Abstract
The thalamus and central dopamine signaling have been shown to play important roles in high-level cognitive processes including impulsivity. However, little is known about the role of dopamine receptors in the thalamus in decisional impulsivity. In the present study, rats were tested using a delay discounting task and divided into three groups: high impulsivity (HI), medium impulsivity (MI), and low impulsivity (LI). Subsequent in vivo voxel-based magnetic resonance imaging revealed that the HI rats displayed a markedly reduced density of gray matter in the lateral thalamus compared with the LI rats. In the MI rats, the dopamine D1 receptor antagonist SCH23390 or the D2 receptor antagonist eticlopride was microinjected into the lateral thalamus. SCH23390 significantly decreased their choice of a large, delayed reward and increased their omission of lever presses. In contrast, eticlopride increased the choice of a large, delayed reward but had no effect on the omissions. Together, our results indicate that the lateral thalamus is involved in decisional impulsivity, and dopamine D1 and D2 receptors in the lateral thalamus have distinct effects on decisional impulsive behaviors in rats. These results provide a new insight into the dopamine signaling in the lateral thalamus in decisional impulsivity.
Keywords: Lateral thalamus, Decisional impulsivity, D1 receptor, D2 receptor
Introduction
Impulsivity is simply defined as the tendency to act prematurely without foresight [1], and is generally separated into: (1) decisional impulsivity, defined as “actions initiated without due deliberation of other possible options or outcome behaviors”; and (2) motor impulsivity, defined as behaviors that are “premature, mistimed, difficult to control and suppress” [2]. Many diseases are closely associated with impulsivity, such as attention deficit hyperactivity disorder, substance abuse disorder, Parkinson’s disease, conduct disorder, and personality disorders [3–7]. Here, we focused on the decisional impulsivity for large rewards in a delay discounting task [8, 9], which has been validated in humans and animals.
Traditionally, the thalamus is considered as a “relay” for highly-processed sensory-motor information that is conveyed to the cortices [10, 11] and the information through integration in the cortex is progressively processed at striatal, pallidal/nigral, and thalamic levels [12]. However, in recent years, the thalamus has been repeatedly implicated in high-level cognitive functions such as decision making, attention, learning, and memory, beyond a mere role in relaying sensory-motor information to cortical domains [13–20]. Several studies have demonstrated that thalamic activity is closely associated with motor impulsivity in humans [21, 22], however, the subregions of the thalamus underlying decisional impulsivity remain poorly understood.
In addition, dopaminergic receptors have been reported to be involved in impulsivity. Systemic administration or local brain infusions of the D1 receptor antagonist SCH23390 result in increased impulsivity in rats [23–26], while D1 receptor stimulation in the medial prefrontal cortex or nucleus accumbens (NAc) promotes the choice of delayed large rewards or premature ‘impulsive’ responding [25, 27]. Moreover, infusions of the D2/3 receptor antagonist nafadotride into the NAc core (NAcC) decrease impulsivity in rats with high impulsivity (HI) [28]. Clearly, these results suggest that different dopamine receptors in different brain regions have different effects on impulsive behavior. However, there is limited research on the pharmacological modulation of dopaminergic receptors in the thalamus. Actually, dopaminergic inputs have been reported in the thalamus, and thalamic neurons expressing dopamine receptors in humans, primates, and rats [29–37]. An intermediate level of D1 receptors is distributed in the lateral thalamus and the distribution and density of thalamic D2 receptor are similar to that of other brain areas in rats [30, 31, 37].
Here, we used in vivo voxel-based magnetic resonance imaging (MRI) to investigate structural differences in the thalamus of HI and low impulsivity (LI) rats. Then, medium impulsivity (MI) rats were bilaterally infused with the D1 receptor antagonist SCH23390 or the D2 receptor antagonist eticlopride into the region regarded as a key part of the thalamus in decisional impulsivity, in order to investigate the role of thalamic dopaminergic modulation in decisional impulsivity in rats.
Materials and Methods
Animals
Forty-three male Sprague-Dawley rats (Animal Center of Peking University, Beijing, China) weighing 250–280 g at the start of the experiments were habituated for 1 week before training. Food was available ad libitum and each rat was housed individually under a 12:12 h light-dark cycle (lights off at 06:30) at a controlled room temperature (22 ± 3 °C) and a relative humidity of 60 ± 15%. All experiments were approved by the Animal Care Committee of Peking University (protocol No. LA2010-053), and met the requirements of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Delay Discounting Task
Apparatus
Rats were trained in operant chambers (40 cm × 20 cm × 40 cm) equipped with a 6 W house light above the chamber and two retractable levers (Med Associates, St. Albans, VT), each 4 cm wide, 5 cm from the Plexiglas floor and 10 cm apart, and equally distant from the side wall. A metal water-containing dish 2 cm above the floor was located in the center between the two levers. Water reward was delivered by a pump (40 μL per drop). A computer programmed the components of the chamber and collected the behavioral data (Magnet, USA).
Procedures
The delay discounting task used in our study was modified from the experiment model used by Yanfang Zuo and Yijing Li [8, 9]. Rats were trained once per day, considered to be a session. They were trained to learn the delay discounting task according to a few steps, until a stable behavioral performance was achieved. Behavioral data from the last three consecutive sessions were analyzed by repeated measures ANOVA. If the main effect of ‘session’ was not significant but the effect of ‘delay’ was significant at the P < 0.05 level, rats were considered to have reached a stable baseline of performance.
Each session lasted ~45 min and was divided into 6 blocks. Each block consisted of 12 trials. In the first two trials (forced-choice trial), only one lever was extended (either the right or the left, counterbalanced in the group) after the house light came on, and the rat had to press a lever to obtain a corresponding reward. The other 10 free-choice trials began with the house light switching on, and the two levers were extended 2 s later. A response on one lever (right or left) resulted in a small, immediate reward (one drop of water) while a response on the other lever resulted in a large, delayed reward (five drops of water) after different delays across blocks (right or left, counterbalanced in the group). Then the levers were retracted and the rats waited for the corresponding reward delivery. The house light was still on during the delay period and turned off after the corresponding reward was delivered. The trial ended and entered into the inter-trial interval (ITI). If the rat did not respond during the free-choice within 10 s, the trial directly entered into the ITI and was recorded as an omission. The levers associated with the large and small reward were the same for each rat during training. The small reward remained at a 2 s delay within all blocks and the delay to the large reward increased from 2 to 4, 8, 12, 16, and 20 s across blocks. The ITI was [40 – response latency + delay duration)] seconds. The rats were allowed to drink water freely for 15 min after training, but they did not have access to water during the rest of the day. Each rat obtained 40–50 mL water during the training and free water times per day, which was enough for their daily needs. The rats were trained for 5–6 days per week.
Morphological Assessment by MRI
MRI scanning was carried out in HI and LI rats after stable behavioral performance was achieved (n = 8/group). Rats were anesthetized with 10% chloral hydrate and transferred to the MRI suite. Two-dimensional T2-weighted fast spin-echo images were acquired in three orthogonal planes to investigate the structure of the thalamus in the HI and LI groups. The scanning used a 7T Bruker Bioclinscan system with parameters of repetition time, 10500 ms; echo time, 41 ms; echo train length, 7; matrix size, 256 × 256; field of view, 36 × 36 mm; slice thickness, 0.5 mm (no slice gap); number of excitations/averages: 2. The heart and breathing rate were closely monitored throughout the MRI scans. Scanning lasted ~10 min for each rat; they were sacrificed after scanning.
Surgery
Twenty-seven MI rats were implanted with bilateral cannulas. Each rat was anesthetized with 40 mg/kg sodium pentobarbital (i.p.) and the head fixed to a stereotaxic apparatus with the incisor set at −3.3 for a flat skull position. Bilateral stainless-steel guide cannulas (OD 0.64 mm) were implanted into the lateral thalamus according to the following stereotaxic coordinates: anteroposterior −2.4 mm (from bregma), mediolateral ±3 mm, and dorsoventral −5 mm from dura. The cannulas were secured to the skull using three bone screws and dental cement. The tips of the guide cannulas were directed to 1.5 mm above the intended sites of injection. Internal stylets were replaced with dummy stylets, which were 0.5 mm longer than the guide cannulas, to keep the cannulas patent and to prevent infection. After surgery, rats were housed individually and rested for 1 week. Following the recovery period, they were re-trained in the delay discounting task.
Drugs
All drug doses were calculated as the salt and dissolved in 0.9% sterile saline. SCH23390 hydrochloride and eticlopride hydrochloride were from Sigma-Aldrich (St. Louis, MO). The dose ranges for all drugs were based on published reports [25, 27, 38, 39].
Microinfusion Procedure
The infusion procedure was adapted from Winstanley et al. [38]. Following re-establishment of stable post-operative performance over 3 sessions, rats were habituated to the infusion procedure with one mock infusion. Infusions were given on a 4-day cycle, starting with a 2-day baseline session for grouping rats, and on the following day the rats received a drug or vehicle infusion prior to testing on the delay discounting task, ending with another session to test the elimination of drug effect.
The rats moved freely while the internal cannula was removed and an injector was inserted with its tip extending 1.5 mm beyond the guide cannula. A 0.5 μL solution was then infused for 2 min. The injector was left in place for an additional 2 min to allow the drug to diffuse in the vicinity of the injector tip. Then the injector was removed and the internal stylet replaced. The rats were put into the operant chambers and the delay discounting task started 5 min later.
Histology
Rats were sacrificed to check the cannula locations after completion of the behavioral experiments. Rats were perfused with 0.9% saline (200 mL) followed by 4% formalin (300 mL), then the brain was post-fixed in 4% formalin overnight and transferred into 20% and 30% sucrose (in 0.01 mol/L PBS) until sectioning. Coronal sections (30 μm) were cut on a freezing microtome and stained with cresyl violet to visualize the location of the guide cannulas and injection sites.
Data Analysis
MRI Data Processing
Voxel-based morphology (VBM) analysis, based on an in-house toolbox, was used to reveal differences in the thalamus between the HI and LI rats. First, MRI images were corrected for intensity non-homogeneity by N3 [40]. Then, the brain images were extracted from the corrected images by skull stripping. Afterwards, these extracted images were segmented into gray matter (GM), white matter, and cerebrospinal fluid using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk) [41]. Moreover, the GM of each rat in the native space was normalized to the standard rat brain atlas [42, 43]. After normalization, the resulting images were smoothed with a Gaussian filter of 800 μm isotropic Gaussian kernel. Next, we conducted voxel-by-voxel comparisons of GM voxels between HI and LI rats using a two tailed two-sample t-test. A voxel level threshold was set at P < 0.001 and clusters >10. To describe the relationship between relative GM density in the bilateral lateral thalamus and decisional impulsivity, the non-linear exponential function logk = (maxlogk − Plateau) * exp (-A * GM) + Plateau was chosen to fit the data, where Plateau represents the maximum value of logk and A represents the rate constant (calculated in Prism 5.0; GraphPad Software, San Diego, CA).
Behavioral Data Processing
In order to estimate the level of decisional impulsivity, the k value of the hyperbolic discounting V = V0/(1+kD) was calculated, where V is the percentage of large reward after a delay of D in seconds, V0 is the percentage of large reward at D = 2 s (when the delay of small and large reward is the same) and the free parameter k describes how rapidly V declines with increasing delay. The calculated k values are shown as logk, which represents the level of decisional impulsivity. The discount factor, k, was used to characterize each rat as LI, MI, or HI.
All analyses used SPSS (ver. 20, IBM Corp., Armonk, NY). As described in detail in previous reports [8, 44], the following variables were analyzed: percentage of large reward, operating rate of all free trials, and number of omissions. Data are presented as mean ± SEM. Behavioral data were analyzed using single dependent variable factor analysis of variance (ANOVA) with group and delay (three groups, six delays) as factors. Results of all drug studies were analyzed using single dependent variable factor ANOVA with dose and delay (vehicle plus 2 doses, six delays) as factors. The LSD post hoc test was used for pairwise comparisons. The operating rate and the number of omissions were analyzed using one-way ANOVA with dose as a between-group factor and the LSD post hoc test was applied. Graphs were produced using GraphPad Prism 5.0. Statistical significance was set at P < 0.05. Only the significant effects that were critical for data interpretation are reported. During the analysis, the homogeneity of variance across groups was determined using Tamhanes’ T2.
Results
Gray Matter Density of the Lateral Thalamus is Lower in HI Rats
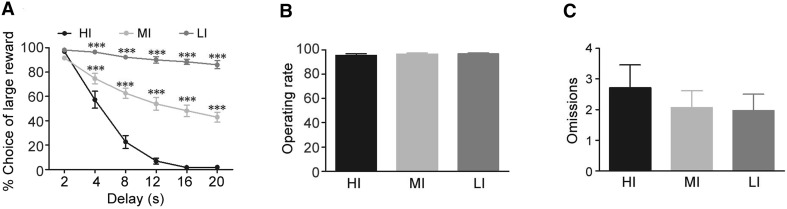
After all rats underwent 42 days of training in the delay discounting task, they were divided into HI, MI, and LI groups based on the logk values of the stable behavioral performance in the last three sessions (Fig. 1A). The top 20% (mean logk value, −0.774; n = 8) were selected as the HI group, the bottom 20% (mean logk value, −1.97; n = 8) as the LI group, and the rest (mean logk value, −1.19; n = 27) as the MI group. With an increased delay of a large reward, the HI rats showed more preference for the small immediate reward than LI and MI rats during the baseline task (group F(2, 246) = 18.10, P < 0.001; delay F(5, 246) = 131.64, P < 0.01; group × delay F(10, 246) = 7.24, P < 0.01), especially at delays of 4, 8, 12, 16, and 20 s (P < 0.001) (Fig. 1A). However, the operating rate and omissions of lever presses throughout all free trials did not differ among the three groups (group F(2, 40) = 0.23, P = 0.80) (Fig. 1B, C). These results suggest that HI, LI, and MI rats are significantly different in the level of impulsivity, but not the level of basic movement.
Fig. 1.
Baseline task performance of HI, MI, and LI rats in the delay discounting task. A Percentage choice of a large reward by HI rats was lower than that of LI and MI rats as the delay of a large reward increased (group: P < 0.001; delay: P < 0.01; interaction: P < 0.01). The operating rate (B) and omissions (C) throughout all free trials did not significantly differ among the three groups (F = 0.23, P = 0.80). HI and LI rats: n = 8/group; MI rats: n = 27, ***P < 0.001 compared to HI group.
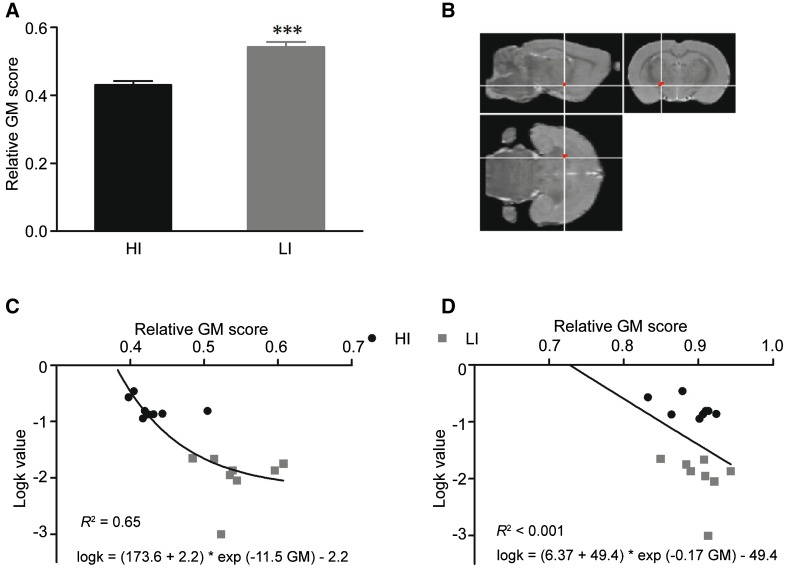
Subsequently, in vivo voxel-based MRI was used to assess structural differences between HI and LI rats, and showed that GM differed between HI and LI rats only in the lateral thalamus and cerebellum. The results showed a bilateral reduction of the relative GM density in the lateral thalamus in the HI rats compared with the LI rats (Fig. 2A, P < 0.001), and the specific difference in the lateral thalamus between HI and LI rats was superposed unilaterally on an averaged MRI template using VBM analysis of orthogonal coronal, sagittal, and horizontal sections (Fig. 2B). The relative GM density of the bilateral lateral thalamus was moderately associated with the logk values, which represent the degree of impulsivity (Fig. 2C, R 2 = 0.65) (non-linear fit of the exponential function, logk = (173.6 + 2.2) * exp (−11.5GM) – 2.2). However, no relationship between the relative GM density of the bilateral medial thalamus and the logk values was found (Fig. 2D, R 2 < 0.001) (non-linear fit of the exponential function, logk = (6.4 + 49.4) * exp (−0.17GM) – 49.4). These results implied that more impulsive rats have a decreased GM density in the lateral thalamus, but not the medial thalamus, so subsequent experiments on dopamine receptors in MI rats focused on the lateral thalamus.
Fig. 2.
HI rats showed a reduced density of gray matter (GM) in the lateral thalamus. A The relative GM score of HI rats was lower than that of LI rats (***P < 0.001). B VBM analysis of orthogonal coronal, sagittal, and horizontal sections superposed unilaterally on an averaged MRI template. C Moderate positive correlation between logk values and GM scores of the most significant voxels in the lateral thalamus (R 2 = 0.65). D No significant relationship of the logk values and GM scores of the most significant voxels in the medial thalamus. HI and LI rats: n = 8/group.
Effects of Intra-lateral Thalamic Infusions of SCH23390 or Eticlopride on Decisional Impulsivity in MI Rats
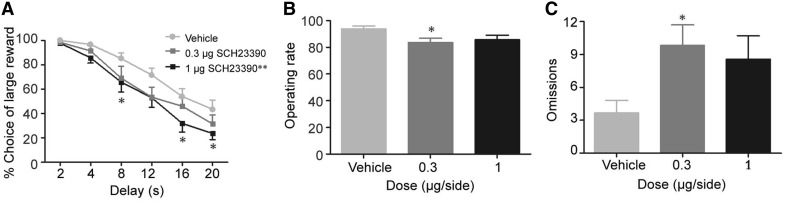
Bilateral infusions of the dopamine D1 receptor antagonist SCH23390 into the lateral thalamus in MI rats increased their preference for the small-immediate reward (dose: F(2, 70) = 10.49, P < 0.001; delay: F(2, 165) = 57.04, P < 0.001); dose × delay: F(10, 350) = 0.57, P = 0.84) (Fig. 3A). Post hoc comparisons revealed that SCH23390 at 1 μg/side shifted the preference from the large-delayed reward towards the small-immediate reward compared to vehicle (P < 0.01), but 0.3 μg/side did not (P = 0.13). Compared with vehicle, an effect of 1 μg/side SCH23390 was found at delays of 8, 16, and 20 s (P < 0.05). Moreover, the movement of rats was influenced by SCH23390 (Fig. 3B, C; dose: F(2, 33) = 3.60, P < 0.05). Further comparisons revealed that only 0.3 μg/side SCH23390 decreased the operating rate and increased the omissions compared to vehicle infusion (P < 0.05).
Fig. 3.
Effects of dopamine D1 receptor blockade in the lateral thalamus on performance of MI rats. A The percentage choice of a large reward declined with 0.3 or 1 µg/side SCH23390 (dose: P < 0.001; delay: P < 0.001; interaction: P = 0.84). Infusions at 1 µg/side shifted the preference towards the small-immediate reward at delays of 8, 16, and 20 s (P < 0.05). B, C SCH23390 at 0.3 µg/side decreased the operating rate (B; P < 0.05) and increased the omissions of lever presses (C; P < 0.05) during all free-choice trials. n = 12/SCH23390 group. *P < 0.05 compared to vehicle.
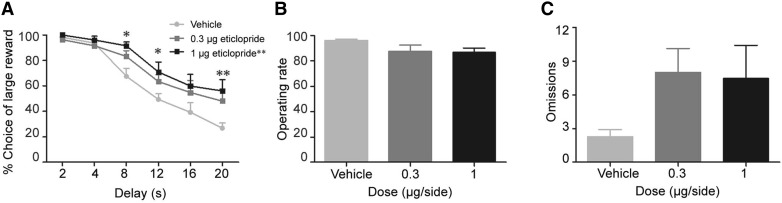
Bilateral infusions of the dopamine D2 receptor antagonist eticlopride into the lateral thalamus in MI rats increased the preference for a large-delayed reward (dose: F(2, 64) = 11.97, P < 0.001; delay: F(5, 160) = 43.81, P < 0.001); dose × delay: F(10, 320) = 1.12, P = 0.35) (Fig. 4A). Further post hoc comparisons revealed that 1 μg/side but not 0.3 μg/side shifted the preference from the small-immediate reward towards the large-delayed reward compared to vehicle group (P < 0.01). Compared with vehicle, a significant effect of 1 μg/side eticlopride was found at delays of 8 s (P < 0.05), 12 s (P < 0.05), and 20 s (P < 0.01). Unlike SCH23390, eticlopride had no effect on the operating rate or the omissions during all free-choice trials (dose: F(2, 33) = 2.20, P = 0.13) (Fig. 4B, C). These results suggested that antagonism of D1 and D2 receptors in the lateral thalamus of MI rats has distinct effects on decisional impulsivity.
Fig. 4.
Effects of dopamine D2 receptor blockade in the lateral thalamus on MI rats. A The percentage choice of a large reward increased with SCH23390 at 0.3 and 1 µg/side (dose: P < 0.001; delay: P < 0.001; interaction: P = 0.35). Infusions at 1 µg/side significantly shifted the preference towards the large-delayed reward at delays of 8, 12, and 20 s (P < 0.05, P < 0.05, P < 0.01). B, C Infusions at 0.3 or 1 µg/side had no effect on operating rate (B; P = 0.13) and omissions (C; P = 0.13) during all free-choice trials. n = 11/eticlopride group, *P < 0.05 compared to vehicle.
Histological Verification of Cannula Placement
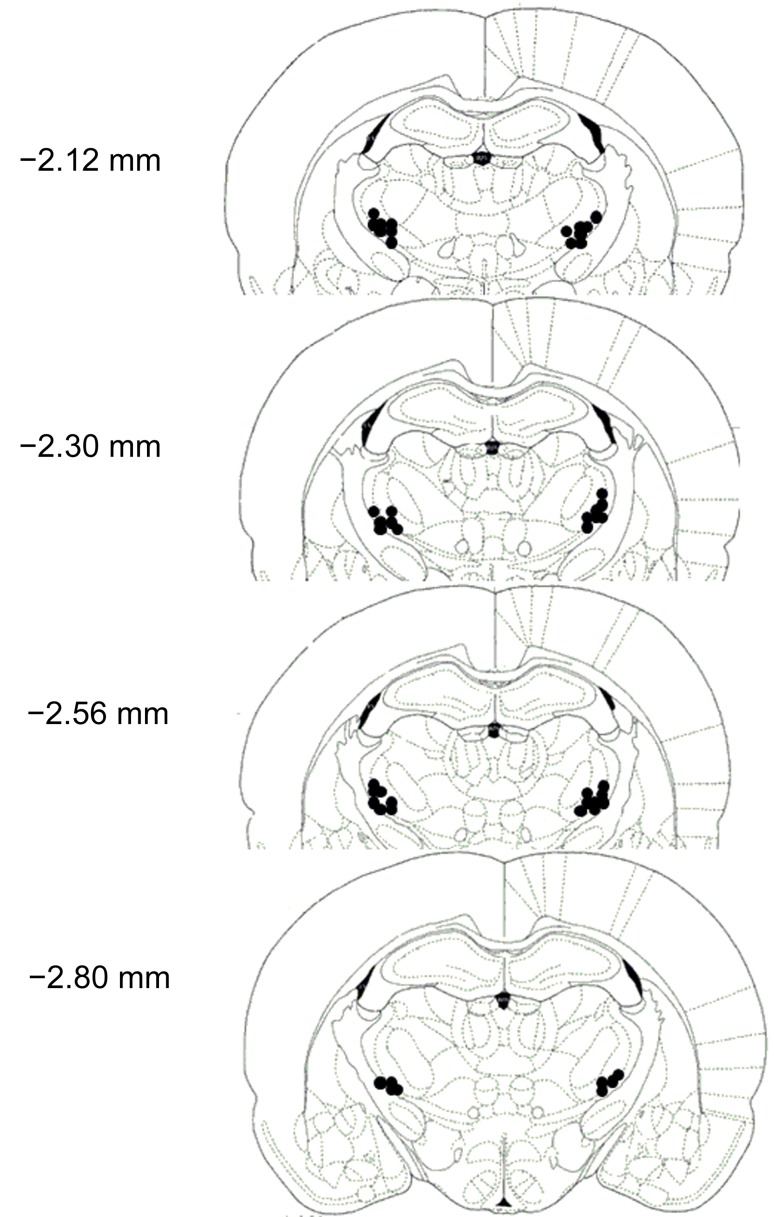
The correct placement of cannulas in the lateral thalamus was checked for all rats after completion of the experiments. All injector tips were mapped onto standardized sections of the rat brain (Fig. 5). Two rats were excluded from analyses because of incorrect cannula placement. One rat died during surgery. Therefore, 24 rats in total were included in the drug administration analysis. Sometimes, the rats failed to finish the drug administration and their data were also excluded. Animal numbers are given in the figure legends.
Fig. 5.
Schematic of coronal sections depicting injection sites in the lateral thalamus at −2.12, −2.30, −2.56, and −2.80 mm rostral to bregma.
Discussion
To the best of our knowledge, this is the first study to investigate the functional involvement of dopamine signaling in the rodent lateral thalamus in decisional impulsivity. We found a lower GM density in the lateral thalamus of HI rats than in LI rats. Infusions of the D1 receptor antagonist SCH23390 or the D2 receptor antagonist eticlopride into the lateral thalamus in MI rats had opposing effects on choice of the small-immediate and the large-delayed rewards in a delay-discounting task.
In this study, the lateral thalamus illustrated by MRI mainly included the ventral posterolateral thalamic nuclei (VPL) and reticular thalamic nuclei (RTN). The RTN is a thin sheet surrounded by other thalamic nuclei [45]. RTN neurons receive collateral innervation from the cerebral cortex and thalamocortical pathway, while they do not send direct projections to the cerebral cortex, but rather send dense, inhibitory GABAergic projections to the other thalamic nuclei [46–48]. The VPL receives projections from the dorsal horn and sends projections to somatosensory cortical areas, which are important components of the sensorimotor pathway [49–52]. Clearly, the structural characteristics of the lateral thalamus suggest that it is involved in regulating the inflow and outflow of information between the cortex and other thalamic nuclei.
Recently, it has been reported that the GM density in the nucleus accumbens core (NAcC) in rats with high motor impulsivity is lower, but the number of NAcC neurons is not changed, although the structural integrity and the density of dendritic spines in this region are affected [53]. In addition, the mesolimbic dopamine system has been strongly linked to impulsive decision-making; interference with D1 or D2 receptors in different brain regions impairs the performance of several impulsive decisional tasks, such as the delay discounting task [8, 23–25, 44, 51, 54–56], probabilistic discounting task [57], and effort discounting task [58]. In the present study, the GM density in the lateral thalamus of HI rats was significantly lower than that in LI rats, and this may be related to changes in the structure and function of dopamine receptors in the lateral thalamus.
As expected, the choice of small-immediate and large-delayed rewards was changed by inhibiting either D1 or D2 receptors in the lateral thalamus in MI rats. In particular, infusions of the D1 receptor antagonist SCH23390 into the lateral thalamus of MI rats promoted the choice for small-immediate over larger-delayed reward, while the D2 receptor antagonist eticlopride had an opposite effect, reducing the choice for small-immediate over larger-delayed reward. These results are consistent with previous reports that small-immediate reward increases when SCH23390 is administered systemically [39] and small-immediate reward is decreased by systemic infusion of raclopride [55]. These opposite effects on decisional impulsive performance in MI rats may result from differences in the distribution and function of D1 and D2 receptors themselves.
It is known that D1 receptors are almost exclusively localized post-synaptically on neurons, while D2 receptors are found both pre- and post-synaptically [59, 60]. It is worth noting that a substantial proportion of D2 receptors is primarily expressed in presynaptic membranes as autoreceptors, suggesting that D1 and D2 receptors play distinct roles in dopamine signaling [61]. On the other hand, our results showing that infusion of D1 and D2 receptor antagonists into the lateral thalamus had distinct effects on the decisional impulsive performance in MI rats, are consistent with previous pharmacological interventions in the medial prefrontal cortex [54, 57]. Moreover, a body of evidence has accumulated to support the hypothesis that D1 and D2 receptors act differently in cognitive processes, such as motor impulsivity [27], learning and memory [62], and attention [63]. Taken together, these studies indicate that a critical balance of intracerebral D1 and D2 receptor activity may contribute to normal decisional impulsivity, and altering this balance induces dissociable changes in impulsive behaviors.
In the present study, SCH23390 infusions into the lateral thalamus were accompanied by a decrease in operating rate and an increment in omissions (1 μg/side: P = 0.056; 0.3 μg/side: P = 0.018), which might be for two reasons. First, previous research has shown that both the dopamine antagonist α-flupenthixol and SCH23390 decrease the number of completed trials and increase omissions in delay discounting tasks [23, 25, 55], perhaps indicating changes in the motivational value of food or the motivation to respond to conditioned reinforcers. In a delay discounting task similar to that in the current study, the change of motivational value was found to increase the response latency and omissions, but the choice of reward was not affected [23]. Second, it has been reported that activity in the lateral thalamic nuclei is modified by shifts of visual attention, and these changes affect visual cognitive processing [64]. Moreover, the visual-projection neurons in the lateral thalamus show a reduced firing rate during a visual detection task using an optogenetic strategy [65]. These results imply that neurons in the lateral thalamus play an important role in attention. Thus it cannot be excluded that slight inattention after blocking D1 receptors in the lateral thalamus produced the reduction in operating rate and increment in omissions in MI rats here. Above all, though the decrease in operating rate and increment in omissions induced by infusions of SCH23390 at 0.3 μg/side and 1 μg/side might be associated with altered motivation or inattention, the effects of D1 receptor antagonists in the lateral thalamus in MI rats on decisional impulsivity cannot be excluded since both doses of SCH23390 were modest and the operating rate was still >75%.
In the present study, an infusion volume of 0.5 μL delivered over 2 min was used in the pharmacology experiments. Based on previous studies, the estimated spread was ~1 mm [27, 66], which was within the lateral thalamus. Furthermore, to demonstrate site specificity, the locations of the cannulas in each rat were checked and data from rats with inaccurate locations were excluded. Thus, the effects of drug infusions on decisional impulsivity were mediated by inhibiting dopamine receptors within the lateral thalamus.
Of course, some limitations in our work need to be pointed out. First, the MI group was not included in MRI data acquisition. Second, compared to LI rats, a lower GM density was found in the lateral thalamus of HI rats, but structural and functional differences in neurons in the lateral thalamus with dopamine receptors between HI and LI animals were not further investigated, such as numbers of spines and firing rates of neurons; these will be investigated in subsequent research. Third, based on the literature, we selected the doses of the dopamine receptor antagonists (0.3 and 1.0 μg/side) [8, 24, 44], which had similar effects on the choice of small-immediate or large-delayed reward in MI rats. The results indicated 1 μg/side was better than 0.3 μg/side. Although there are some flaws, this is still the first study to report the effects of pharmacological manipulation of D1 and D2 receptors in the lateral thalamus on decisional impulsivity.
Altogether, our results extend previous findings indicating that the lateral thalamus plays an important role in decisional impulsivity, and D1 or D2 receptors in the lateral thalamus have distinct effects on cognitive impulsivity. The findings may contribute to further unraveling the neural correlates of decisional impulsivity, improving the understanding of psychiatric disorders in which impulsive decision-making is disturbed, such as substance use disorders and Parkinson’s disease.
Acknowledgements
This work was supported by the National Natural Science Foundation (81471353), the National Basic Research Program of China (2015CB553500), and the Science Fund for Creative Research Groups from of National Natural Science Foundation of China (81521063).
References
- 1.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Basar K, Sesia T, Groenewegen H, Steinbusch HW, Visser-Vandewalle V, Temel Y. Nucleus accumbens and impulsivity. Prog Neurobiol. 2010;92:533–557. doi: 10.1016/j.pneurobio.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Nombela C, Wolpe N, Barker RA, Rowe JB. Time on timing: Dissociating premature responding from interval sensitivity in Parkinson’s disease. Mov Disord. 2016;31:1163–1172. doi: 10.1002/mds.26631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn WY, Ramesh D, Moeller FG, Vassileva J. Utility of machine-learning approaches to identify behavioral markers for substance use disorders: impulsivity dimensions as predictors of current cocaine dependence. Front Psychiatry. 2016;7:34. doi: 10.3389/fpsyt.2016.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izci F, Fındıklı EK, Zincir S, Zincir SB, Koc MI. The differences in temperament-character traits, suicide attempts, impulsivity, and functionality levels of patients with bipolar disorder I and II. Neuropsychiatr Dis Treat. 2016;12:177–184. doi: 10.2147/NDT.S90596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma CL, Sun X, Luo F, Li BM. Prefrontal cortical α2A-adrenoceptors and a possible primate model of attention deficit and hyperactivity disorder. Neurosci Bull. 2015;31:227–234. doi: 10.1007/s12264-014-1514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Zuo Y, Yu P, Ping X, Cui C. Role of basolateral amygdala dopamine D2 receptors in impulsive choice in acute cocaine-treated rats. Behav Brain Res. 2015;287:187–195. doi: 10.1016/j.bbr.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 9.Zuo Y, Wang X, Cui C, Luo F, Yu P, Wang X. Cocaine-induced impulsive choices are accompanied by impaired delay-dependent anticipatory activity in basolateral amygdala. J Cogn Neurosci. 2012;24:196–211. doi: 10.1162/jocn_a_00131. [DOI] [PubMed] [Google Scholar]
- 10.Butler EG, Horne MK, Rawson JA. Sensory characteristics of monkey thalamic and motor cortex neurons. J Physiol. 1992;445:1–24. doi: 10.1113/jphysiol.1992.sp018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 12.Alexander GE. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 13.Shany UT, Lin N, Rosen HJ, Sollberger M, Miller BL, Rankin KP. Self-awareness in neurodegenerative disease relies on neural structures mediating reward-driven attention. Brain. 2014;137:2368–2381. doi: 10.1093/brain/awu161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Bourbon-Teles J, Bentley P, Koshino S, Shah K, Dutta A, Malhotra P, et al. Thalamic control of human attention driven by memory and learning. Curr Biol. 2014;24:993–999. doi: 10.1016/j.cub.2014.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorobyev V, Kwon MS, Moe D, Parkkola R, Hämäläinen H. Risk-taking behavior in a computerized driving task: brain activation correlates of decision-making, outcome, and peer influence in male adolescents. PLoS One. 2015;10:e0129516. doi: 10.1371/journal.pone.0129516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/S0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, Weingart D, Bonner T, Watson B, Park MT, Sharp W, et al. Defining the neuroanatomic basis of motor coordination in children and its relationship with symptoms of attention-deficit/hyperactivity disorder. Psychol Med. 2016;46:2363–2373. doi: 10.1017/S0033291716000660. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Chen C, Cai Y, Li S, Zhao X, Zheng L, et al. Dissociated neural substrates underlying impulsive choice and impulsive action. Neuroimage. 2016;134:540–549. doi: 10.1016/j.neuroimage.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Wong CW, Olafsson V, Plank M, Snider J, Halgren E, Poizner H, et al. Resting-state fMRI activity predicts unsupervised learning and memory in an immersive virtual reality environment. PLoS One. 2014;9:e109622. doi: 10.1371/journal.pone.0109622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty S, Kolling N, Walton ME, Mitchell AS. Critical role for the mediodorsal thalamus in permitting rapid reward-guided updating in stochastic reward environments. Elife. 2016;5:e13588. doi: 10.7554/eLife.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miedl SF, Wiswede D, Marco-Pallarés J, Ye Z, Fehr T, Herrmann M, et al. The neural basis of impulsive discounting in pathological gamblers. Brain Imaging Behav. 2015;9:887–898. doi: 10.1007/s11682-015-9352-1. [DOI] [PubMed] [Google Scholar]
- 22.Amlung M, Sweet LH, Acker J, Brown CL, MacKillop J. Dissociable brain signatures of choice conflict and immediate reward preferences in alcohol use disorders. Addict Biol. 2014;19:743–753. doi: 10.1111/adb.12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Koffarnus MN, Newman AH, Grundt P, Rice KC, Woods JH. Effects of selective dopaminergic compounds on a delay-discounting task. Behav Pharmacol. 2011;22:300–311. doi: 10.1097/FBP.0b013e3283473bcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loos M, Pattij T, Janssen MC, Counotte DS, Schoffelmeer AN, Smit AB, et al. Dopamine receptor D1/D5 gene expression in the medial prefrontal cortex predicts impulsive choice in rats. Cereb Cortex. 2010;20:1064–1070. doi: 10.1093/cercor/bhp167. [DOI] [PubMed] [Google Scholar]
- 26.Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- 27.Pezze MA, Dalley JW, Robbins TW. Differential roles of dopamine D1 and D2 receptors in the nucleus accumbens in attentional performance on the five-choice serial reaction time task. Neuropsychopharmacology. 2007;32:273–283. doi: 10.1038/sj.npp.1301073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, et al. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savasta M, Dubois A, Scatton B. Autoradiographic localization of D1 dopamine receptors in the rat brain with [3H]SCH 23390. Brain Res. 1986;375:291–301. doi: 10.1016/0006-8993(86)90749-3. [DOI] [PubMed] [Google Scholar]
- 31.Dubois A, Savasta M, Curet O, Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986;19:125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 32.Sun J, Xu J, Cairns NJ, Perlmutter JS, Mach RH. Dopamine D1, D2, D3 receptors, vesicular monoamine transporter type-2 (VMAT2) and dopamine transporter (DAT) densities in aged human brain. PLoS One. 2012;7:e49483. doi: 10.1371/journal.pone.0049483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pifl C, Hornykiewicz O, Blesa J, Adánez R, Cavada C, Obeso JA. Reduced noradrenaline, but not dopamine and serotonin in motor thalamus of the MPTP primate: relation to severity of parkinsonism. J Neurochem. 2013;125:657–662. doi: 10.1111/jnc.12162. [DOI] [PubMed] [Google Scholar]
- 34.García-Cabezas MA, Rico B, Sánchez-González MA, Cavada C. Distribution of the dopamine innervation in the macaque and human thalamus. Neuroimage. 2007;34:965–984. doi: 10.1016/j.neuroimage.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-González MA, García-Cabezas MA, Rico B, Cavada C. The primate thalamus is a key target for brain dopamine. J Neurosci. 2005;25:6076–6083. doi: 10.1523/JNEUROSCI.0968-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.García-Cabezas MA, Martínez-Sánchez P, Sánchez-González MA, Garzón M, Cavada C. Dopamine innervation in the thalamus: monkey versus rat. Cereb Cortex. 2009;19:424–434. doi: 10.1093/cercor/bhn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young KA, Wilcox RE. Characterization of D2 receptors and dopamine levels in the thalamus of the rat. Life Sci. 1991;48:1845–1852. doi: 10.1016/0024-3205(91)90240-C. [DOI] [PubMed] [Google Scholar]
- 38.Winstanley CA, Zeeb FD, Bedard A, Fu K, Lai B, Steele C, et al. Dopaminergic modulation of the orbitofrontal cortex affects attention, motivation and impulsive responding in rats performing the five-choice serial reaction time task. Behav Brain Res. 2010;210:263–272. doi: 10.1016/j.bbr.2010.02.044. [DOI] [PubMed] [Google Scholar]
- 39.Fernando AB, Economidou D, Theobald DE, Zou MF, Newman AH, Spoelder M, et al. Modulation of high impulsivity and attentional performance in rats by selective direct and indirect dopaminergic and noradrenergic receptor agonists. Psychopharmacology (Berl) 2012;219:341–352. doi: 10.1007/s00213-011-2408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 41.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 42.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Nie B, Chen K, Zhao S, Liu J, Gu X, Yao Q, et al. A rat brain MRI template with digital stereotaxic atlas of fine anatomical delineations in paxinos space and its automated application in voxel-wise analysis. Hum Brain Mapp 2013, 34: 1306–1318. [DOI] [PMC free article] [PubMed]
- 44.Pattij T, Schetters D, Schoffelmeer AN. Dopaminergic modulation of impulsive decision making in the rat insular cortex. Behav Brain Res. 2014;270:118–124. doi: 10.1016/j.bbr.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 45.Mitrofanis J, Guillery RW. New views of the thalamic reticular nucleus in the adult and the developing brain. Trends Neurosci. 1993;16:240–245. doi: 10.1016/0166-2236(93)90163-G. [DOI] [PubMed] [Google Scholar]
- 46.Deng J, Elberger A. Corticothalamic and thalamocortical pathfinding in the mouse: Dependence on intermediate targets and guidance axis. Anat Embryol (Berl) 2003;207:177–192. doi: 10.1007/s00429-003-0338-1. [DOI] [PubMed] [Google Scholar]
- 47.Ferrarelli F, Tononi G. The thalamic reticular nucleus and schizophrenia. Schizophr Bull. 2011;37:306–315. doi: 10.1093/schbul/sbq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pratt JA, Morris BJ. The thalamic reticular nucleus: a functional hub for thalamocortical network dysfunction in schizophrenia and a target for drug discovery. J Psychopharmacol. 2015;29:127–137. doi: 10.1177/0269881114565805. [DOI] [PubMed] [Google Scholar]
- 49.Price DD, Verne GN. Does the spinothalamic tract to ventroposterior lateral thalamus and somatosensory cortex have roles in both pain sensation and pain-related emotions? J Pain. 2002;3:105–108. doi: 10.1054/jpai.2002.122950. [DOI] [PubMed] [Google Scholar]
- 50.Willis WD, Zhang X, Honda CN, Giesler GJ. Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267–276. doi: 10.1016/S0304-3959(01)00268-8. [DOI] [PubMed] [Google Scholar]
- 51.Pagano RL, Fonoff ET, Dale CS, Ballester G, Teixeira MJ, Britto LR. Motor cortex stimulation inhibits thalamic sensory neurons and enhances activity of PAG neurons: possible pathways for antinociception. Pain. 2012;153:2359–2369. doi: 10.1016/j.pain.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Carvell GE, Simons DJ. Motor modulation of afferent somatosensory circuits. Nat Neurosci. 2008;11:1430–1438. doi: 10.1038/nn.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caprioli D, Sawiak SJ, Merlo E, Theobald DE, Spoelder M, Jupp B, et al. Gamma aminobutyric acidergic and neuronal structural markers in the nucleus accumbens core underlie trait-like impulsive behavior. Biol Psychiatry. 2014;75:115–123. doi: 10.1016/j.biopsych.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pardey MC, Kumar NN, Goodchild AK, Cornish JL. Catecholamine receptors differentially mediate impulsive choice in the medial prefrontal and orbitofrontal cortex. J Psychopharmacol. 2013;27:203–212. doi: 10.1177/0269881112465497. [DOI] [PubMed] [Google Scholar]
- 55.Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- 56.Tian L, Qin X, Sun J, Li X, Wei L. Differential effects of co-administration of oxotremorine with SCH 23390 on impulsive choice in high-impulsive rats and low-impulsive rats. Pharmacol Biochem Behav. 2016;142:56–63. doi: 10.1016/j.pbb.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 57.St Onge JR, Abhari H, Floresco SB. Dissociable contributions by prefrontal D1 and D2 receptors to risk-based decision making. J Neurosci. 2011;31:8625–8633. doi: 10.1523/JNEUROSCI.1020-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schweimer J, Hauber W. Dopamine D1 receptors in the anterior cingulate cortex regulate effort-based decision making. Learn Mem. 2006;13:777–782. doi: 10.1101/lm.409306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beaulieu JM, Gainetdinov RR. The physiology signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 60.Govindaiah G, Wang T, Gillette MU, Crandall SR, Cox CL. Regulation of inhibitory synapses by presynaptic D4 dopamine receptors in thalamus. J Neurophysiol. 2010;104:2757–2765. doi: 10.1152/jn.00361.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.De Mei C, Ramos M, Iitaka C, Borrelli E. Getting specialized: presynaptic and postsynaptic dopamine D2 receptors. Curr Opin Pharmacol. 2009;9:53–58. doi: 10.1016/j.coph.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.André MA, Manahan-Vaughan D. Involvement of dopamine D1/D5 and D2 receptors in context-dependent extinction learning and memory reinstatement. Front Behav Neurosci. 2016;9:372. doi: 10.3389/fnbeh.2015.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diaz E, Medellín J, Sánchez N, Vargas JP, López JC. Involvement of D1 and D2 dopamine receptor in the retrieval processes in latent inhibition. Psychopharmacology (Berl) 2015;232:4337–4346. doi: 10.1007/s00213-015-4063-2. [DOI] [PubMed] [Google Scholar]
- 64.McAlonan K, Cavanaugh J, Wurtz RH. Attentional modulation of thalamic reticular neurons. J Neurosci. 2006;26:4444–4450. doi: 10.1523/JNEUROSCI.5602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Halassa MM, Chen Z, Wimmer RD, Brunetti PM, Zhao S, Zikopoulos B, et al. State-dependent architecture of thalamic reticular subnetworks. Cell. 2014;158:808–821. doi: 10.1016/j.cell.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Routtenberg A. Intracranial chemical injection and behavior: a critical review. Behav Biol. 1972;7:601–641. doi: 10.1016/S0091-6773(72)80073-7. [DOI] [PubMed] [Google Scholar]