Abstract
Activation of presynaptic group II metabotropic glutamate receptors (mGluR2/3) inhibits drug reward and drug-seeking behavior, but the role of N-acetylaspartylglutamate (NAAG), an agonist of endogenous mGluR2/3, in heroin reward and heroin-seeking behavior remained unclear. Here, we aimed to explore the effects of exogenous NAAG on heroin self-administration and heroin-seeking behavior. First, rats were trained to self-administer heroin under a fixed ratio 1 (FR1) schedule for 10 days, then received NAAG (50 or 100 μg/10 μL in each nostril) in the absence or presence of LY341495 (1 mg/kg, i.p.), an antagonist of mGluR2/3, on day 11 and the effects of NAAG on heroin self-administration under FR1 were recorded for 3 consecutive days. Motivation was assessed in heroin self-administration under a progressive ratio schedule on day 11 in another 5 groups with the same doses of NAAG. Additional rats were withdrawn for 14 days after 14 days of heroin self-administration, then received the same pharmacological pretreatment and were tested for heroin-seeking behaviors induced by heroin priming or cues. The results showed that intranasal administration of NAAG significantly decreased intravenous heroin self-administration on day 12, but not on day 11. Pretreatment with LY341495 prior to testing on day 12 prevented the inhibitory effect of NAAG on heroin reinforcement. The break-point for reward motivation was significantly reduced by NAAG. Moreover, NAAG also significantly inhibited the heroin-seeking behaviors induced by heroin priming or cues and these were restored by pretreatment with LY341495. These results demonstrated that NAAG, via activation of presynaptic mGluR2/3, attenuated the heroin reinforcement, heroin motivational value, and heroin-seeking behavior, suggesting that it may be used as an adjunct treatment for heroin addiction.
Keywords: Glutamate, Opioid, Metabotropic glutamate receptor, Reward, Addiction
Introduction
Glutamate in the corticomesolimbic circuitry plays an important role in heroin addiction, including heroin reward and heroin-seeking behavior [1, 2]. Long-term use of drugs induces alterations in glutamatergic transmission within the nucleus accumbens (NAc), and this may underlie the relapse to heroin-seeking behaviors upon re-exposure to drugs or drug-associated stimuli following withdrawal [3–5]. Generally, activation of glutamatergic transmission, particularly at presynaptic group II metabotropic glutamate receptors (mGluR2/3), inhibits the release of dopamine (DA) and glutamate [6, 7]. Several reports have shown that systemic administration of LY379268, a well-characterized mGluR2/3 agonist [8], attenuates some of the behavioral and neurochemical effects of psychomimetic agents including phencyclidine and amphetamine in animal models [9, 10], decreases intravenous nicotine self-administration [11], and reduces alcohol reinforcement [12] and the discriminative stimulus effect of alcohol [13]. Moreover, LY379268 significantly inhibits the reinstatement of heroin-seeking behavior induced by contextual or discrete cues which had been associated with the heroin reward [14, 15]. Therefore, activation of mGluR2/3 by their agonists can regulate drug reward and drug-seeking behavior.
N-acetylaspartylglutamate (NAAG) is the most abundant and widely-distributed dipeptide transmitter in the mammalian nervous system [16]. A number of studies have demonstrated that NAAG acts as an endogenous agonist for mGluR2/3 [17, 18]. NAAG is hydrolyzed into glutamate and N-acetyl-L-aspartate by glutamate carboxypeptidase II (GCP-II). A highly consistent distribution of NAAG and GCP-II in brain tissue has been confirmed by neurochemical data [19]. Systemic administration of 2-(phosphonomethyl)pentanedioic acid (2-PMPA), a selective inhibitor of GCP-II, elevates NAAG levels in the brain and subsequently prevents excessive glutamate release by activation of presynaptic mGluR2/3 [20]. Moreover, 2-PMPA inhibits cocaine-induced behavioral sensitization, conditioned place-preference (CPP), and cocaine-induced kindled seizures [21–23]. 2-PMPA also attenuates morphine tolerance and reward but not the expression of morphine dependence [24]. Recent evidence has demonstrated that 2-PMPA and NAAG inhibit the reward motivational value of cocaine under a progressive ratio (PR) and reduce the reinstatement of cocaine-seeking behavior induced by cues [25, 26]. Thus, we hypothesized that NAAG, as an endogenous agonist of mGluR2/3, may play a key role in heroin self-administration and heroin-seeking behavior after withdrawal.
In the present study, we first investigated the effects of intranasal NAAG on intravenous heroin self-administration under fixed ratio 1 (FR1) or PR. Meanwhile, we investigated the effects of LY341495, an mGluR2/3 antagonist, on the inhibitory action of NAAG on heroin reward and motivational value. Second, we evaluated the effects of NAAG treatment in the absence or presence of LY341495 on heroin priming and cue-induced heroin-seeking behaviors in rats after withdrawal from heroin self-administration.
Materials and Methods
Animals
One hundred and fifty-eight male Sprague-Dawley rats (Zhejiang Experimental Animal Center, Hangzhou, China), weighing 250–280 g at the beginning of the experiment, were housed individually in a temperature-controlled, ventilated colony room with a 12-h light/dark cycle (lights onset 19:00, offset 07:00). All experiments were conducted during the dark period according to the specifications of the National Institute of Health Guide for the Care and Use of Laboratory Animals (Eighth edition). Food and water were available ad libitum.
Heroin Self-administration
Rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and implanted with chronically indwelling intravenous catheters as described previously [27]. The catheters were flushed daily with 0.3 mL saline containing penicillin B (20,000 units) and heparin (5 units) to prevent bacterial infection and maintain catheter patency. All rats were allowed to recover for at least 7 days.
After recovery from surgery, the animals were placed into operant chambers from Med Associates Inc. (Saint Albans, VT) for daily 4-h training sessions of heroin self-administration. Every session was started with a blue light on inside the active nose-poke hole. The rat received a single heroin infusion (0.05 mg/kg in each infusion) following completion of an active nose-poke under the FR1 schedule. Each infusion was paired with a 20-s illumination of the house light in combination with the noise of the infusion pump, which therefore served as the discrete conditioned stimulus (CS) paired with drug infusion. A timeout period was imposed for 20 s, during which a response had no programmed consequences but was still recorded. Illumination of the blue light in the active nose-poke again signaled the end of the 20-s timeout period. Touching the inactive nose-poke was recorded but had no programmed consequences. All rats were trained for at least 10 consecutive days to reach stable response activity.
Experiment 1. Effects of NAAG on Heroin Self-Administration Under FR1 Procedure
After 10 days of heroin self-administration training, rats were randomly divided into 5 groups (8/group). The control group received bilateral intranasal saline (10 μL/side), while those in the NAAG 50 and NAAG 100 groups received bilateral intranasal NAAG (50 and 100 μg/10 μL on each side) 1 h before testing. The rats in the NAAG+LY341495 group received bilateral intranasal NAAG (100 μg/10 μL) as above, and LY341495 (1 mg/kg, i.p.) 30 min prior to testing. Rats in the LY341495 group received the same LY341495 treatment only, as above. Then heroin self-administration under FR1 was tested in all rats on day 11. To investigate the long-term effect of NAAG on heroin self-administration under the FR1 schedule, all groups of rats received another 2 consecutive days of heroin self-administration until baseline response activity was re-established.
Another 4 groups of rats (7/group; control, NAAG, LY341495, and NAAG+LY341495 groups) were first trained in heroin self-administration for 10 days as described above, and then given one more day (day 11) of heroin self-administration training. On day 11, the NAAG and NAAG+LY341495 groups received bilateral intranasal NAAG (100 μg/10 μL on each side) 1 h prior to the session. On day 12, the LY341495 and NAAG+LY341495 groups received an i.p. injection of LY341495 (1 mg/kg) 30 min prior to testing. Then the heroin self-administration under FR1 reinforcement was tested.
Experiment 2. Effect of NAAG on Motivation of Heroin Self-Administration
The break-point was defined as the reward motivational value and underlined the incentive effect of heroin-taking in rats. The PR reinforcement schedule required animals to increase active nose-poking progressively for each successive infusion in the following series, within a self-administration session. The progression of ratio was calculated as follows for 28 reinforcements [28]: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 603, 737, 901, 1102, and 1347. The last ratio successfully completed was registered as the break-point for that session [28]. In this experiment, rats were first trained for heroin self-administration under the FR1 schedule for 10 consecutive days to ensure the rats with the establishment of stable self-administration. Then the rats with the establishment of stable self-administration based on the numbers of active pokes on day 10 were randomly divided into 5 groups (6/group)—control, NAAG 50, NAAG 100, NAAG+LY341495, and LY341495 groups—with the same administrations as in Experiment 1. All rats underwent a 4-h test session of heroin self-administration under the PR reinforcement schedule on day 11.
Heroin-Seeking Behaviors
In these procedures, all rats were first trained for heroin self-administration for 14 days under the FR1 schedule, and the general procedure for self-administration was the same as described previously. Rats were then placed into the home chambers and withdrawn for another 14 days. After completion of withdrawal, all rats were tested for heroin priming- or cue-induced heroin-seeking behaviors.
Experiment 3. Effects of NAAG on Heroin Priming-Induced Seeking Behavior
Five groups of rats (6/group) were again placed into the operant chambers after completion of withdrawal. The control, NAAG 50, NAAG 100, NAAG+LY341495, and LY341495 groups received the same pharmacological pretreatment as described in the motivation testing, then each rat was injected with heroin (0.25 mg/kg, s.c.) 10 min before the beginning of the test. In the testing, one active nose-poke response resulted in another presentation of the CS but no heroin infusion occurred, while an inactive nose-poke response had no programmed consequences. Nose-pokes including both active and inactive during this test were accumulated over 120 min.
Experiment 4. Effects of NAAG on Cue-Induced Heroin-Seeking Behavior
Rats were randomly divided into the same 5 groups as in Experiment 3 (6/group). In the 2-h cue-induced heroin-seeking testing, each rat was exposed to the discrete CS for 5 s at the beginning of testing in the operant chamber, after which each active nose-poke response resulted in another presentation of the CS but without heroin infusion. An inactive nose-poke response had no programmed consequences. Active and inactive nose-pokes during this test were calculated for comparison.
Reagents
Heroin (diacetylmorphine HCl) was obtained from the National Institute of Forensic Science (Beijing, China). The heroin dose (0.05 mg/kg in each injection) used for self-administration was selected based on our previous study [29]. Heroin was dissolved in sterile saline at 0.2 mg/mL. LY341495 from Tocris Bioscience (Ellisville, MO) was dissolved in sterile saline with gentle warming for systemic administration (i.p.). NAAG (purity: >99%) synthesized by Sangon Biotech (Shanghai) Co., Ltd, China, was dissolved in sterile saline for intranasal administration.
Statistics
Data are expressed as mean ± SD. The mean numbers of active pokes, inactive pokes, and infusions during self-administration and seeking tests were analyzed with one-way ANOVA followed by Bonferroni post-hoc test or two-way ANOVA with repeated measures. If two-way ANOVA resulted in a significant main effect or a significant interaction, a one-way ANOVA with Bonferroni post-hoc test was performed for multiple comparisons. P < 0.05 was considered to be statistically significant.
Results
NAAG Gradually Inhibits Heroin Self-Administration
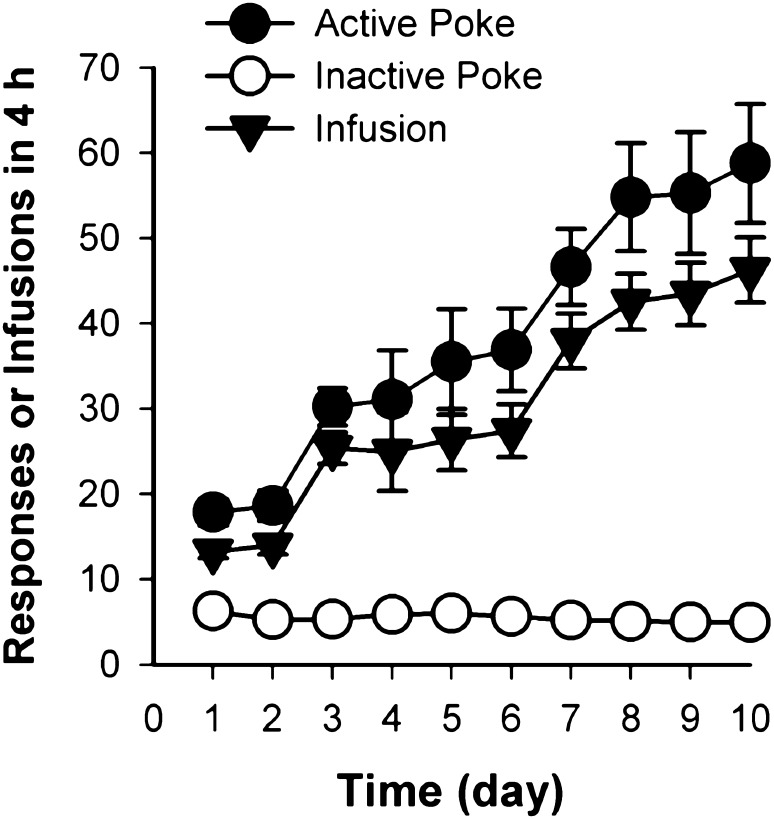
Similar to our previous finding [29], the active pokes and heroin infusions reached a stable high level within 10 days. On the contrary, inactive pokes remained at a low level throughout the 10 days of training (Fig. 1).
Fig. 1.
Effects of 10 days of training on the pokes and infusions during heroin self-administration in rats. The active pokes and heroin infusions reached a stable and high level within 10 days of heroin self-administration, while the inactive pokes remained at a low level.
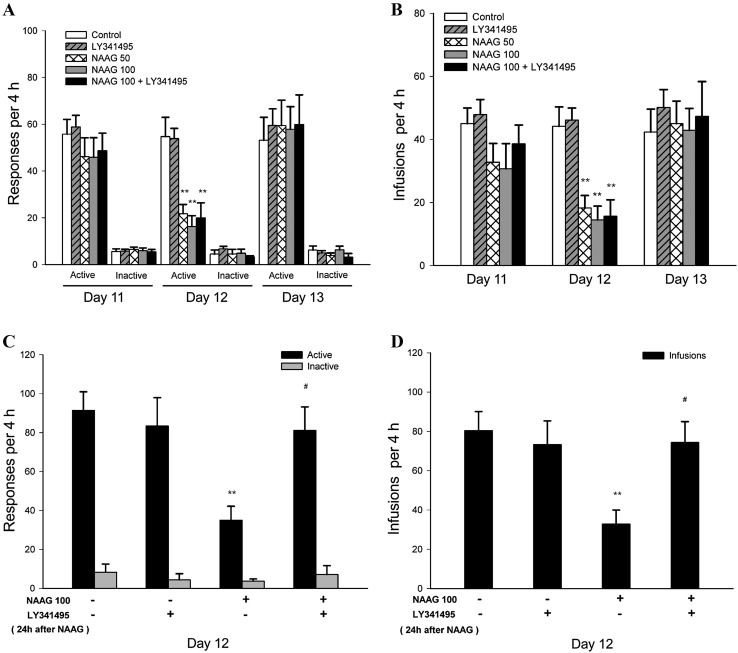
The effect of NAAG on heroin administration was assessed in Experiment 1 under FR1 reinforcement for 3 consecutive days. Two-way ANOVA for repeated measures revealed that the active pokes had a treatment main effect (F 4,35 = 4.18, P = 0.007) and time main effect (F 2,70 = 14.22, P = 0.00007), but no treatment × time interaction effect (F 8,70 = 1.99, P = 0.06). However, the inactive pokes had no treatment main effect (F 4,35 = 0.45, P = 0.77), time main effect (F 2,70 = 0.39, P = 0.68), or treatment × time interaction effect (F 8,70 = 0.56, P = 0.81). On day 11, post-hoc Bonferroni comparisons revealed no difference in the active pokes among all groups (P > 0.05). On day 12, multiple comparisons showed a decrease in the active pokes in the NAAG 50 (P < 0.01), NAAG 100 (P < 0.01), and NAAG + LY341495 (P < 0.01) groups, but not in the LY341495 group (P > 0.05), when compared with the control. There was also no difference in the active pokes between NAAG 100 and NAAG+LY341495 groups (P > 0.05). On day 13, there was no difference in the active pokes among all groups (P > 0.05, Fig. 2A). Two-way ANOVA also revealed that heroin infusions had a treatment main effect (F 4,35 = 6.59, P = 0.0005) and time main effect (F 2,70 = 9.72, P = 0.0002), but no treatment × time interaction effect (F 8,70 = 1.64, P = 0.13). On day 11, post-hoc comparisons revealed no difference in heroin infusions among all groups (all P > 0.05). On day 12, individual group comparisons showed an inhibition of heroin infusions in the NAAG 50 (P < 0.01), NAAG 100 (P < 0.01), and NAAG + LY341495 (P < 0.01) groups, but not in the LY341495 group (P > 0.05), when compared with the control. There was no difference in heroin infusions between the NAAG 100 and NAAG+LY341495 groups (P > 0.05). On day 13, there was no difference in heroin infusions among all the groups (P > 0.05, Fig. 2B).
Fig. 2.
Effects of NAAG on heroin self-administration under the FR1 schedule. A, B. Pretreatment with NAAG (50 and 100 μg/10 μL in each nostril) or NAAG (100 μg/10 μL in each nostril) plus LY341495 (1 mg/kg, i.p.) had no effects on the active pokes and heroin infusions on day 11, but significantly inhibited the active pokes and heroin infusions on day 12. The active pokes and heroin infusions in these groups recovered to normal levels on day 13. C, D. NAAG (100 μg/10 μL in each nostril) was administered on day 11 but LY341495 (1 mg/kg, i.p.) was injected 24 h after NAAG in this experiment. The active pokes and heroin infusions were significantly inhibited by NAAG but not with LY341495. The inhibitory effects on the active pokes and heroin infusions of NAAG were restored by the combined treatment with LY341495. (*P < 0.05, **P < 0.01 compared with control group; # P < 0.05 compared with NAAG group).
In the experimental setting in which LY341495 was injected 24 h after NAAG administration on day 12, two-way ANOVA revealed that the active pokes had a main effect of NAAG (F 1,12 = 4.92, P = 0.047) and an NAAG × LY341495 interaction effect (F 1,12 = 7.42, P = 0.018), but no LY341495 main effect (F 1,12 = 3.70, P = 0.078). Post-hoc comparisons showed an inhibition of active pokes in the NAAG group (P < 0.01) but not in the LY341495 group (P > 0.05), compared with control. The NAAG+LY341495 group showed a recovery effect on the active pokes (P < 0.05) compared with the NAAG group. There was no difference in the inactive pokes among all groups (P > 0.05, Fig. 2C). Two-way ANOVA also revealed that heroin infusions had an NAAG main effect (F 1,12 = 5.50, P = 0.037) and an NAAG × LY341495 interaction effect (F 1,12 = 7.89, P = 0.016), but no LY341495 main effect (F 1,12 = 4.50, P = 0.055). Post-hoc comparisons showed an inhibition of heroin infusions in the NAAG group (P < 0.01) but not in the LY341495 group (P > 0.05), compared with control. The NAAG + LY341495 group showed a recovery effect on heroin infusions (P < 0.05) compared with the NAAG group (Fig. 2D).
NAAG Inhibits Heroin Motivation Under the PR Procedure
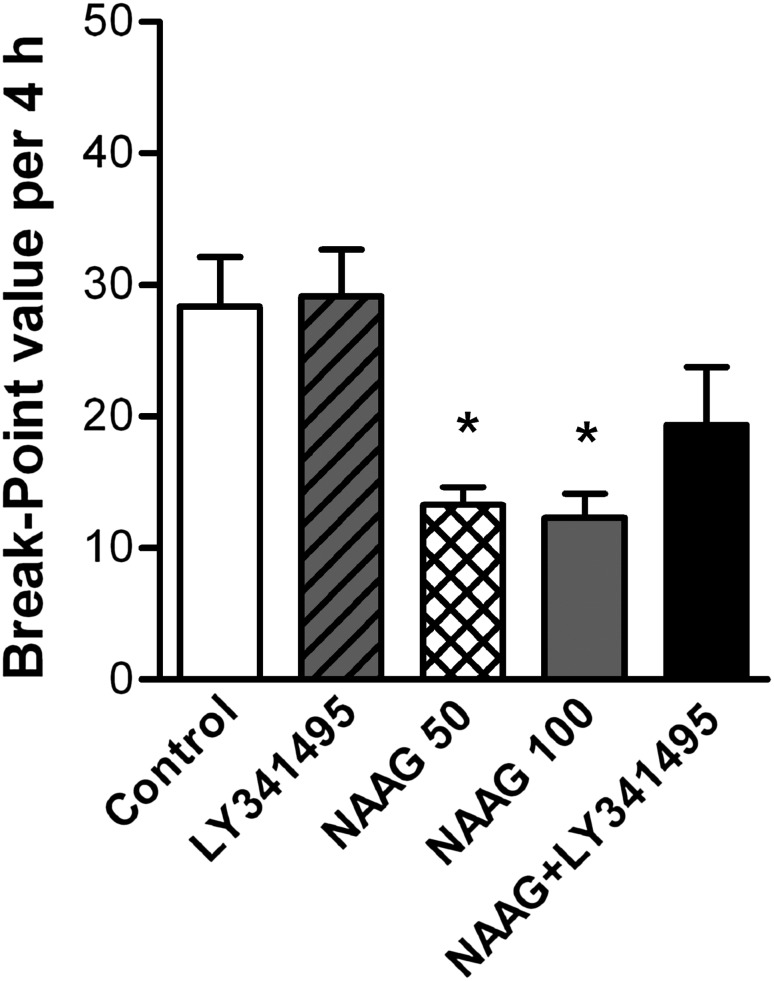
One-way ANOVA revealed an inhibition of the break-point after NAAG administration (F4,25 = 6.21, P = 0.001). Post-hoc comparisons showed an inhibition of the break-point in the NAAG 50 (P < 0.05) and NAAG 100 groups (P < 0.05), but not in the LY341495 or NAAG+LY341495 groups, when compared with control. The NAAG+LY341495 group showed a slight recovery effect on the break-point but without a statistically significant difference (P > 0.05) when compared with the NAAG 100 group (Fig 3).
Fig. 3.
Effects of NAAG on the motivation of heroin self-administration under the PR schedule. Pretreatment with NAAG (50 and 100 μg/10 μL in each nostril) but not LY341495 alone (1 mg/kg, i.p.) significantly inhibited the break-point. The inhibitory effect on the break-point of NAAG (100 μg/10 μL in each nostril) slightly recovered but had no statistically significant difference from the combined treatment with LY341495. (*P < 0.05 compared with control group).
NAAG Inhibits Heroin Priming- and Cue-Induced Heroin-Seeking Behavior
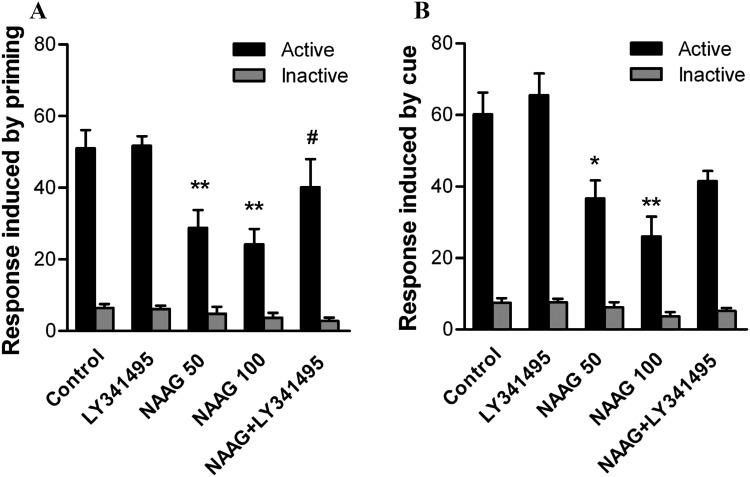
The intranasal administration of NAAG inhibited both heroin priming-induced and cue-induced heroin-seeking behavior after withdrawal in Experiments 3 and 4 (Fig. 4). As shown in Fig. 4A, one-way ANOVA revealed an inhibition of the active pokes after NAAG administration (F 4,25 = 11.61, P = 0.0002). Post-hoc comparisons revealed a reduction in the active pokes in the NAAG 50 (P < 0.01) and NAAG 100 groups (P < 0.01), but not in the LY341495 group when compared with control. The NAAG+LY341495 group showed a recovery effect on the active pokes (P < 0.05) compared with NAAG 100 group. There was no difference in the inactive pokes among all groups (P > 0.05)
Fig. 4.
Effects of NAAG on heroin priming- and cue-induced heroin-seeking behaviors. (A) Active pokes were significantly inhibited by pretreatment with NAAG (50 and 100 μg/10 μL in each nostril) but not LY341495 alone (1 mg/kg, i.p.) during heroin priming-induced drug-seeking tests. The inhibitory effect of NAAG (100 μg/10 μL in each nostril) on the active pokes was reversed by combined treatment with LY341495. (B) Active pokes were inhibited by pretreatment with NAAG (50 and 100 μg/10 μL in each nostril) but not LY341495 alone (1 mg/kg, i.p.) during cue-induced heroin-seeking tests. (*P < 0.05, **P < 0.01 compared with control group; # P < 0.05 compared with the NAAG 100 group).
Similarly, as shown in Fig. 4B, one-way ANOVA also revealed an inhibition of the active pokes after NAAG administration (F 4,25 = 9.73, P = 0.0007). Multiple comparisons revealed a reduction in the active pokes in the NAAG 50 (P < 0.05) and NAAG 100 groups (P < 0.01), but not in the LY341495 group compared with control. Pretreatment with LY341495 slightly reversed the NAAG-induced inhibition of active pokes during cue-induced heroin-seeking behavior. There was no difference in the inactive pokes among all groups (P > 0.05).
Discussion
In the present study, we found that the onset time of inhibition of heroin self-administration under FR1 was 1 day, rather than 1 h, after intranasal administration of NAAG. The break-point value was immediately decreased by NAAG on heroin self-administration under the PR procedure. This inhibitory effect of NAAG on heroin self-administration was reversed by pretreatment with LY341495, a selective antagonist of mGluR2/3. In addition, NAAG inhibited both the heroin priming-induced and cue-induced heroin-seeking behaviors after withdrawal. These data demonstrated that exogenous NAAG inhibits heroin reward, heroin motivation, and heroin-seeking behavior.
Drug self-administration is the most robust and reliable model with which to measure the rewarding effect of drugs [30]. Here, we noted that NAAG at 50 and 100 μg in each nostril only weakly affected the heroin self-administration under FR1 on the first testing day, but remarkably inhibited the self-administration on the second day. The absence of an effect of NAAG on the heroin reward on the first testing day is consistent with a previous report that intranasal administration of NAAG (100 or 300 μg in each nostril) has no effect on cocaine self-administration under the FR2 schedule [24]. One possible reason is that the rapidly cumulative infusions of heroin under the FR1 schedule induce high levels of DA in the corticomesolimbic regions. DA release in the NAc, one of the most important corticomesolimbic nuclei, has long been thought to play a critical role in mediating the drug reward effect [31]. Thus, the stronger rewarding effect produced by the higher cumulative heroin dose during the FR1 reinforcement schedule may have overcome the NAAG antagonism of the rewarding effect of heroin. This deduction is directly confirmed by a recent report that 2-PMPA, an inhibitor of GCP-II, elevates the endogenous NAAG level, and inhibits intravenous cocaine self-administration only at lower doses, but not at higher doses of cocaine [25]. During the second day, it seems that the robust increase in DA due to the direct heroin effect would exist during the test given 24 h after NAAG delivery. However, an elevated NAAG level in the brain may combat the gradual release of DA at the beginning of heroin administration during the second day of training, and subsequently NAAG may play an inhibitory role in the heroin reward. NAAG at doses that effectively inhibit cocaine reward and relapse behavior fails to affect sucrose self-administration [24, 25]. Thus, NAAG may specifically inhibit the heroin reward at a slow rate.
PR reinforcement has been used to measure the degree of reinforcing efficacy [32]. The break-point value represents the motivation value of drug-taking. NAAG clearly inhibited the heroin motivation value under the PR reinforcement procedure. That the cumulative dose of heroin in the PR reinforcement schedule was lower than in the FR1 reinforcement schedule, and the FR1 schedule demands less work to achieve the reward effects [33], may provide a possible explanation for the absence of effective action of NAAG on heroin self-administration on the first testing day. A previous study has shown that LY341495, a selective antagonist of mGluR2/3, has no effect on locomotor activity at 1 mg/kg [34]. The inhibitory action of NAAG on both the heroin self-administration on the second testing day and the heroin motivation was partially reversed by pretreatment with LY341495, showing a specific reversal action of LY341495 on the inhibitory effect of NAAG in heroin reward and motivation. mGluR2/3 is mainly localized presynaptically on glutamate nerve terminals, and reduces the stimulated release of glutamate. The evidence shows that glutamate is co-released with DA in the NAc after injection of various drugs [35, 36], and the release of DA in the NAc is directly or indirectly regulated by the glutamate terminals of axons projecting from the prefrontal cortex, amygdala, or hippocampus [1]. Thus, NAAG might decrease the glutamate and DA levels in the NAc via activation of presynaptic mGluR2/3 and inhibit the heroin rewarding effect by negative feedback.
Glutamate release in the corticomesolimbic regions is critical for heroin- and cue-induced heroin-seeking [3]. N-acetylcysteine, which keeps the extracellular concentration of glutamate in the corticomesolimbic regions in the normal physiological range by restoring cystine–glutamate exchange, induces an enduring reduction in heroin- and cue-induced heroin-seeking behavior [5]. In the present study, the rats were withdrawn from heroin administration but the contextual seeking behaviors were not extinguished. Contextual cues may also contribute to the heroin-seeking behavior induced by heroin-priming or cues after withdrawal. Elevation of endogenous NAAG by 2-PMPA prevents excessive glutamate release by activating presynaptic mGluR2/3 [37]. Moreover, 2-PMPA at doses that inhibit cocaine reward and relapse does not affect the sucrose-seeking behavior [24, 25]. LY379268, at doses effective in attenuating heroin-seeking behavior, also attenuates sucrose-seeking but not sucrose self-administration in rats [38]. The pharmacological effects of LY379268 may be mediated predominantly by activation of a subtype of mGlu2 receptors although it has a relatively higher selectivity for mGlu2 (EC50 = 2.69 nmol/L) then for mGlu3 (EC50 = 4.48 nmol/L) receptors [39]. On the other hand, stimulation of postsynaptic mGluR2/3 modulates neuronal excitability and plasticity via intracellular mechanisms such as the modulation of ion channels. However, it was not clear whether postsynaptic mGluR2/3 is involved in the drug reward or drug-seeking behavior [40]. Thus, the present data provide evidence that exogenous NAAG inhibits heroin-seeking behavior via activation of presynaptic mGluR2/3.
NAAG here was given intranasally and not intraperitoneally, due to the peptide’s poor bioavailability and difficulty in penetrating the blood-brain barrier. The literature shows that a wide variety of therapeutic compounds such as peptides and proteins can be delivered intranasally [41]. This mode of drug delivery provides numerous benefits, including a large surface area for absorption, no first-pass metabolism, and direct delivery into the CNS. Compared to LY379268, NAAG has several unique pharmacological properties. First, LY379268, at the doses used, not only inhibits heroin relapse, but also inhibits natural (sucrose-seeking) behavior [14]. Increasing the brain NAAG level dose not affect natural seeking behavior at its effective doses to inhibit drug-seeking behavior [25, 42]. Second, the therapeutic effects and the suppression of motor activity of LY379268 have rapid tolerance [8, 43]. However, a crucial caveat in the present study is that the rate of absorption of NAAG into the brain and level of endogenous NAAG after intranasal administration need to be elucidated.
In conclusion, the results of the present study demonstrated that the exogenous delivery of NAAG had a slow inhibitory effect on heroin reward, produced rapid inhibitory effects on the heroin motivation and heroin- and cue-induced heroin-seeking behaviors. Elevation of the brain NAAG level may activate the presynaptic mGluR2/3 and subsequently reduce the heroin reward and heroin-seeking, suggesting the potential use of NAAG in the treatment of heroin addiction.
Acknowledgements
This work was supported by the National Basic Research Program of China (2015CB553504), the National Natural Science Foundation of China (81471350 and 81671321), and the Natural Science Foundation of Ningbo Municipality, Zhejiang Province, China (2015A610193).
References
- 1.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza MS. Glutamatergic transmission in drug reward: implications for drug addiction. Front Neurosci. 2015;9:404. doi: 10.3389/fnins.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LaLumiere RT, Kalivas PW. Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lou ZZ, Chen LH, Liu HF, Ruan LM, Zhou WH. Blockade of mGluR5 in the nucleus accumbens shell but not core attenuates heroin seeking behavior in rats. Acta Pharmacol Sin. 2014;35:1485–1492. doi: 10.1038/aps.2014.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu G, Duffy P, Swanson C, Ghasemzadeh MB, Kalivas PW. The regulation of dopamine transmission by metabotropic glutamate receptors. J Pharmacol Exp Ther. 1999;289:412–416. [PubMed] [Google Scholar]
- 7.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 8.Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13:444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- 10.Dd DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: novel agents for schizophrenia? Curr Drug Targets CNS Neurol Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- 11.Liechti ME, Markou A. Metabotropic glutamate 2/3 receptor activation induced reward deficits but did not aggravate brain reward deficits associated with spontaneous nicotine withdrawal in rats. Biochem Pharmacol. 2007;74:1299–1307. doi: 10.1016/j.bcp.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Sidhpura N, Weiss F, Martin-Fardon R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol Psychiatry. 2010;67:804–811. doi: 10.1016/j.biopsych.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cannady R, Grondin JJ, Fisher KR, Hodge CW, Besheer J. Activation of group II metabotropic glutamate receptors inhibits the discriminative stimulus effects of alcohol via selective activity within the amygdala. Neuropsychopharmacology. 2011;36:2328–2338. doi: 10.1038/npp.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bossert JM, Busch RF, Gray SM. The novel mGluR2/3 agonist LY379268 attenuates cue-induced reinstatement of heroin seeking. Neuroreport. 2005;16:1013–1016. doi: 10.1097/00001756-200506210-00026. [DOI] [PubMed] [Google Scholar]
- 15.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neale JH, Bzdega T, Wroblewska B. N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem. 2000;75:443–452. doi: 10.1046/j.1471-4159.2000.0750443.x. [DOI] [PubMed] [Google Scholar]
- 17.Neale JH. N-acetylaspartylglutamate is an agonist at mGluR(3) in vivo and in vitro. J Neurochem. 2011;119:891–895. doi: 10.1111/j.1471-4159.2011.07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olszewski RT, Bzdega T, Neale JH. mGluR3 and not mGluR2 receptors mediate the efficacy of NAAG peptidase inhibitor in validated model of schizophrenia. Schizophr Res. 2012;136:160–161. doi: 10.1016/j.schres.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Fuhrman S, Palkovits M, Cassidy M, Neale JH. The regional distribution of N-acetylaspartylglutamate (NAAG) and peptidase activity against NAAG in the rat nervous system. J Neurochem. 1994;62:275–281. doi: 10.1046/j.1471-4159.1994.62010275.x. [DOI] [PubMed] [Google Scholar]
- 20.Slusher BS, Vornov JJ, Thomas AG, Hurn PD, Harukuni I, Bhardwaj A, et al. Selective inhibition of NAALADase, which converts NAAG to glutamate, reduces ischemic brain injury. Nat Med. 1999;5:1396–1402. doi: 10.1038/70971. [DOI] [PubMed] [Google Scholar]
- 21.Shippenberg TS, Rea W, Slusher BS. Modulation of behavioral sensitization to cocaine by NAALADase inhibition. Synapse. 2000;38:161–166. doi: 10.1002/1098-2396(200011)38:2<161::AID-SYN7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Slusher BS, Thomas A, Paul M, Schad CA, Ashby CR., Jr Expression and acquisition of the conditioned place preference response to cocaine in rats is blocked by selective inhibitors of the enzyme N-acetylated-alpha-linked-acidic dipeptidase (NAALADASE) Synapse. 2001;41:22–28. doi: 10.1002/syn.1056. [DOI] [PubMed] [Google Scholar]
- 23.Witkin JM, Gasior M, Schad C, Zapata A, Shippenberg T, Hartman T, et al. NAALADase (GCP II) inhibition prevents cocaine-kindled seizures. Neuropharmacology. 2002;43:348–356. doi: 10.1016/S0028-3908(02)00124-7. [DOI] [PubMed] [Google Scholar]
- 24.Popik P, Kozela E, Wrobel M, Wozniak KM, Slusher BS. Morphine tolerance and reward but not expression of morphine dependence are inhibited by the selective glutamate carboxypeptidase II (GCP II, NAALADase) inhibitor, 2-PMPA. Neuropsychopharmacology. 2003;28:457–467. doi: 10.1038/sj.npp.1300048. [DOI] [PubMed] [Google Scholar]
- 25.Xi ZX, Kiyatkin M, Li X, Peng XQ, Wiggins A, Spiller K, et al. N-acetylaspartylglutamate (NAAG) inhibits intravenous cocaine self-administration and cocaine-enhanced brain-stimulation reward in rats. Neuropharmacology. 2010;58:304–313. doi: 10.1016/j.neuropharm.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xi ZX, Li X, Peng XQ, Li J, Chun L, Gardner EL, et al. Inhibition of NAALADase by 2-PMPA attenuates cocaine-induced relapse in rats: a NAAG-mGluR2/3-mediated mechanism. J Neurochem. 2010;112:564–576. doi: 10.1111/j.1471-4159.2009.06478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai M, Chen W, Zhu H, Zhou X, Liu H, Zhang F, et al. Low dose risperidone attenuates cue-induced but not heroin-induced reinstatement of heroin seeking in an animal model of relapse. Int J Neuropsychopharmacol. 2013;16:1569–1575. doi: 10.1017/S1461145712001563. [DOI] [PubMed] [Google Scholar]
- 28.Duvauchelle CL, Sapoznik T, Kornetsky C. The synergistic effects of combining cocaine and heroin (“speedball”) using a progressive-ratio schedule of drug reinforcement. Pharmacol Biochem Behav. 1998;61:297–302. doi: 10.1016/S0091-3057(98)00098-7. [DOI] [PubMed] [Google Scholar]
- 29.Lai M, Zhu H, Sun A, Zhuang D, Fu D, Chen W, et al. The phosphodiesterase-4 inhibitor rolipram attenuates heroin-seeking behavior induced by cues or heroin priming in rats. Int J Neuropsychopharmacol. 2014;17:1397–1407. doi: 10.1017/S1461145714000595. [DOI] [PubMed] [Google Scholar]
- 30.O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 31.Wise RA. Role of brain dopamine in food reward and reinforcement. Philos Trans R Soc Lond B Biol Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold JM, Roberts DC. A critique of fixed and progressive ratio schedules used to examine the neural substrates of drug reinforcement. Pharmacol Biochem Behav. 1997;57:441–447. doi: 10.1016/S0091-3057(96)00445-5. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, et al. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology (Berl) 2009;203:677–684. doi: 10.1007/s00213-008-1414-2. [DOI] [PubMed] [Google Scholar]
- 34.O’Neill MF, Heron-Maxwell C, Conway MW, Monn JA, Ornstein P. Group II metabotropic glutamate receptor antagonists LY341495 and LY366457 increase locomotor activity in mice. Neuropharmacology. 2003;45:565–574. doi: 10.1016/S0028-3908(03)00232-6. [DOI] [PubMed] [Google Scholar]
- 35.Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. doi: 10.1523/JNEUROSCI.3128-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neale JH, Olszewski RT, Gehl LM, Wroblewska B, Bzdega T. The neurotransmitter N-acetylaspartylglutamate in models of pain, ALS, diabetic neuropathy, CNS injury and schizophrenia. Trends Pharmacol Sci. 2005;26:477–484. doi: 10.1016/j.tips.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 39.Collado I, Pedregal C, Mazon A, Espinosa JF, Blanco-Urgoiti J, Schoepp DD, et al. (2S,1’S,2’S,3’R)-2-(2’-carboxy-3’-methylcyclopropyl) glycine is a potent and selective metabotropic group 2 receptor agonist with anxiolytic properties. J Med Chem. 2002;45:3619–3629. doi: 10.1021/jm0110486. [DOI] [PubMed] [Google Scholar]
- 40.Hetzenauer A, Corti C, Herdy S, Corsi M, Ferraguti F, Singewald N. Individual contribution of metabotropic glutamate receptor (mGlu) 2 and 3 to c-Fos expression pattern evoked by mGlu2/3 antagonism. Psychopharmacology (Berl) 2008;201:1–13. doi: 10.1007/s00213-008-1236-2. [DOI] [PubMed] [Google Scholar]
- 41.Costantino HR, Illum L, Brandt G, Johnson PH, Quay SC. Intranasal delivery: physicochemical and therapeutic aspects. Int J Pharm. 2007;337:1–24. doi: 10.1016/j.ijpharm.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 42.Peng XQ, Li J, Gardner EL, Ashby CR, Jr, Thomas A, Wozniak K, et al. Oral administration of the NAALADase inhibitor GPI-5693 attenuates cocaine-induced reinstatement of drug-seeking behavior in rats. Eur J Pharmacol. 2010;627:156–161. doi: 10.1016/j.ejphar.2009.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cartmell J, Monn JA, Schoepp DD. Tolerance to the motor impairment, but not to the reversal of PCP-induced motor activities by oral administration of the mGlu2/3 receptor agonist, LY379268. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:39–46. doi: 10.1007/s002109900151. [DOI] [PubMed] [Google Scholar]