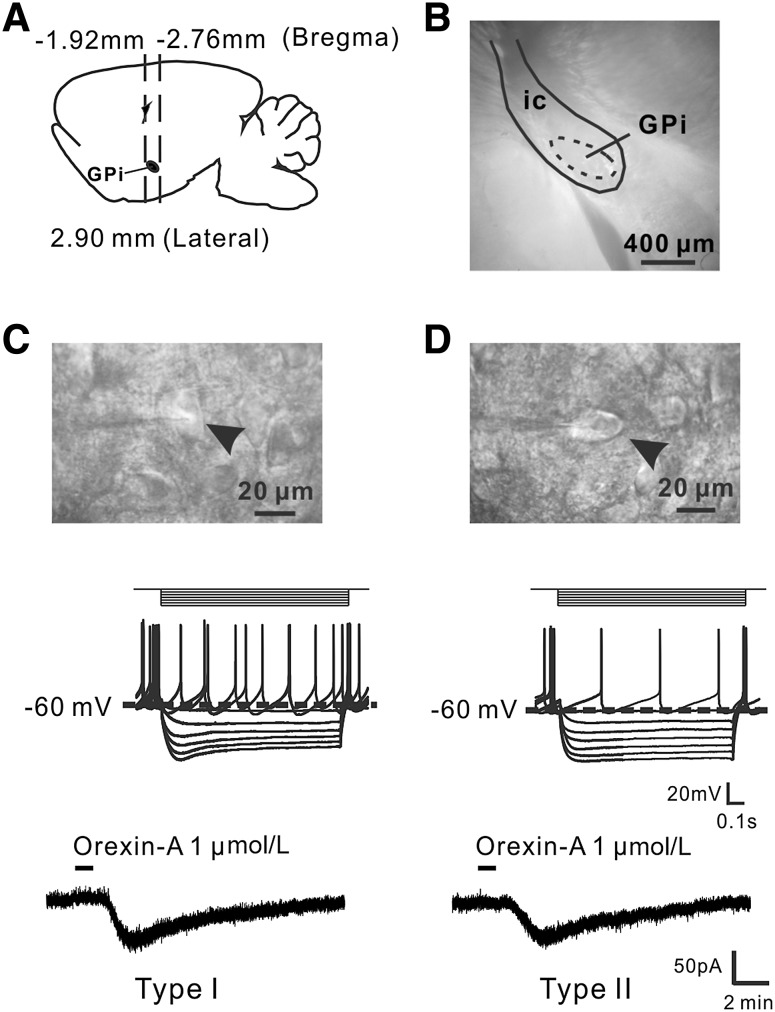
We carried out whole-cell patch clamp recordings on brain slices containing the GPi (Fig. 1A and B) to determine the effect of orexin-A on GPi neurons. According to whether a neuron exhibited spontaneous firing and hyperpolarization-activated inward rectification [22, 23], characteristic of HCN channels [26, 27], all the recorded GPi neurons (n = 76) with a diameter >20 µm were classified into two subgroups. Type I (n = 68, 89.5%) showed a voltage sag during injection of a negative current step, whereas Type II (n = 8, 10.5%) exhibited little or no voltage sag during negative current injection (Fig. 1C, D). The ratio of the two types was consistent with previous reports [22].
Fig. 1.
Orexin-A excites two types of GPi neurons. A Diagram of rat brain in sagittal view showing the location of the GPi between 1.92 and 2.76 mm from bregma. B A coronal brain slice containing the GPi. C, D Electrophysiological identification of Types I and II neurons in the GPi and the effect of orexin-A. The diameters of both types were >20 µm (arrowheads). Type I neurons showed a voltage sag during injection of a negative current step, whereas Type II neurons (n = 8, 10.5%) showed little or none. GPi, internal globus pallidus; ic, internal capsule.
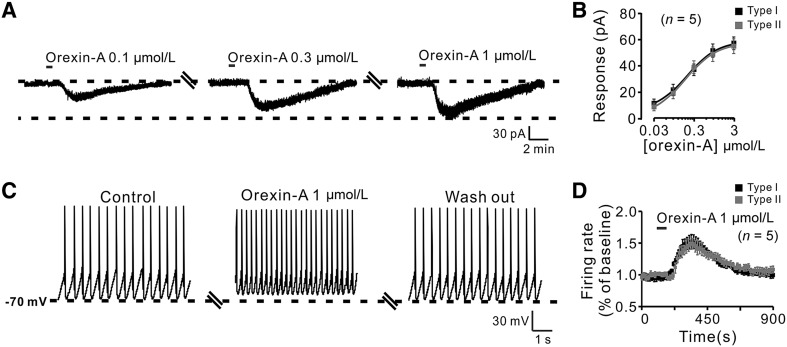
Of the 76 GPi neurons recorded, 59 (77.6%) neurons including both Type I (n = 52) and Type II (n = 7) showed an excitatory response to bath application of orexin-A (1 µmol/L), whereas the remaining 17 (22.4%; Type I, n = 16; Type II, n = 1) had no response. Notably, in voltage-clamp recordings, orexin-A induced a significant inward current in both types (Fig. 1C, D). Moreover, orexin-A (0.1, 0.3, and 1 µmol/L) elicited a concentration-dependent inward current in Type I neurons (Fig. 2A). The concentration–response curves showed that orexin-A (0.03, 0.1, 0.3, 1, and 3 µmol/L) elicited an inward current in both types of neuron in a concentration-dependent manner at 10.4 ± 2.4, 21.4 ± 3.4, 38.2 ± 5.6, 51.3 ± 5.1, and 59.0 ± 5.8 pA, respectively, for Type I (n = 5), and 9.8 ± 2.2, 20.0 ± 4.3, 39.4 ± 6.5, 50.0 ± 5.2, and 56.6 ± 4.7 pA for Type II (n = 5) (Fig. 2B). On the other hand, in current-clamp recordings, orexin-A (1 µmol/L) depolarized both types, increasing the firing rate of Type I from 6.2 ± 0.9 to 9.8 ± 1.2 spikes/s (n = 5) and of Type II neurons from 6.5 ± 1.0 to 9.4 ± 1.3 spikes/s (n = 5) (Fig. 2C, D). These results indicate that orexin-A excites both Types I and II neurons in the GPi.
Fig. 2.
Excitatory responses of GPi neurons induced by orexin-A. A Orexin-A elicited inward currents in a GPi neuron in a concentration-dependent manner at a holding potential of −60 mV. B Group data of the two types of neuron (n = 5/group). C Orexin-A depolarized the membrane potential and increased the firing rate of a GPi neuron. D Peri-stimulus time histogram of the two types of neuron (n = 5/group).
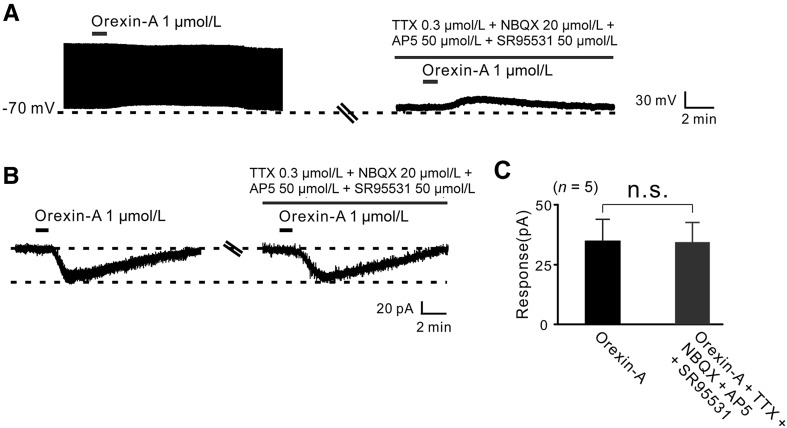
Orexin-Induced Excitation of GPi Neurons is a Direct Postsynaptic Effect
To clarify whether the effect of orexin-A on GPi neurons is a direct postsynaptic effect, we assessed the effect of TTX (0.3 µmol/L), NBQX (20 µmol/L, a potent AMPA receptor antagonist), AP5 (50 µmol/L, a potent NMDA receptor antagonist) and SR95531 (50 µmol/L, a GABAA receptor antagonist) on the orexin-induced excitation of GPi neurons. Although TTX blocked the GPi neuronal firing, TTX, together with NBQX, AP5, and SR95531 did not block the orexin-A-induced depolarization (Fig. 3A). Furthermore, the inward current elicited by orexin-A (35.0 ± 4.5 pA) was not influenced by co-application of TTX, NBQX, AP5, and SR95531 (32.0 ± 4.9 pA, n = 5, P > 0.05; Fig. 3B, C). These data demonstrate that the effect of orexin on GPi neurons is a direct postsynaptic excitatory action.
Fig. 3.
The excitation of GPi neurons induced by orexin-A is a direct postsynaptic effect. A In current-clamp recordings, TTX, together with NBQX, AP5, and SR95531, did not block the depolarization induced by orexin-A. B In voltage-clamp recordings, TTX, NBQX, AP5, and SR95531 did not block the inward current induced by orexin-A. C Group data from tested neurons (n = 5; n.s. not significant).
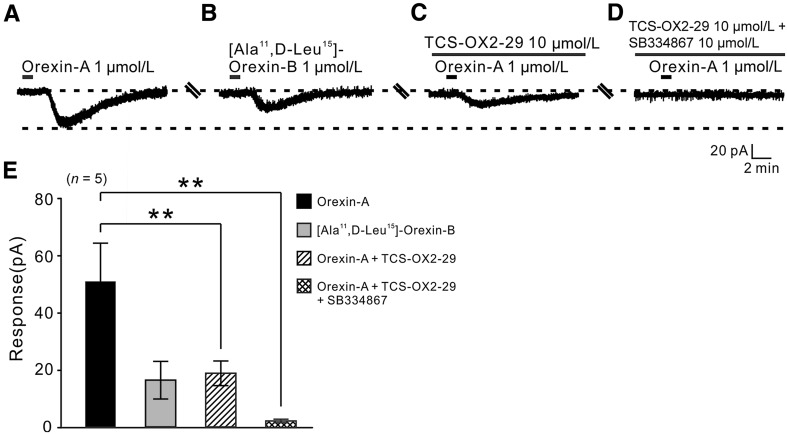
OX1 and OX2 Receptors Co-mediate the Excitatory Effect of Orexin on GPi Neurons
Two subtypes of orexin receptor have been identified, OX1 and OX2 [28, 29]; it is known that they have distinct distribution patterns and may mediate different actions of orexin [5]. Therefore, we used selective agonists and antagonists for each receptor to determine which subtype(s) mediate(s) the orexin-induced excitation of both types of GPi neuron. Application of 1 µmol/L orexin-A elicited an inward current (51.0 ± 13.45 pA, n = 5; Fig. 4A, E), and [Ala11, D-Leu15]-orexin-B (1 μmol/L, a selective OX2 receptor agonist) mimicked the orexin-A-induced inward current (16.6 ± 6.56 pA, n = 5; Fig. 4B, E), indicating an involvement of OX2 receptors. On the other hand, TCS-OX2-29 (10 µmol/L), a selective OX2 receptor antagonist, partially attenuated the orexin-A-elicited inward current (19.0 ± 4.30 pA, n = 5, P < 0.01; Fig. 4C, E). Furthermore, TCS-OX2-29 (10 µmol/L) combined with SB334867 (10 µmol/L), a selective OX1 receptor antagonist, almost totally blocked the orexin-A-induced inward current (1.02 ± 0.18, n = 5, P < 0.01; Fig. 4D, E), indicating that OX1 receptors also participate in the orexin-A-induced excitation of GPi neurons. All these results demonstrate that OX1 and OX2 receptors co-mediate the excitatory effect of orexin on GPi neurons. In addition, the receptor mechanisms underlying the orexin-induced excitation of both types of GPi neurons are the same.
Fig. 4.
OX1 and OX2 receptors co-mediate the inward currents induced by orexin-A in GPi neurons. A The orexin-A-induced inward current recorded from a GPi neuron. B [Ala11, D-Leu15]-orexin B mimicked the effect of orexin-A. C, D TCS-OX2-29, a selective OX2 receptor antagonist, partly blocked the orexin-A-induced inward current, whereas TCS-OX2-29 combined with SB334867, a selective OX1 receptor antagonist, almost totally blocked it. E Group data from tested neurons (n = 5; **P < 0.01).
OX1 and OX2 Receptors are Co-localized in the GPi
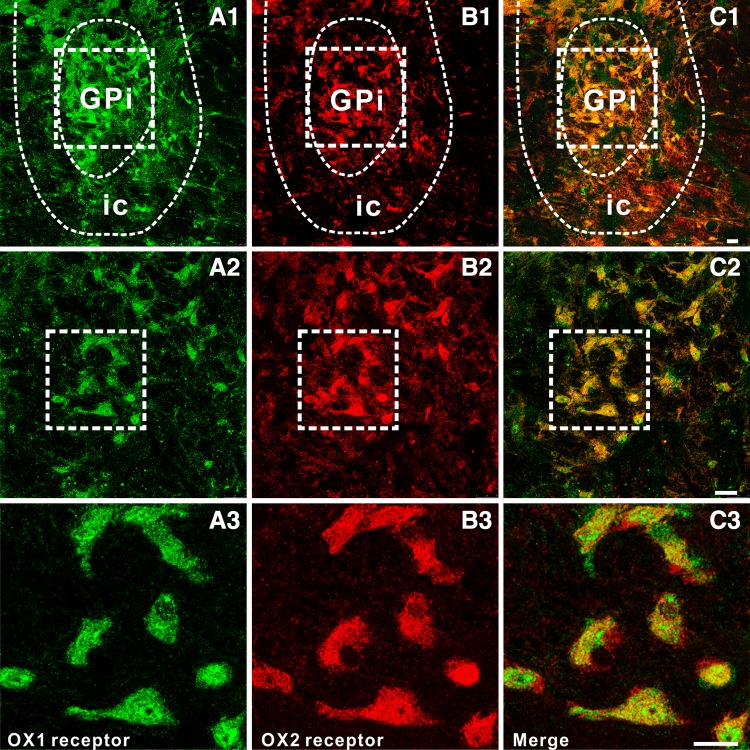
To map the distributions of OX1 and OX2 receptors in the GPi, we performed double immunofluorescence staining of rat brain slices containing the GPi. We found that the two receptors were not only expressed in the GPi (Fig. 5A1–A3 and B1–B3), but also co-localized in the same neurons (Fig. 5C1–C3), consistent with the electrophysiological results.
Fig. 5.
Double immunofluorescence staining for OX1 and OX2 receptors in rat GPi neurons. A1–A3 OX1 receptor staining. B1–B3 OX2 receptor staining. C1–C3 Merged images showing co-localization of OX1 and OX2 receptors in the same GPi neurons. Scale bars: A1, B1, C1, 150 µm; A2, B2, C2, 60 µm; A3, B3, C3, 25 µm. GPi, internal globus pallidus; ic, internal capsule.
Discussion
Previous in vitro and/or in vivo studies have demonstrated that orexin excites neurons in various brain regions, including the arcuate nucleus [30], lateral and medial hypothalamus [31], nucleus accumbens [32], locus coeruleus [33], hippocampus [34], and prefrontal cortex [35]. Recently, studies from our and other laboratories have also revealed that orexin exerts an excitatory effect on neurons in many subcortical motor structures, such as the cerebellum [36], vestibular nuclei [12, 37], ventral horn of the spinal cord [38], and several components in the basal ganglia [39–41]. In this study, using whole-cell patch-clamp recording and immunostaining, we demonstrated that orexin-A postsynaptically depolarized and excited the two types of neurons in the GPi, an important output nucleus in the basal ganglia circuitry and a target for the deep brain stimulation treatment of dyskinesias in PD [42, 43]. Both OX1 and OX2 receptors were expressed on the same GPi neurons and co-mediated the orexin-induced excitatory effect.
The whole-cell patch-clamp recordings allowed us to identify and classify the two types of GPi neurons based on their different membrane properties, so that we could assess the effect of orexin on the different types. However, orexin had a uniformly excitatory effect on both Type I and Type II neurons and their underlying receptor mechanisms were also the same. On both types, TTX combined with antagonists for glutamate and GABA receptors did not block the orexin-induced excitation, strongly suggesting that the excitatory effect of orexin on GPi neurons is a direct postsynaptic action. Therefore, although the exact physiological functions of the two types of neurons remain enigmatic, orexin may extensively modulate neuronal activity in the GPi and consequently influence the functions mediated by these two types of neurons.
Accumulating clinical reports have implied a close correlation between the central orexinergic system and PD. PD patients have an increasing loss of orexinergic neurons in the hypothalamus with disease progression and a substantial decrease in the concentration of orexin in ventricular cerebrospinal fluid [44, 45]. Since the central orexinergic system plays a critical role in regulating the sleep-wakefulness cycle, and orexin deficiency results in narcolepsy, the loss of orexinergic neurons is hypothesized to be responsible for sleep disturbances [46], such as excessive daytime sleepiness, nocturnal insomnia, and REM sleep behavior disorder, among the non-motor symptoms of PD. However, our present results that orexin excites GPi neurons, together with other data that orexin modulates neurons in various components of the basal ganglia [39–41, 47] and induces a larger increase in the firing rates of pallidal neurons in PD than normal rats [19], suggest that orexin loss may also directly contribute to the parkinsonian motor pathophysiology.
In fact, the generation and execution of behavioral responses depend on the coordination and integration of somatic and non-somatic components. The central orexinergic system, strictly originating from the hypothalamus but extensively bridging various somatic motor and non-somatic centers, may constitute the neural substrate underlying somatic-non-somatic integration. Considering that the loss of orexin in PD may be linked to both parkinsonian motor and non-motor symptoms and that orexin deficiency results in not only narcolepsy but also cataplexy, which is actually a simultaneous somatic (motor) and non-somatic (sleep and emotional) dysfunction, we suggest the central orexinergic system and orexin may play an essential role in somatic-non-somatic integration.
In conclusion, in the present study we demonstrate that orexin postsynaptically excites Types I and II GPi neurons via the co-activation of OX1 and OX2 receptors. The orexinergic innervation of the GPi may actively participate in motor control and parkinsonian pathophysiology via modulation of the final output of the basal ganglia.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (NSFC; 31330033, 91332124, 31471112, 31500848, 81671107, 31600834, J1210026), and the NSFC/Research Grants Council (RGC) Joint Research Scheme (31461163001); the Ministry of Education of China (Specialized Research Fund for the Doctoral Program of Higher Education/RGC Earmarked Research Grant 20130091140003, and Fundamental Research Funds for the Central Universities); the Natural Science Foundation of Jiangsu Province, China (BK2011014, BK20140599, and BK20151384); and the Postdoctoral Sciences Foundation, China (2013T60520).
Footnotes
He-Ren Gao, Qian-Xing Zhuang, and Yong-Xiao Zhang contributed equally to this work.
Contributor Information
Jian-Jun Wang, Email: jjwang@nju.edu.cn.
Jing-Ning Zhu, Email: jnzhu@nju.edu.cn.
References
- 1.de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92:1 page following 696. [DOI] [PubMed]
- 3.Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–C1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- 5.Zhang XY, Yu L, Zhuang QX, Zhu JN, Wang JJ. Central functions of the orexinergic system. Neurosci Bull. 2013;29:355–365. doi: 10.1007/s12264-012-1297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cun Y, Tang L, Yan J, He C, Li Y, Hu Z, et al. Orexin A attenuates the sleep-promoting effect of adenosine in the lateral hypothalamus of rats. Neurosci Bull. 2014;30:877–886. doi: 10.1007/s12264-013-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/S0092-8674(00)81973-X. [DOI] [PubMed] [Google Scholar]
- 8.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu M, Zaborszky L, Hajszan T, van den Pol AN, Alreja M. Hypocretin/orexin innervation and excitation of identified septohippocampal cholinergic neurons. J Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takakusaki K, Takahashi K, Saitoh K, Harada H, Okumura T, Kayama Y, et al. Orexinergic projections to the cat midbrain mediate alternation of emotional behavioural states from locomotion to cataplexy. J Physiol. 2005;568:1003–1020. doi: 10.1113/jphysiol.2005.085829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Li B, Yu L, He YC, Li HZ, Zhu JN, et al. A role for orexin in central vestibular motor control. Neuron. 2011;69:793–804. doi: 10.1016/j.neuron.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 13.Nambu A. Globus pallidus internal segment. Prog Brain Res. 2007;160:135–150. doi: 10.1016/S0079-6123(06)60008-3. [DOI] [PubMed] [Google Scholar]
- 14.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 15.Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/S0166-2236(97)01151-X. [DOI] [PubMed] [Google Scholar]
- 16.Stroupe KT, Weaver FM, Cao L, Ippolito D, Barton BR, Burnett-Zeigler IE, et al. Cost of deep brain stimulation for the treatment of Parkinson’s disease by surgical stimulation sites. Mov Disord. 2014;29:1666–1674. doi: 10.1002/mds.26029. [DOI] [PubMed] [Google Scholar]
- 17.Singh A, Gutekunst CA, Uthayathas S, Finberg JP, Mewes K, Gross RE, et al. Effects of fibroblast transplantation into the internal pallidum on levodopa-induced dyskinesias in parkinsonian non-human primates. Neurosci Bull. 2015;31:705–713. doi: 10.1007/s12264-015-1559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, et al. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 2012;217:233–256. doi: 10.1007/s00429-011-0343-8. [DOI] [PubMed] [Google Scholar]
- 19.Xue Y, Yang YT, Liu HY, Chen WF, Chen AQ, Sheng Q, et al. Orexin-A increases the activity of globus pallidus neurons in both normal and parkinsonian rats. Eur J Neurosci. 2016;44:2247–2257. doi: 10.1111/ejn.13323. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. The Rat Atlas in Stereotaxic Coordinates. New York: Academic Press; 2007. [Google Scholar]
- 21.Gao HR, Zhuang QX, Li B, Li HZ, Chen ZP, Wang JJ, et al. Corticotropin releasing factor excites neurons of posterior hypothalamic nucleus to produce tachycardia in rats. Sci Rep. 2016;6:20206. doi: 10.1038/srep20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi H, Kita H, Kitai ST. Intracellular study of rat entopeduncular nucleus neurons in an in vitro slice preparation: electrical membrane properties. Brain Res. 1990;527:81–88. doi: 10.1016/0006-8993(90)91063-M. [DOI] [PubMed] [Google Scholar]
- 23.Shindou T, Mori A, Kase H, Ichimura M. Adenosine A(2A) receptor enhances GABA(A)-mediated IPSCs in the rat globus pallidus. J Physiol. 2001;532:423–434. doi: 10.1111/j.1469-7793.2001.0423f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Zhuang QX, Li B, Wu GY, Yung WH, Zhu JN, et al. Selective modulation of histaminergic inputs on projection neurons of cerebellum rapidly promotes motor coordination via HCN channels. Mol Neurobiol. 2016;53:1386–1401. doi: 10.1007/s12035-015-9096-3. [DOI] [PubMed] [Google Scholar]
- 25.Li B, Zhuang QX, Gao HR, Wang JJ, Zhu JN. Medial cerebellar nucleus projects to feeding-related neurons in the ventromedial hypothalamic nucleus in rats. Brain Struct Funct. 2017;222:957–971. doi: 10.1007/s00429-016-1257-2. [DOI] [PubMed] [Google Scholar]
- 26.Qiu DL, Chu CP, Shirasaka T, Nabekura T, Kunitake T, Kato K, et al. Neuromedin U depolarizes rat hypothalamic paraventricular nucleus neurons in vitro by enhancing IH channel activity. J Neurophysiol. 2003;90:843–850. doi: 10.1152/jn.00225.2003. [DOI] [PubMed] [Google Scholar]
- 27.Qiu DL, Chu CP, Shirasaka T, Tsukino H, Nakao H, Kato K, et al. Corticotrophin-releasing factor augments the I(H) in rat hypothalamic paraventricular nucleus parvocellular neurons in vitro. J Neurophysiol. 2005;94:226–234. doi: 10.1152/jn.01325.2004. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/S0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 29.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 30.Rauch M, Riediger T, Schmid HA, Simon E. Orexin A activates leptin-responsive neurons in the arcuate nucleus. Pflugers Arch. 2000;440:699–703. doi: 10.1007/s004240000342. [DOI] [PubMed] [Google Scholar]
- 31.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–7971. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baldo BA, Gual-Bonilla L, Sijapati K, Daniel RA, Landry CF, Kelley AE. Activation of a subpopulation of orexin/hypocretin-containing hypothalamic neurons by GABAA receptor-mediated inhibition of the nucleus accumbens shell, but not by exposure to a novel environment. Eur J Neurosci. 2004;19:376–386. doi: 10.1111/j.1460-9568.2004.03093.x. [DOI] [PubMed] [Google Scholar]
- 33.Horvath TL, Peyron C, Diano S, Ivanov A, Aston-Jones G, Kilduff TS, et al. Hypocretin (orexin) activation and synaptic innervation of the locus coeruleus noradrenergic system. J Comp Neurol. 1999;415:145–159. doi: 10.1002/(SICI)1096-9861(19991213)415:2<145::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Chen XY, Chen L, Du YF. Orexin-A increases the firing activity of hippocampal CA1 neurons through orexin-1 receptors. J Neurosci Res 2016. doi: 10.1002/jnr.23975. [DOI] [PubMed]
- 35.Xia J, Chen X, Song C, Ye J, Yu Z, Hu Z. Postsynaptic excitation of prefrontal cortical pyramidal neurons by hypocretin-1/orexin A through the inhibition of potassium currents. J Neurosci Res. 2005;82:729–736. doi: 10.1002/jnr.20667. [DOI] [PubMed] [Google Scholar]
- 36.Yu L, Zhang XY, Zhang J, Zhu JN, Wang JJ. Orexins excite neurons of the rat cerebellar nucleus interpositus via orexin 2 receptors in vitro. Cerebellum. 2010;9:88–95. doi: 10.1007/s12311-009-0146-0. [DOI] [PubMed] [Google Scholar]
- 37.Yu L, Zhang XY, Chen ZP, Zhuang QX, Zhu JN, Wang JJ. Orexin excites rat inferior vestibular nuclear neurons via co-activation of OX1 and OX 2 receptors. J Neural Transm (Vienna) 2015;122:747–755. doi: 10.1007/s00702-014-1330-z. [DOI] [PubMed] [Google Scholar]
- 38.Yamuy J, Fung SJ, Xi M, Chase MH. Hypocretinergic control of spinal cord motoneurons. J Neurosci. 2004;24:5336–5345. doi: 10.1523/JNEUROSCI.4812-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–89. doi: 10.1016/S0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- 40.Rasmussen K, Hsu MA, Yang Y. The orexin-1 receptor antagonist SB-334867 blocks the effects of antipsychotics on the activity of A9 and A10 dopamine neurons: implications for antipsychotic therapy. Neuropsychopharmacology. 2007;32:786–792. doi: 10.1038/sj.npp.1301239. [DOI] [PubMed] [Google Scholar]
- 41.Shin HS, Cho HS, Sung KW, Yoon BJ. Orexin-A increases cell surface expression of AMPA receptors in the striatum. Biochem Biophys Res Commun. 2009;378:409–413. doi: 10.1016/j.bbrc.2008.11.051. [DOI] [PubMed] [Google Scholar]
- 42.Zahodne LB, Okun MS, Foote KD, Fernandez HH, Rodriguez RL, Wu SS, et al. Greater improvement in quality of life following unilateral deep brain stimulation surgery in the globus pallidus as compared to the subthalamic nucleus. J Neurol. 2009;256:1321–1329. doi: 10.1007/s00415-009-5121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Follett KA, Torres-Russotto D. Deep brain stimulation of globus pallidus interna, subthalamic nucleus, and pedunculopontine nucleus for Parkinson’s disease: which target? Parkinsonism Relat Disord. 2012;18(Suppl 1):S165–S167. doi: 10.1016/S1353-8020(11)70051-7. [DOI] [PubMed] [Google Scholar]
- 44.Fronczek R, Overeem S, Lee SY, Hegeman IM, van Pelt J, van Duinen SG, et al. Hypocretin (orexin) loss in Parkinson’s disease. Brain. 2007;130:1577–1585. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 45.Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA. Sleep disorders, obesity, and aging: the role of orexin. Ageing Res Rev. 2015;20:63–73. doi: 10.1016/j.arr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, et al. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142:29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]