Abstract
Accumulation and aggregation of β-amyloid (Aβ) peptides result in neuronal death, leading to cognitive dysfunction in Alzheimer’s disease. The self-assembled Aβ molecules form various intermediate aggregates including oligomers that are more toxic to neurons than the mature aggregates, including fibrils. Thus, one strategy to alleviate Aβ toxicity is to facilitate the conversion of Aβ intermediates to larger aggregates such as fibrils. In this study, we designed a peptide named A3 that significantly enhanced the formation of amorphous aggregates of Aβ by accelerating the aggregation kinetics. Thioflavin T fluorescence experiments revealed an accelerated aggregation of Aβ monomers, accompanying reduced Aβ cytotoxicity. Transgenic Caenorhabditis elegans over-expressing amyloid precursor protein exhibited paralysis due to the accumulation of Aβ oligomers, and this phenotype was attenuated by feeding the animals with A3 peptide. These findings suggest that the Aβ aggregation-promotion effect can potentially be useful for developing strategies to reduce Aβ toxicity.
Keywords: Aβ, Alzheimer’s disease, Aggregation, Aβ oligomer, Amorphous aggregate, Cell death, C. elegans, β-sheet
Introduction
Aggregation of amyloid peptides is known to play a key role in Alzheimer’s disease (AD). These amyloid aggregates are composed mainly of β-amyloid (Aβ) peptide. In vivo, Aβ is produced by endoproteolytic cleavage of amyloid precursor protein (APP) [1–5]. Aβ monomers self-assemble into variable intermediate aggregates, such as oligomers, protofibrils, annular aggregates, and the insoluble mature fibrils [6–8]. Recent studies suggest that the soluble Aβ oligomers, rather than Aβ monomers or the insoluble large Aβ fibrils, are the most toxic, leading to neuronal pathology and the clinical manifestations of AD [9–12]. Extensive efforts have been directed towards Aβ reduction, either by inhibiting the enzymes that generate Aβ peptide or neutralizing Aβ antibodies. These strategies have not been successful so far [13]. An alternative strategy to alleviate Aβ toxicity is to promote Aβ oligomers to further aggregate into Aβ fibers or other insoluble aggregates. There have been reports that [G3]-Mor (a gallic acidtriethylene glycol dendrimer, functionalized with 27 terminal morpholinoethyl groups) and PEI-P (polyethylenimineperphenazine-P) accelerate aggregation, which would significantly reduce the toxicity of Aβ aggregates [14, 15]. Aggregation of proteins can be affected by various environmental conditions, such as temperature, salt concentration, or the presence of other catalysts [16]. In addition, surface chemistry has also been shown to appreciably affect the aggregation kinetics of proteins and peptides [17, 18].
Attempts have been made to find molecular species that can interfere with the aggregation kinetics of β amyloid. These molecules can be categorized as polypeptides, dyes, and organic or inorganic compounds [19–26]. Amyloid-binding dyes such as thioflavin T (ThT) have been reported to reduce the aggregation of Aβ (3–42) and significantly extend the lifespan of adult Caenorhabditis elegans [22]. Phage display experiments have shown that some peptides have a high affinity for either monomeric or aggregated Aβ [27]. These findings have improved our understanding of the molecular pathways of the protein aggregation process. However, most of these studies were conducted in vitro. Transgenic mouse models of AD exhibit some of the pathological and behavioral changes seen in humans, but such models are expensive, time-consuming, and not amenable to drug screening [28, 29]. Simple invertebrate models of Aβ aggregation may offer advantages, not only in drug screening but also in studying the basic cellular processes conserved among animals. Transgenic C. elegans can express human Aβ and exhibit pathological and behavioral abnormalities relevant to Aβ [30, 31].
In the present study, we set out to identify peptide modulators that significantly affect the aggregation kinetics of Aβ, leading to the attenuation of β-amyloid toxicity.
Materials and Methods
Synthetic Peptides and Aβ
Four peptide modulators were designed and custom-synthesized by GL Biochem Ltd (Shanghai, China). The peptide purity (>98%) was verified by high-performance liquid chromatography and mass spectrum analyses. Aβ42 was from GL Biochem. The peptide modulators were dissolved in triple-distilled H2O and Aβ was dissolved in 1% dimethyl sulfoxide (DMSO) for in vivo experiments.
ThT Fluorescence
Aβ was incubated in sterile phosphate-buffered saline (PBS) with or without the peptide modulators for up to 4.5 days at 37 °C. The final concentration of Aβ was 40 μmol/L. Aggregation was recorded by fluorescence after addition of ThT. A fluorescent dye, ThT identifies structures containing β-sheets. We chose a series of time points for quantitative analysis, and Aβ aggregation was accompanied by a characteristic increase in fluorescence intensity at 480 nm in Corning 96-well black plates (New York, NY) using a PerkinElmer Victor microplate reader (Waltham, MA). To exclude background fluorescence, the fluorescence intensity of a blank solution (ThT alone in PBS) was subtracted from all readings.
Transmission Electron Microscopy (TEM)
We incubated Aβ (40 μmol/L) with the peptide modulators (10 μmol/L) at 37 °C for 3 days in PBS. Aβ or peptide modulators alone were set up as control groups. At 6 and 72 h, a 50 μL sample was dropped onto carbon-coated copper grids (200 mesh) for 20 min and dried by air flow after removal of the excess sample drop. Afterwards, the samples were negatively stained with 20 μL phosphotungstic acid (1 mg/mL) for 10 min and the excess solution was removed. Then the grids were washed three times with triple-distilled water and dried under vacuum overnight. All experiments were performed using a FEI Tecnai G2 20 S-TWIN TEM (Hillsboro, TX) with an accelerating voltage of 200 kV.
Atomic Force Microscopy (AFM)
Samples were diluted in the same way as for TEM. At 72 h, ~10 μL of the solution was applied to a freshly-cleaved mica surface and dried with air flow. AFM measurements were performed on a Bruker Dimension 3100 scanning probe microscope (Karlsruhe, Germany) in tapping mode at room temperature. A silicon probe was oscillated at ~330 kHz and images were collected at a scan rate of 1 Hz.
Cell Line and Culture
The human neuroma cell line SH-SY5Y was purchased from the Cell Culture Center of the Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences. Cells were cultured in Hyclone RPMI-1640 medium (Logan, UT) supplemented with 15% Gibco newborn bovine serum (Grand Island, NY), penicillin G (100 kU/L), and kanamycin (0.1 g/L). The cells were routinely subcultured once every 3 days and maintained at 37 °C in 5% CO2 and 95% air in 75-cm2 tissue culture flasks.
MTT Assay
SH-SY5Y cells were plated in full medium on 96-well plates in a 100 μL total volume at 1 × 104 cells/mL and cultured at 37 °C in 5% CO2 for 24 h. Then 10 μL Aβ (final concentration, 40 µmol/L) and 10 μL peptide modulator (final concentration as indicated) were added and incubated for another 30 h. Then the medium was carefully removed from each well and 150 μL DMSO was added. Cell viability was determined based on the mitochondrial conversion of MTT dye to formazan crystals. Absorbance was read at 540 nm on a Thermo microplate reader (Waltham, MA). Data are expressed as percentage cell viability compared to a 100% signal from untreated cells.
C. elegans Strains
The wild-type strain N2, and the transgenic nematode strains CL4176 and CL802 were kindly provided by professor Guangjun Nie (National Center for Nanoscience and Technology, China). The CL4176 strain constitutively produces body-wall muscle-specific Aβ42, the expression of which depends on upshifting the temperature from 16 to 23 °C. The CL802 strain does not express body-wall muscle-specific Aβ42 and was used as a control. The wild-type N2 and the transgenic CL4176 and CL802 were propagated at 16 °C on solid nematode growth medium seeded with 200 μL spots of Escherichia coli (OP50) for food. After obtaining age-synchronized C. elegans, the worms were fed with the designed peptides either from stage L1 (1 day old) or starting from the egg stage.
Paralysis Assays
The CL4176 strain maintained at 16 °C was egg-synchronized onto 50 × 10 mm2 culture plates containing either vehicle or drug. Transgene expression was induced by up-shifting the temperature from 16 to 23 °C, starting on the 24th hour after egg-laying and lasting until the end of the paralysis assay. Paralysis was scored at 2-h intervals until the last worm became paralyzed.
Results
Effect of A3 Peptide on Aβ Aggregation Kinetics
Aβ peptide has several core amyloidogenic sequences, regarded as self-recognition regions, by which Aβ peptides interact with each other and can further assemble into β-sheet-rich fibrils [32, 33]. Several studies have shown that the onset of aggregation (nucleation) is accelerated by the addition of peptides with pro-aggregative activity [34–36]. In addition, some small molecular inhibitors have been shown to reduce Aβ aggregation by targeting the hydrophobic residues of Aβ sequences [15, 37].
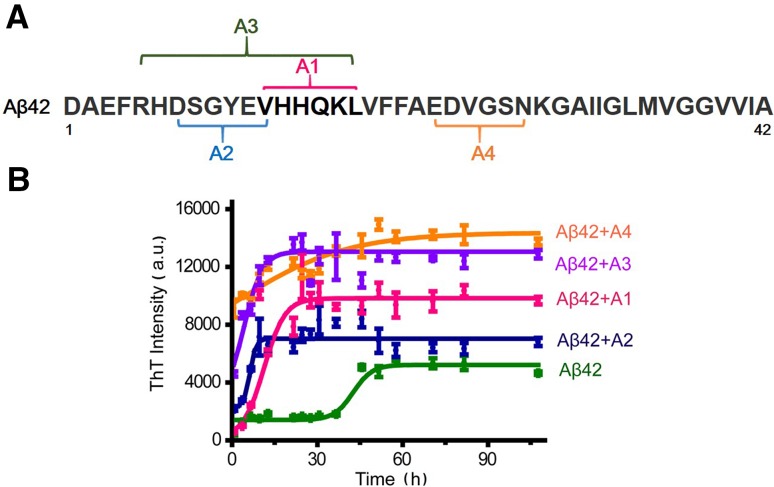
In the present study, we set out to develop peptide-based Aβ modulators, focusing on the core amyloidogenic sequences of the Aβ42 molecule. The main principle of our design was based on the amino-amino electrostatic forces, but we also considered the hydrophilic and hydrophobic interactions, as well as steric hindrance. We designed four peptides with the following sequences (Fig. 1A):
A1: VDDQEL
A2: KSGYKV
A3: DDKSGYKVDDQD
A4: RKVGSN
Fig. 1.
Schematic of the design of peptide modulators. A Specific Aβ sequences with specific targets of the peptide modulators indicated. B The peptide modulators altered Aβ42 aggregation. ThT fluorescence was monitored during incubation of 40 μmol/L Aβ42 in the absence or presence of 10 μmol/L peptide modulator. Error bars are standard deviations of the mean of three replicates.
It is believed that Aβ fibrils contain many more β-sheet motifs than Aβ oligomers [38]. Thus, an increase in β-sheets can be viewed as an indicator of oligomer-to-fibril conversion. The ThT fluorescence assay has been widely used to detect the β-sheet conformation in Aβ42 aggregates [39, 40]. The fluorescence intensity of ThT depends strictly on the presence of β-sheet structures in the system—the more β-sheets are formed, the higher the ThT fluorescence intensity. Newly-synthesized Aβ42 was incubated for 72 h in the absence or presence of each of the peptide modulators, and the aggregation process was monitored over time by ThT fluorescence.
In the absence of any peptide, the ThT fluorescence was 0 a.u. or slightly lower (data not shown). Aβ42 alone exhibited detectable fluorescence at time point 0, valued at ~1000 a.u. (Fig. 1B), and then increased to reach a maximum at 50 h (Fig. 1B). In the presence of any of the four peptide modulators, the ThT fluorescence not only reached much higher levels but also increased at a faster rate. Among the four modulators, A3 appeared to have the greatest effect on ThT fluorescence (Fig. 1B). At 0 h, the ThT intensity was already high (~4000 a.u.) and it only took 16 h to reach the peak. At the plateau, the intensity was almost three times that with Aβ alone (Aβ alone, 4500 a.u.; Aβ + A3, 13000 a.u.). Thus, the Aβ aggregation process was significantly accelerated by the A3 peptide. Interestingly, the ThT fluorescence of the Aβ42 + A3 solution had no lag time (Fig. 1B). It should be pointed out that the starting point was not really 0; the time interval from mixing the Aβ42 and A3 peptides to finishing the measurement was ~5 min. Moreover, the A3 peptide might act as a nucleating agent, leading to much faster nucleation of the A3–Aβ complexes.
Morphological Change of Aβ Aggregates in the Presence of A3 Peptide
TEM and AFM experiments were carried out to observe the morphological changes of Aβ intermediates incubated in the presence or absence of the A3 peptide (Fig. 2). TEM revealed that while Aβ42 peptide appeared as small granular particles without a clear form or shape at 6 h, it clearly formed a typical fibril structure after 3 days (72 h) in vitro, as reported previously [41] (Fig. 2A, D). The A3 peptide alone did not form any discernible structure under TEM (Fig. 2B, E). We expected that Aβ oligomers would be converted to long fibrils after co-incubation with the A3 peptide for 6 h and more so for 72 h. To our surprise, we found a time-dependent accumulation of smaller amorphous aggregates (Fig. 2C, F). Compared with Aβ alone, the amount of oligomer-like structures was significantly reduced and small amorphous aggregates were observed instead. AFM experiments provided a different way of looking at protein aggregates. Consistent with the TEM results, Aβ42 alone exhibited clustering structures indicative of Aβ oligomers. Co-incubation of Aβ with the A3 peptide revealed almost no Aβ oligomer-like structures, but a marked increase in small amorphous aggregates at 72 h (Fig. 2G–I). The EM and ThT experiments together support the notion that the A3 peptide interacts with Aβ at the early stage of aggregation and promotes Aβ aggregation into amorphous rather than fibrillary structures. The accelerated aggregation with faster nucleation and cross-linking effects gave rise to a less-ordered morphology, but their secondary structures were still mainly β-sheets.
Fig. 2.
A3 peptide facilitated Aβ aggregation into amorphous aggregates, as revealed by TEM and AFM. A–F Negatively-stained TEM images of Aβ, A3 peptide, and Aβ + A3 after 6 h and 3 days incubation in vitro. Scale bars, 100 nm. G–I AFM images of Aβ, A3 peptide, and Aβ + A3 after 3 days of incubation. Scale bars, 500 nm.
Reduced Cytotoxicity of Aβ Aggregates in Association with A3 Peptide
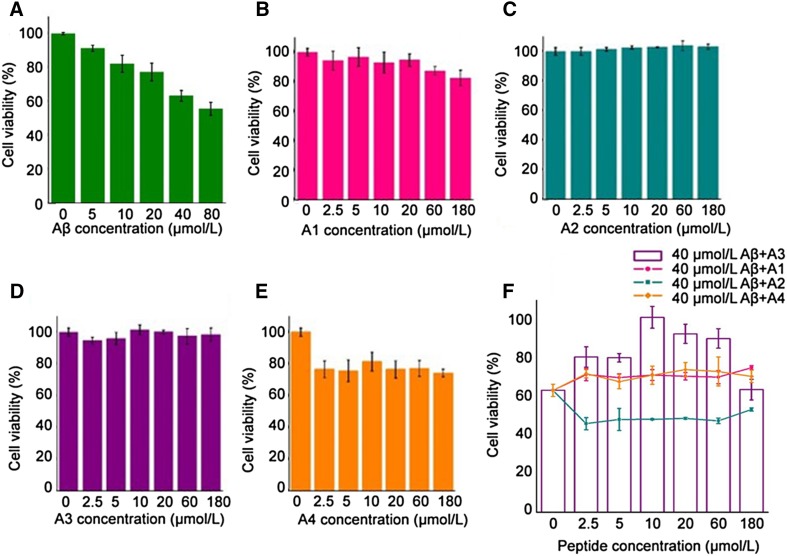
Next, we determined whether the A3 peptide counters Aβ toxicity by using SH-SY5Y cells. Application of Aβ aggregates to the cultured cells induced a dose-dependent decrease in viability (Fig. 3A). At 40 μmol/L, Aβ reduced the viability to nearly 60%. In contrast, exposure of SYHY-5Y cells to the A1, A2, or A3 peptide alone induced no change in viability (Fig. 3B–D). The A4 peptide appeared to have a small effect on cell survival even at 2.5 µmol/L, so it was not useful in cytotoxicity experiments (Fig. 3E).
Fig. 3.
The peptides affected Aβ aggregation-induced cytotoxicity in SHSY-5Y cells at different concentrations. SHSY-5Y cell viability was measured by MTT assay after incubation. A Aβ42 reduced the viability of SHSY-5Y cells in a concentration-dependent manner. B–E Cytotoxicity of designed peptides in SH-SY5Y cells. F Viability changed in the presence of designed peptides over a wide range of concentrations (2.5–180 μmol/L). The cell survival in PBS controls was taken to be 100%. Error bars correspond to standard deviations of six sets of experiments.
In addition, we found that the A3 peptide inhibited Aβ-induced cell death over a wide range of concentrations, from 2.5 to 60 μmol/L (Fig. 3F). At the highest concentration (180 μmol/L), cell viability dropped to the control level (60%). This “biphasic” viability curve might be due to a pH decrease in the medium, when too much A3 peptide (isoelectric point 3.85) was added. In contrast, none of the other peptides (A1, A2, and A4) countered the effect of Aβ on viability (Fig. 3F). Taken together, our experiments suggest that the A3 peptide attenuates Aβ-induced cytotoxicity, possibly by facilitating Aβ aggregation.
A3 Peptide Alleviates Aβ-Induced Paralysis in Transgenic C. elegans
We further investigated whether the A3 peptide specifically protects against Aβ-induced toxicity in vivo, in a transgenic C. elegans model of human AD, the CL4176 strain (smg-1ts [myo-3/Aβ1–42long 3’-untranslated region]). The CL4176 strain over-expresses human Aβ in muscle cells, leading to an Aβ-dependent paralysis phenotype (Fig. 4A). As a control, we used the CL802 strain that does not express Aβ. A3 peptide solution (50 mg/mL) was fed to CL4176 and CL802 animals. Aβ expression was induced by a temperature upshift from 16 to 23 °C at 24 h [22]. Paralysis did not occur in the CL802 control (Fig. 4B). The CL4176 line fed with the A3 peptide showed a notable delay of paralysis compared with those without A3 (Fig. 4B). For quantitative analysis, we defined PT50 as the time interval at which 50% of the worms were paralyzed. Treatment with the A3 peptide caused an approximately 5 h delay of PT50, compared with the untreated CL4176 as the control group (P < 0.03; n = 9 assays; 100 worms in each assay group). Moreover, the peptide feeding was found to delay paralysis at different concentrations (data not shown). These results suggest that the A3 peptide is capable of attenuating the behavioral phenotype induced by Aβ.
Fig. 4.
The A3 peptide delayed the rate of paralysis in the muscle Aβ strain CL4176. A Normal C. elegans can move freely (above). C. elegans with transgene expression paralysis cannot move or only move the head (below). B Time course of paralysis in CL4176 worms fed with the peptide (50 mg/mL). Paralysis was scored at 2-h intervals. Data from three experiments with 100 worms in each group. Plots are representative of three independent experiments.
Discussion
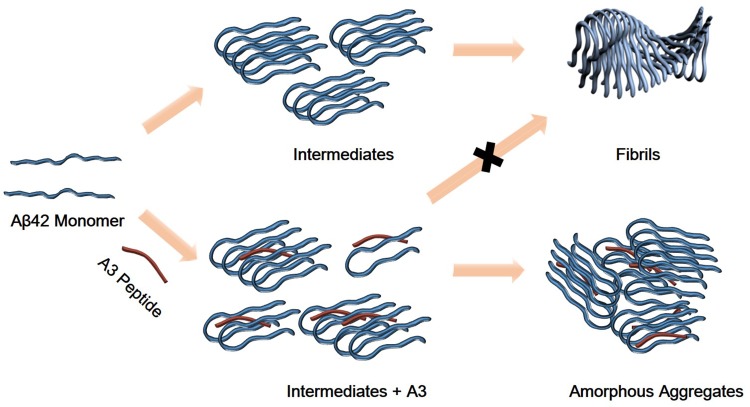
The present study aimed at identifying molecular species that interfere with the Aβ aggregation process. Our results showed that the A3 peptide accelerated the conversion of Aβ oligomers into β-sheet-rich amorphous aggregates, rather than Aβ fibrils. A cartoon illustrating how the A3 peptide modulates Aβ aggregation is shown in Fig. 5. In parallel, the A3 peptide also led to a reduction in the Aβ-induced toxicity in cultured SH-SY5Y cells and muscle paralysis in C. elegans in vivo. Taken together, our results reveal a new approach to attenuating Aβ-induced cell death, and raise the possibility of a new strategy for Aβ-related disease, including AD therapy.
Fig. 5.
Cartoon of Aβ42 aggregation with or without A3 peptide. Aβ42 monomers assemble into cross-β structure oligomeric intermediates and finally into amyloid fibrils (above). In the presence of A3 peptide, Aβ42 aggregation is accelerated into amorphous aggregates (below).
The ThT fluorescence assay was used to quantify the amount of β-sheet structure, and therefore indirectly the aggregation kinetics of Aβ intermediates. In prior experiments, we had measured the ThT fluorescence of the A3 peptide alone, and none was detectable (data not shown). Thus, the ThT fluorescence in the mixture was derived from the β-sheet structures of Aβ alone, or the A3 peptide-Aβ complex. A faster increase and higher intensity of ThT fluorescence was measured in the Aβ + A3 peptide mixture than with Aβ alone. However, at the starting point of the Aβ + A3 peptide curve, the ThT fluorescence was already quite high (~4000 a.u.), and there was no lag time (Fig. 1B). Two factors might contribute to the higher fluorescence at the start. First, the starting point was not really “0” time—the interval from mixing to finishing the measurement was ~5 min. Second, the A3 peptide might act as a nucleating agent, leading to much faster nucleation of A3 peptide-Aβ complexes.
It is generally believed that the Aβ42 peptide does not exist as a monomer in the brain. Instead, it aggregates into more toxic Aβ oligomers, which in turn can be converted into larger and insoluble Aβ fibrils [41] (Fig. 5). TEM and AFM images at different time points revealed that, in the presence of the A3 peptide, Aβ oligomers form amorphous structures rather than fibril structures (Fig. 2). It is unclear how the A3 peptide directs Aβ to form amorphous aggregates rather than fibrils. We speculate that the nucleation and crosslinking capacity of the A3 peptide might accelerate Aβ aggregation, giving rise to a less-ordered amorphous morphology. Interestingly, the main secondary structures of amorphous aggregates remained largely β-sheets. It is possible that the A3 peptide interacts with the β-sheets of Aβ molecules by electrostatic attraction, Van der Waals attraction, and hydrophobic force, which would neutralize the electrostatic repulsion to prevent the self-assembly of Aβ oligomers.
The cytoprotective effect of the A3 peptide against Aβ is quite different from the previously reported inhibitors, which prevent neuronal death by inhibiting Aβ aggregation [42]. Our results support the notion that the A3 peptide reduced the toxicity of Aβ aggregation in SH-SY5Y cells and C. elegans by accelerating the conversion of Aβ oligomers to amorphous aggregates, which are less toxic than Aβ oligomers or even fibrils. However, the A3 peptide had its protective effects at low concentrations (up to 10 μmol/L), but not a high concentration (180 μmol/L). Since the A3 peptide alone had little cytotoxicity, the decrease in cell viability was most likely due to changes induced by the A3 peptide-Aβ complex. At a high concentration (180 μmol/L), the A3 peptide (with an isoelectric point of 3.85) may change the pH of the culture medium, affecting cell viability. Moreover, Aβ42 (with an isoelectric point of 5.43) has polar chains with a potential for negative and positive charges at neutral pH. A large increase in the A3 peptide-to-Aβ ratio (180:40 μmol/L) may alter their charge interaction, making it difficult to form the less toxic amorphous structures.
Our studies indicate that the A3 peptide has the ability to significantly accelerate Aβ aggregation, leading to changes in the morphology of Aβ aggregates. Instead of fibrils, amorphous structures, which contained more β-sheets, were observed in the mature aggregates. Furthermore, the A3 peptide protected cells from Aβ-associated toxicity in vitro and alleviated Aβ-induced paralysis of transgenic C. elegans in vivo. The cytoprotective effect of the A3 peptide is quite different from that of inhibitors, which protect neurons by inhibiting Aβ aggregation. This aggregation-promotion effect may benefit the design of anti-amyloid therapy and ultimately benefit the treatment of amyloid diseases.
Acknowledgements
We gratefully acknowledge Prof. Bai Lu of Tsinghua University for his help and thoughtful suggestions on the manuscript. This work was supported by the National Natural Science Foundation of China (91127043, 31600803, and 21273051).
References
- 1.Selkoe Dennis J. Physiological production of the β-amyloid protein and the mechanism of Alzheimer’s disease. Trends Neurosci. 1993;16:403–409. doi: 10.1016/0166-2236(93)90008-A. [DOI] [PubMed] [Google Scholar]
- 2.Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: The role of peptide assembly state. J Neurosci . 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer’s disease–genotypes, phenotype, and treatments. Science. 1997;275:322–330. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 4.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 5.Drouet B, Pinçon-Raymond M, Chambaz J, Pillot T. Molecular basis of Alzheimer’s disease. Cell Mol Life Sci. 2000;57:705–715. doi: 10.1007/s000180050035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 7.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 8.Morgado I, Fändrich M. Assembly of Alzheimer’s Aβ peptide into nanostructured amyloid fibrils. CurrOpin Colloid Interface Sci. 2011;16:508–514. doi: 10.1016/j.cocis.2011.06.016. [DOI] [Google Scholar]
- 9.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Klein WL, et al. Diffusible, nonfibrillar ligands derived from A1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallitto MM, Ghanta J, Heinzelman P, Kiessling LL, Murphy RM. Recognition sequence design for peptidyl modulators of beta-amyloid aggregation and toxicity. Biochemistry. 1999;38:3570–3578. doi: 10.1021/bi982119e. [DOI] [PubMed] [Google Scholar]
- 11.Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Howlett DR, et al. Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of beta-amyloid peptide. Biochem J. 2000;348:137–144. doi: 10.1042/bj3480137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer’s disease and other neurodegenerative disorders: The emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 13.Lu B, Nagappan G, Guan X, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 14.Klajnert B, Wasiak T, Ionov M, Fernandez-Villamarin M, Sousa-Herves A, Correa J, et al. Dendrimers reduce toxicity of Aβ 1-28 peptide during aggregation and accelerate fibril formation. Nanomedicine. 2012;8:1372. doi: 10.1016/j.nano.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Zhu LJ, Song Y, Cheng PN, Jeffrey SM. Molecular design for dual modulation effect of amyloid protein aggregation. J Am Chem Soc. 2015;137:8062–8068. doi: 10.1021/jacs.5b01651. [DOI] [PubMed] [Google Scholar]
- 16.Fändrich M, Fletcher MA, Dobson CM. Amyloid fibrils from muscle myoglobin. Nature. 2001;410:165–166. doi: 10.1038/35065514. [DOI] [PubMed] [Google Scholar]
- 17.Kowalewski T, Holtzman DM. In situ atomic force microscopy study of Alzheimer’s β-amyloid peptide on different substrates: New insights into mechanism of β-sheet formation. Proc Natl Acad Sci U S A. 1999;96:3688–3693. doi: 10.1073/pnas.96.7.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X, Wang Y, Liu L, Niu L, Yang YL, Wang W. Molecular-level evidence of the surface-induced transformation of peptide structures revealed by scanning tunneling microscopy. Langmuir. 2009;25:8849–8853. doi: 10.1021/la901342r. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzo A, Yankner BA. β-amyloid neurotoxicity requires fibril formation and is inhibited by Congo Red. Proc Natl Acad Sci U S A. 1995;91:12243–12247. doi: 10.1073/pnas.91.25.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woods LA, Platt GW, Hellewell AL, Hewitt EW, Homans SW, Ashcroft AE, et al. Ligand binding to distinct states diverts aggregation of an amyloid-forming protein. Nat Chem Biol. 2011;7:730–739. doi: 10.1038/nchembio.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geng J, Li M, Ren JS, Wang EB, Qu XG. Polyoxometalates as inhibitors of the aggregation of amyloid β peptides associated with Alzheimer’s disease. Angew Chem Int Ed Engl. 2011;50:4184–4188. doi: 10.1002/anie.201007067. [DOI] [PubMed] [Google Scholar]
- 22.Alavez S, Vantipalli MC, Zucker DJS, Klang IM, Lithgow GJ. Amyloid-binding compounds maintain protein homeostasis during ageing and extend lifespan. Nature. 2011;472:226–229. doi: 10.1038/nature09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fändrich M. Oligomeric intermediates in amyloid formation: Structure determination and mechanisms of toxicity. J Mol Biol. 2012;421:427–440. doi: 10.1016/j.jmb.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Morgado I, Fändrich M. Assembly of Alzheimer’s Aβ peptide into nanostructured amyloid fibrils. Curr Opin Colloid Interface Sci. 2011;16:508–514. doi: 10.1016/j.cocis.2011.06.016. [DOI] [Google Scholar]
- 25.Choi JS, Braymer JJ, Nanga RPR, Ramamoorthy A, Lim MH. Design of small molecules that target metal-A species and regulate metal-induced A aggregation and neurotoxicity. Proc Natl Acad Sci U S A. 2010;107:21990–21995. doi: 10.1073/pnas.1006091107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoo SI, Yang M, Brender JR, Subramanian V, Sun K, Joo NE. Innentitelbild: inhibition of amyloid peptide fibrillation by inorganic nanoparticles: functional similarities with proteins (Angew. Chem. 22/2011) Angew Chem. 2011;123:5096. doi: 10.1002/ange.201102689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klajnert B, Wasiak T, Ionov M, Fernandez-Villamarin M, Sousa-Herves A, Correa J, et al. Dendrimers reduce toxicity of Aβ 1-28 peptide during aggregation and accelerate fibril formation. Nanomedicine. 2012;8:1372–1378. doi: 10.1016/j.nano.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Moran PM, Higgins LS, Cordell B. Age-related learning deficits in transgenic mice expressing the 751-amino acid isoform of human beta-amyloid precursor protein. Proc Natl Acad Sci U S A. 1995;92:5341–5345. doi: 10.1073/pnas.92.12.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno M, Chang L, Tseng W. Temporal memory deficits in Alzheimer’s mouse models: rescue by genetic deletion of BACE1. Eur J Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- 30.Link CD. Expression of human beta-amyloid peptide in transgenic Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1995;92:9368–9372. doi: 10.1073/pnas.92.20.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Link CD, Taft A, Kapulkin V, Duke K, Kim S, Fei Q, et al. Gene expression analysis in a transgenic Caenorhabditis elegans. Alzheimer’s disease model. Neurobiol Aging. 2003;24:397–413. doi: 10.1016/S0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 32.Cheng B, Gong H, Xiao H, Petersen RB, Zheng L, Huang K, et al. Inhibiting toxic aggregation of amyloidogenic proteins: a therapeutic strategy for protein misfolding diseases. Biochim Biophys Acta. 2013;1830:4860–4871. doi: 10.1016/j.bbagen.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 33.Pike CJ, Walencewiczwasserman AJ, Kosmoski J, Cribbs DH, Glabe CG, Cotman CW, et al. Structure-activity analyses of beta-amyloid peptides: contributions of the beta 25-35 region to aggregation and neurotoxicity. J Neurochem. 1995;64:253–265. doi: 10.1046/j.1471-4159.1995.64010253.x. [DOI] [PubMed] [Google Scholar]
- 34.Scrocchi LA, Ha K, Chen Y, Wu L, Wang F, Fraser PE. Identification of minimal peptide sequences in the (8-20) domain of human islet amyloid polypeptide involved in fibrillogenesis. J Struct Biol. 2003;141:218–227. doi: 10.1016/S1047-8477(02)00630-5. [DOI] [PubMed] [Google Scholar]
- 35.Du HN, Li HT, Zhang F, Lin XJ, Shi JH, Shi YH, et al. Acceleration of alpha-synuclein aggregation by homologous peptides. FEBS Lett. 2006;580:3657–3664. doi: 10.1016/j.febslet.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 36.Kim JR, Murphy RM. Mechanism of accelerated assembly of beta-amyloid filaments into fibrils by KLVFFK(6) Biophys J. 2004;86:3194–3203. doi: 10.1016/S0006-3495(04)74367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Re F, Airoldi C, Zona C, Masserini M, La FB, Quattrocchi N, et al. Beta amyloid aggregation inhibitors: small molecules as candidate drugs for therapy of Alzheimer’s disease. Curr Med Chem. 2010;17:2990–3006. doi: 10.2174/092986710791959729. [DOI] [PubMed] [Google Scholar]
- 38.Matthew B, Shohei K. Molecular mechanism of thioflavin-T binding to amyloid fibrils. Biochim Biophys Acta. 2010;1804:1405–1412. doi: 10.1016/j.bbapap.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krebs MR, Bromley EH, Donald AM. The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struct Biol. 2005;149:30–37. doi: 10.1016/j.jsb.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Wu Y, Wu Z, Butko P, Christen Y, Lambert MP, Klein WL, et al. Amyloid-beta-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans. J Neurosci. 2006;26:13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bharadwaj PR, Dubey AK, Masters CL, Martins RN, Macreadie IG. Aβ aggregation and possible implications in Alzheimer’s disease pathogenesis. J Cell Mol Med. 2009;13:412–421. doi: 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bieschke J, Lindquist S. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc Natl Acad Sci U S A. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]