Abstract
Schizophrenia is considered to be a disorder of brain connectivity, which might result from a disproportionally impaired rich-club organization. The rich-club is composed of highly interconnected hub regions that play crucial roles in integrating information between different brain regions. Few studies have yet investigated whether the structural rich-club organization is impaired in patients and their first-degree relatives. In this study, we established a weighted network model of white matter connections using diffusion tensor imaging of 19 patients and 39 unaffected parents, 22 young healthy controls for the patients, and 25 old healthy controls for the parents. Feeder edges between rich-club nodes and non-rich-club nodes were significantly decreased in both schizophrenic patients and their unaffected parents compared with controls. Furthermore, the feeder edges showed significant positive correlations with the scores in Category Fluency Test—animal naming in the unaffected parents. Specific feeder edges exhibited discriminative power with accuracy of 84.4% in distinguishing unaffected parents from old healthy controls. Our findings suggest that impaired rich-club organization, especially impaired feeder edges, may be related to familial vulnerability to schizophrenia, possibly reflecting a genetic predisposition for schizophrenia.
Keywords: Structural brain network, Diffusion tensor imaging, Rich-club, Familial vulnerability, Schizophrenia
Introduction
Schizophrenia is a serious neuropsychiatric disease characterized by complex phenotypes including positive, negative, and cognitive symptoms [1, 2]. Dysfunctions in cognition (attention, memory, executive function, and information integration) have a significant impact on patients’ functional status. Schizophrenia is a highly heritable disorder with an estimated heritability of ~81% [3, 4], implying a significant role of genetic factors in its pathogenesis. Previous studies have shown that impaired cognitive function in schizophrenia is related to familial risk [5, 6].
With the development of diffusion tensor imaging (DTI) and the application of graph theoretical analysis, recent connectome studies have demonstrated that hubs exist in the human brain and are densely interconnected to form a central ‘rich-club’ organization [7–9], which is thought to be essential for the global efficiency of information transfer. Rich-club connectivity and nodes also play crucial roles in neural signaling and are involved in different functional subdomains of the human brain [9, 10].
Schizophrenia is considered to be a disorder of brain connectivity [11–15]. Investigators have shown widespread impairment of the white matter (WM) connectivity in schizophrenic patients [14, 16, 17], and further research has discovered reduced global efficiency and impaired integrity in the frontal and temporal cortex at the system level [18–20]. Studies have shown that the rich-club topology in schizophrenia is destroyed, and greater reductions in rich-club strength are associated with a lower level of network efficiency, an evident alteration of functional dynamics, and a worse outcome [9].
Recently, a new focus on the relationship between familial vulnerability to schizophrenia and brain network connections has gained increasing attention. Given that the unaffected relatives of patients often show network alterations paralleling those in affected probands [21–24], a reasonable hypothesis has been proposed that the potentially heritable aspects of rich-club organization may also be present in unaffected first-degree relatives, resulting in a significantly higher risk of schizophrenia than the general population. To test this hypothesis, Collin et al. [25] showed that unaffected siblings have a similar but milder pattern of rich-club organization abnormality compared with schizophrenic patients, suggesting that the abnormal rich-club connectivity is associated with the familial risk of schizophrenia. However, few studies have revealed the brain network connections in patients with schizophrenia and their unaffected parents. We previously demonstrated compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents [26]. In the present study, we used a different approach to examine the rich-club organization in schizophrenic patients and their unaffected parents, and attempted to define the pathological pattern of change and identify the potential discriminability of rich-club organization in the unaffected parents of schizophrenic patients. The network-based statistic (NBS) method was also used to reveal specific connections in the rich-club organization associated with the heritability of schizophrenia [27]. The relationship between disease-related cognitive function and rich-club organization was also investigated.
Materials and Methods
Participants
Twenty-five nuclear families with schizophrenic probands, and 50 normal controls who reported no first- or second-degree relatives with schizophrenia and well-matched for age and sex with the patients or their parents, were recruited through the Institute of Mental Health, Peking University. Eighteen participants were excluded because of non-cooperation with the scan or contraindications for magnetic resonance imaging (MRI). Finally, 105 individuals entered subsequent analysis: 19 schizophrenic patients (SCH), 39 unaffected parents (SCHP), 22 young healthy controls for the SCH group (HC1) and 25 old healthy controls for the SCHP group (HC2). All participants had been enrolled in our previous fMRI study which approached a different issue using a different method [26]. Patients with schizophrenia all met the ICD-10 diagnostic criteria for paranoid schizophrenia [28], which was ensured by two psychiatrists. All patients were receiving antipsychotic medications when scanned, and the dosages were converted to the equivalent dose for chlorpromazine [29]. Patients treated with electroconvulsive therapy within 6 months or with a history of serious medical illness were excluded. None of the healthy controls reported having first- or second-degree relatives with schizophrenia spectrum disorders. All participants were assessed to be right-handed using the Edinburgh Handedness Inventory [30] and had no intracranial pathology, a history of head injury, neurological disorder, or alcohol/substance abuse.
The demographic and clinical characteristics of the participants are listed in Table 1. This study was approved by the Medical Ethics Committee of the Institute of Mental Health, Peking University. We informed all participants about the purposes and procedures of the study, and all participants gave written consent.
Table 1.
Demographic and clinical characteristics of the patients, their unaffected parents, and healthy controls.
| Variable | SCH (n = 19) | HC1 (n = 22) | P | SCHP (n = 39) | HC2 (n = 25) | P |
|---|---|---|---|---|---|---|
| Sex (male/female) | 8/11 | 12/10 | 0.29a | 19/20 | 13/12 | 0.69a |
| Age (years) | 23.7 (4.3) | 22.7 (3.1) | 0.39 | 51.5 (6/0) | 52.0 (6.7) | 0.79 |
| Education (years) | 13.6 (1.9) | 14.0 (2.0) | 0.59 | 13.2 (3.1) | 13.2 (2.6) | 0.96 |
| Age at disease onset (years) | 19.5 (3.2) | |||||
| Course of illness (months) | 43.8 (32.9) | |||||
| CPZ-eq at scan (mg/day) | 442.5 (293.3) | |||||
| PANSS sum score | 70.2 (9.4) | |||||
| Positive | 16.8 (3.9) | |||||
| Negative | 14.8 (4.9) | |||||
| CTF | 23.1 (6.7) | 35.1 (8.2) | <0.01 | 27.0 (8.2) | 32.9 (9.7) | <0.01 |
| MQ | 94.3 (15.2) | 115.7 (11.7) | <0.01 | 101.5 (13.4) | 111.8 (10.8) | <0.01 |
| TOH | 131.6 (99.4) | 82.7 (57.9) | 0.204 | 138.5 (61.8) | 108.8 (73.5) | 0.368 |
CPZ-eq chlorpromazine equivalents, HC1 healthy controls for schizophrenic patients, HC2 healthy controls for parents, SCH schizophrenic patients, SCHP unaffected parents of schizophrenic patients, CFT processing speed of word information assessed by Category Fluency Test—animal naming, MQ Memory Quotient assessed with Wechsler Memory Scale-Chinese Revised, TOH executive function assessed with Tower of Hanoi. Mean (standard deviation), unless otherwise indicated.
aPearson’s χ2 test.
Assessment of Symptomatology and Cognitive Function
The severity of symptoms was evaluated by two experienced psychiatrists using the Positive and Negative Syndrome Scale (PANSS) [30] within one week of MRI scanning.
Three cognitive fields were chosen: (1) Processing speed of word information assessed by Category Fluency Test—animal naming (CFT) [31]. Participants were asked to name as many animals as possible in 1 minute. A higher number of correct answers reflects a faster processing speed. (2) Executive function was assessed with the Tower of Hanoi (TOH) [32]. The time to finish the TOH task was used as the main assessment index, the faster the better. (3) Memory was assessed as the memory quotient (MQ) from the Wechsler Memory Scale—Chinese Revised [33]. A high score indicates better memory.
Data Acquisition
All participants were scanned in a 3.0-Tesla Magnetom Trio MRI scanner (Siemens Medical System, Erlangen, Germany) at the Department of Radiology of the Third Hospital, Peking University. T1-weighted images were acquired in a sagittal orientation using a 3D-MPRAGE sequence with the following parameters: repetition time (TR) = 2350 ms; echo time (TE) = 3.44 ms; slice thickness = 1 mm; flip angle = 7°; matrix size = 256 × 256; field of view (FOV) = 256 × 256 mm2; 192 slices; and acquisition voxel size = 1.0 × 1.0 × 1.5 mm3. For the diffusion imaging scans, a single-shot echo-planar imaging-based sequence was applied with the following parameters: TR = 5300 ms; TE = 92 ms; thickness/gap = 3/0.3 mm; matrix = 128 × 128; FOV = 230 × 230 mm2; acquisition voxel size = 1.8 × 1.8 × 3.0 mm3; number of excitations = 2; slices = 40; 64 diffusion directions with b = 1000 s/mm2; and an additional image without diffusion weighting (i.e., b = 0 s/mm2).
Data Processing
Diffusion Tensor Image Preprocessing and Tractography
All the DTI data preprocessing and analyses described below were conducted using the Pipeline for Analyzing Brain Diffusion Images (PANDA, http://www.nitrc.org/projects/panda) [34], which makes use of commands in FSL (http://fsl.fmrib.ox.ac.uk/fsl) and the Diffusion Toolkit. First, eddy-current distortions and head-motion artifacts of the diffusion image of each participant were corrected by co-registering each diffusion-weighted image to the b0 image using an affine transformation. The diffusion gradient directions were also adjusted accordingly. Second, the tensor matrix and the diffusion tensor matrices were estimated voxel by voxel. Third, deterministic tractography was performed in the native diffusion space to obtain whole-brain WM tracts using the fiber assignment continuous tracking algorithm. The seed location was set in the center of each voxel. The tracking of each fiber terminated if the turn angle was >45° or entered a voxel with a fractional anisotropy (FA) <0.2.
Brain Network Construction
All steps of the network construction were also implemented in PANDA, and the detailed procedures are described below.
Network Node Definition
To eliminate a prior anatomical bias and obtain an elaborate parcellation of the brain, the random 1024 template in PANDA was used to divide the cerebral cortex into 1024 regions, each representing a node of the cortical network. Briefly, individual T1-weighted images were co-registered to the FA images in the DTI space using an affine transformation. The T1 images were then nonlinearly transformed to the ICBM152 averaged T1 template in MNI (Montreal Neurological Institute) space. The inverse nonlinear transformations were then used to warp the 1024-label atlas from the MNI space to individual DTI space by a nearest-neighbor interpolation method.
Network Edge Definition
To define the network edges, two regions were considered structurally connected only if the two end-points of at least one fiber were located in each of them. The mean FA value of the connected fibers between 2 regions was defined as the weights of the network edges.
Rich-Club Analysis
To describe the rich-club organization of the structural network, we computed the rich-club coefficient according to van den Heuvel and Sporns [15]. All network analyses were performed using GRETNA (GRaph thEoreTical Network Analysis) software (http://www.nitrc.org/projects/gretna/) [35] and the results were visualized using BrainNet Viewer software (https://www.nitrc.org/projects/bnv/) [36]. The detailed definition of rich-club organization was as follows. To identify the rich-club regions of the WM structural networks, we constructed the backbone network by detecting the significant nonzero connections across all participants in each group, with a nonparametric one-tailed sign test (P < 0.05, corrected). Based on each backbone network, the hub regions were defined as the regions with number of edges >mean+ 2 × SD. Then, the rich-club coefficient (RC) and normalized RC were calculated for the backbone network, according to van den Heuvel and Sporns [15]. For the weighted networks, the RC ϕ w(k) is defined as:
with E >k denoting the subset of edges between the hub nodes with a degree >k. In our study, k = mean + 2 × SD, W >k denotes the total sum weights in the total network, with weights W representing the number of fiber streamlines of the edges. ϕ(k) was normalized relative to the ϕ random(k) of a set of comparable random networks (n = 1000) of equal size and degree sequence, giving a normalized RC . On the basis of the categorization of the nodes into hub and non-hub regions, edges of the network were classified onto rich-club connections, linking hub nodes to hub nodes; feeder connections, linking hub nodes to non-hub nodes; and local connections, linking non-hub nodes. Finally, the connection strengths of rich-club, feeder, and local connections were calculated for each participant.
Statistical Analysis
For the cognitive assessment and topological organization of the network, an analysis of covariance model with diagnostic group as the fixed variable was performed separately between the patients, unaffected parents, and their respective controls. Age and sex were included as covariates in order to exclude their potential effects. We further calculated the partial correlation analysis between the network metrics and clinical measures/cognitive performances which showed significant between-group differences using age and sex as covariates. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL) and a significance level at P < 0.05 was used.
NBS Analysis
To localize the specific rich-club organization in which the structural connectivity differed between each pair of groups, we used the NBS approach separately in the abnormal types of edges in the rich-club organization. Briefly, for each type of edge with a significant diagnostic effect (rich-club and feeder edges), a primary cluster-defining threshold was first used to identify supra-threshold connections, within which the size (i.e., number of edges) of any connected components was then determined. A corrected P value was calculated for each component using the null distribution of maximal connected component size, which was derived empirically using a nonparametric permutation approach (10,000 permutations). Notably, before the permutation tests, multiple linear regressions were applied to remove the effects of age and sex. For a detailed description, see Zalesky et al. [27].
Receiver Operating Characteristic (ROC) Analysis
To further explore the discriminating power of the rich-club organization, we performed ROC analysis [37]. Specifically, the disrupted sub-network was localized by the NBS approach separately in each of the two groups. We calculated the average value of the decreased edges of each whole sub-network. Then, these average values were used in ROC analysis to test the predictive power of distinguishing SCH and SCHP from controls. The ROC curve was generated using sequential thresholding on the metric of each participant. The x axis of the ROC curve is 1-specificity, in which the specificity is the proportion of the controls who were classified correctly. The y axis of the ROC curve is the sensitivity, which is the proportion of the SCH/SCHP who were classified correctly. The accuracy is defined as the proportion of subjects who were classified correctly into the control or SCH/SCHP group. For each point in the ROC curve, there is an accuracy combining a sensitivity and a specificity. The point in the northwest represents both the highest sensitivity and specificity, and therefore the highest discriminating accuracy.
We used a relatively strict threshold for the global metric at P < 0.01. And to correct for multiple comparisons in the edge analysis, an NBS corrected approach was used to give each component a corrected P at a threshold of P = 0.05.
Results
Demographic, Clinical, and Cognitive Characteristics
All patients were in the acute stage with symptoms of moderate severity and above. Because only the paranoid subtype was recruited, all patients were mainly characterized by positive symptoms. There were no significant differences in age and sex between the schizophrenic patients, unaffected parents, and their respective controls. Compared with the normal controls, schizophrenic patients and their unaffected parents showed significantly lower cognitive scores in CFT and MQ (P < 0.05; Table 1).
Disrupted Rich-Club Organization
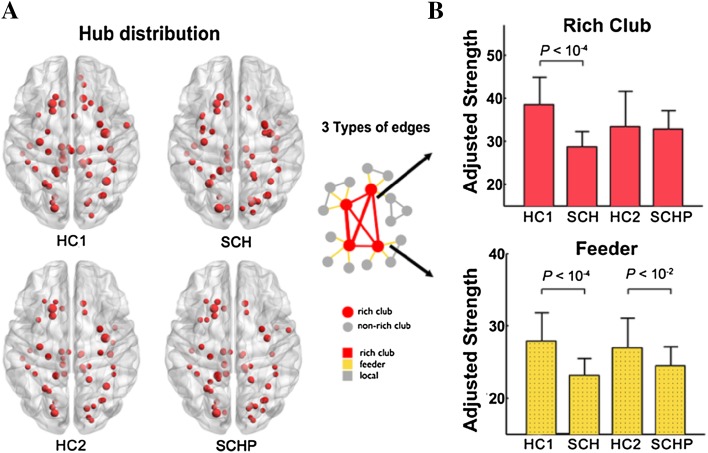
Based on the structural backbone network, similar hub distributions were found in all four groups, mainly in the bilateral posterior cingulate cortex (PCG), precuneus cortex (PCUN), middle temporal gyrus (MTG), and middle occipital gyrus (MOG), as well as subcortical nodes in the thalamus (THA), insula (INS), and caudate (CAU) regions (Fig. 1A). All four groups showed dominant rich-club organization with an RC coefficient >1 (normalized RC in HC1 = 1.35, in SCH = 1.28, in HC2 = 1.37, and in SCHP = 1.30). But disrupted connection strength was found in different types of edges in the rich-club organization related to schizophrenia. The rich-club edges linking rich-club nodes were significantly decreased in SCH patients compared to their normal controls. And the feeder edges connecting rich-club nodes and non-hub nodes were significantly decreased both in SCH patients and their unaffected parents compared to their corresponding controls (Fig. 1B).
Fig. 1.
A Similar hub distributions in all four groups: mainly located in the bilateral posterior cingulate cortex, precuneus cortex, middle temporal gyrus, and middle occipital gyrus, and subcortical nodes in the thalamus, insula, and caudate regions. B The rich-club edges were decreased in SCH patients compared to their normal controls (P < 1×10−4). The feeder edges were decreased both in SCH patients (P < 1×10−4) and their unaffected parents (P < 1×10−2) compared to their corresponding controls
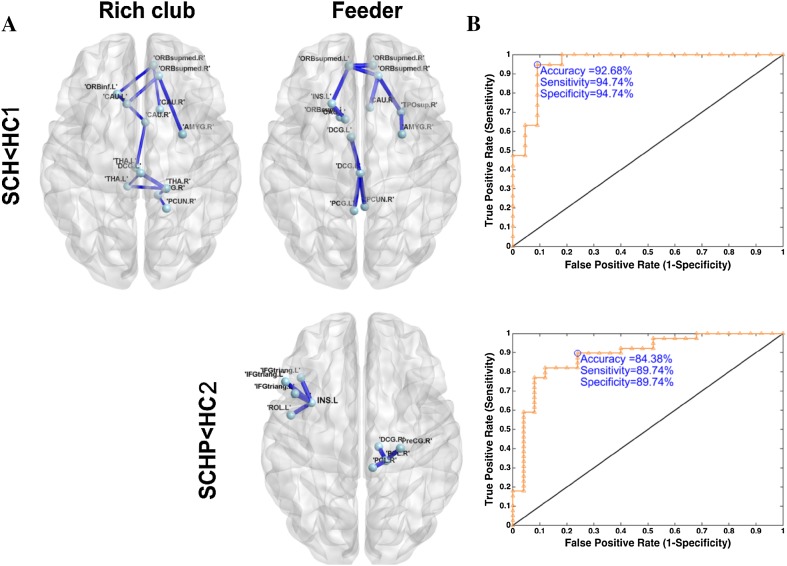
The NBS identified specific disrupted edges with lower connection strength in patients and unaffected parents compared to their normal controls. In schizophrenic patients, 12 rich-club connections between 13 nodes were decreased (P < 0.05, NBS corrected, Fig. 2); these mainly connected the bilateral CAU, right AMYG, bilateral THA, left median cingulate and paracingulate gyri (DCG), and right PCUN; and 11 feeder connections between 13 nodes were disrupted, mainly in the right AMYG, right PCUN, left DCG regions, left PCG, and left INS regions (P < 0.05, NBS corrected, Fig. 2). In unaffected parents, the decreased feeder edges were specific to 7 edges connecting 9 nodes mainly in the left inferior frontal gyrus, triangular part, left Rolandic operculum, left INS, right precentral gyrus (PreCG), and right paracentral lobule (PCL) regions (P < 0.05, NBS corrected, Fig. 2).
Fig. 2.
Network-based statistic (NBS) results. A In schizophrenic patients (SCH), 12 rich-club connections between 13 nodes were decreased (P < 0.05, NBS corrected) and 11 feeder connections between 13 nodes were disrupted (P < 0.05, NBS corrected). In unaffected parents (SCHP), the decreased feeder edges were specific to 7 edges connecting 9 nodes (P < 0.05, NBS corrected). B Disrupted rich-club and feeder edges exhibited better discriminative power with an accuracy of 92.7% in distinguishing SCHs from young healthy controls (HC1). For differentiating SCHPs from old healthy controls (HC2), the feeder edges identified by NBS improved the discriminative power with an accuracy of 84.4%.
Correlation of Rich-Club Measures with Cognitive Function and Clinical Measures
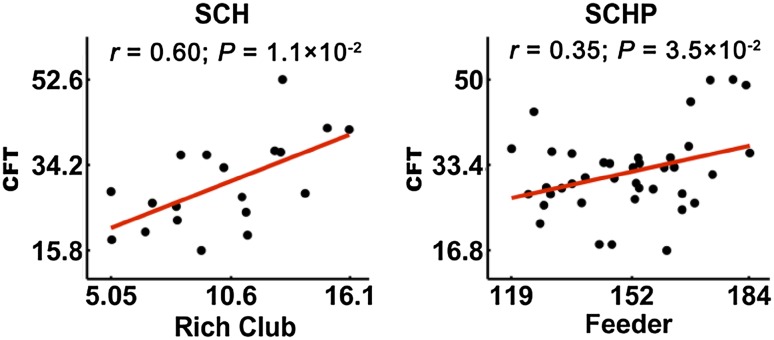
The correlations between cognitive scores and three types of edge in the rich-club organization were investigated. The CFT score showed a positive correlation with the connection strength of rich-club edges in SCHs (r = 0.6, P = 0.01, Fig. 3), and of feeder edges in SCHPs (r = 0.35, P = 0.035, Fig. 3).
Fig. 3.
The CFT score showed a positive correlation with connection strength of rich-club edges in SCH patients (r = 0.60, P = 0.01) and of feeder edges in the SCHP group (r = 0.35, P = 0.035).
In SCHs, we found a positive linear relationship between the connection strength of feeder edges and age at disease onset (r = 0.519, P = 0.033), and a negative linear relationship between connection strength of feeder edges and course of illness (r = −0.588, P = 0.013).
No significant correlations were found between rich-club connectivity and symptom severity/medication.
Results of ROC Analysis
To distinguish SCH from HC1, the average value of all connections within the sub-network containing rich-club and feeder edges, which was calculated using the NBS approach, showed the highest prediction accuracy (92.7%). To differentiate SCHP from HC2, the average value of all connections within the sub-network containing feeder edges, using the NBS approach, represented the highest discriminative power (accuracy 84.4%) (Fig. 2B).
Discussion
Using connectome-based graph theoretical analysis combined with diffusion imaging, we found impaired rich-club and feeder edges in schizophrenic patients and a similar decrease in connection strength in feeder edges in their unaffected parents.
Recent structural network studies focusing on rich-club organization have provided evidence implicating disrupted hub organization in the topological disturbances associated with schizophrenia [8]. Moreover, functional studies have found reduced levels of functional coupling and disrupted low-frequency power of rich-club regions in patients [38, 39]. In our study, similar hub distributions were found in all four groups, mainly located in the bilateral PCG, PCUN, MTG, and MOG, and subcortical nodes in the THA, INS, and CAU, consistent with the findings from previous studies [15, 40, 41]. This result showed that even when structural connections between brain regions are widely damaged in schizophrenia, the major distribution of hub structure in the WM remains stable. Consistent with a previous study, the connectivity strength among frontal, parietal, and insular hub connections was most strongly affected in schizophrenia compared to healthy controls [9]. In the current study, we found decreased connection strength of rich-club edges between hub regions of the bilateral CAU, right AMYG, bilateral THA, left DCG, and right PCUN; and decreased feeder connections with hub regions of the right AMYG, right PCUN, left DCG, left PCG, and left INS. Reduced connectivity in sub-networks of the frontal, parietal, and subcortical regions has been reported frequently in schizophrenic patients [16−18, 42, 43]. Because the “rich-club” and “non-rich-club” nodes might have different roles and relative importance for neural function, the different impaired pattern of rich-club organization in the brain regions of schizophrenic patients [44] might provide new insight into the pathogenesis of schizophrenia. Moreover, in unaffected parents, we found the decreased connection of feeder edges mainly within the left INS and IFG, right PreCG, DCG, and right PCL regions. Connectivity between hubs of the PreCG is known to be decreased in patients [25]. A previous study pointed out that regional centrality in the INS is reduced in schizophrenic patients [42]. Our results showed the regional damage of the brain has some hereditary basis and a potential genetic mechanism in schizophrenia should be further explored.
The main finding of this study was the impaired WM structural rich-club organization in schizophrenic patients and their unaffected parents, i.e., decreased connection strength of feeder edges. Previous WM structural network studies have shown an altered overall network organization and decreased connectivity density of the central rich-club in patients with schizophrenia [45–48]. And impaired rich-club connectivity has also been reported in unaffected siblings of schizophrenic patients [25]. In addition, a recent study showed that, besides siblings of patients, clinically high-risk subjects also show significantly altered structural rich-club organization, and this disruption is associated with the severity of negative psychotic symptoms in such subjects [49]. The decreased feeder edges in both SCHs and SCHPs in our study showed that impaired rich-club organization may be related to familial vulnerability to schizophrenia and further suggested that the disease may show heritable impaired connections between hub and non-hub regions. The pattern of feeder edges appeared to dramatically decline in SCHPs, different from the decreased strength of rich-club edges in schizophrenic siblings [25]. Although they are both first-degree relatives of patients, the cause of the discrepancy may be methodological variation or internal differences in gene dosage that may need further study. Together with previous evidence, our findings suggest that abnormal feeder edges provide a potential endophenotypic marker for schizophrenia.
Genetic influences in the etiology of schizophrenia have been firmly established [50, 51] and structural brain abnormalities may be related to the familial risk for schizophrenia. Furthermore, recent studies have shown that the offspring of schizophrenic patients show reduced connectivity, decreased global efficiency, and fewer hub regions [43, 52]. Twin and family studies have shown that the organization of structural brain networks is influenced by genetic factors [53–55]. Study of the unaffected parents of schizophrenic patients provides a means of understanding whether there are abnormal brain connections in individuals at increased genetic risk but unaffected by schizophrenia. Our previous study has shown that global efficiency tends to be lower in the parents of patients than in matched controls [26]. The current finding is consistent with the results of previous structural network studies, in which unaffected first-degree relatives show a pattern of brain abnormalities similar to those in schizophrenic patients [46, 56]. All of the findings indicate that an abnormal rich-club connectome in schizophrenia reflects an inherited susceptibility to the disease, and genetic influences on the anatomical disorganization of brain networks might increase an individual’s vulnerability to schizophrenia.
Of note, the disrupted feeder connections in SCHPs differed from those in SCHs. Because the hub distribution was not exactly the same in SCHs and SCHPs, the rich-club and feeder connections cannot be identical in the two groups. Therefore, it is understandable that we found different abnormal edges when we specified the abnormal edges in each group using the NBS method. Schizophrenia is a complex genetic disease, and the effect of susceptibility to disease on structural connections may not be completely consistent in patients and their parents. The impaired feeder connections in patients and their parents may be an overall effect of different susceptibility genes on brain connectivity. Nevertheless, how these genes affect the structural network still needs further study. Given that we are concerned with the topological organization of the network, the inconsistent specific abnormal edges in patients and their unaffected parents do not affect our main results and conclusion, i.e., the impaired feeder connectivity is related to a familial risk of schizophrenia.
Significant correlations between the connectivity strength of rich-club connectivity and CFT scores were found in both SCHs and SCHPs. Specifically, the positive correlation between CFT scores and rich-club edge connectivity (r = 0.6, P < 0.01) and feeder edge connectivity (r = 0.35, P = 0.035) was found in SCHs and SCHPs, respectively. That is to say, a slower word information processing speed was associated with a more serious impairment in the structural connectivity of rich-club edges in patients and that of feeder edges in unaffected parents. Previous studies have shown that impaired rich-club organization in patients may contribute to reduced integration of information among different systems of the human brain [9, 57]. Considerable evidence has shown that deficits in regions that rely on the rapid and efficient assimilation of information in schizophrenic patients might lead to slow cognitive processing [15]. The connectivity of rich-club and feeder edges was decreased in patients with schizophrenia. However, only decreased feeder edge connectivity was the neuroimaging phenotype shared between patients and their parents in the current study. The impaired cognitive function in schizophrenia has been demonstrated to be related to familial risk [58, 59]. Therefore, the change of feeder edge connectivity might be the substrate of the impaired ability of word information processing in unaffected parents.
In addition, the feeder edge connectivity was positively correlated with age at disease onset and negatively correlated with the course of illness in schizophrenic patients; that is to say, the earlier the disease appeared and the longer the course, the more seriously the structural connectivity of feeder edges was impaired. Although previous studies have reported a relationship between negative symptoms and rich-club organization [49, 60], we found no correlation between any network measure and symptom severity. The lack of a significant relationship between negative symptoms and rich-club organization may be due to the fact that only paranoid schizophrenic patients were recruited, and the sample size was relatively small. Further studies are needed to clarify the relationship between the altered rich-club organization and the symptoms in schizophrenia.
The specific properties of network organization play important roles in brain function, and their alterations might provide clinically useful diagnostic markers for neuropsychiatric disease. The results of ROC analysis showed that, the sub-network containing rich-club and feeder edges showed the highest prediction accuracy (92.7%) in distinguishing SCH from HC1, while the sub-network containing feeder edges represented the highest discriminative power (accuracy, 84.4%) in differentiating SCHP from HC2. Altered connections between different edges showed the potential power to serve as a biomarker in diagnosis of the disease and the characteristic heritable impairment of schizophrenia. We emphasize that the prediction accuracy was limited in the current sample, and whether this accuracy can be generalized to other novel samples is unknown. In future, we will collect an independent dataset to validate the diagnostic power of the identified connections.
There were some limitations that must be considered in interpreting our results. First, our special participants need to be considered. There may be some heterogeneity among first-degree relatives in terms of genetic predisposition for schizophrenia. To extract this heterogeneity, we need to apply an obligate carrier study in which the unaffected relatives who appear to transmit a genetic predisposition to their affected children are classified as presumed obligate carriers. Due to the limited number of participants, we had to lay aside the classification of the parents of patients. Second, all the patients in our study were taking antipsychotic medications, and although the effect of antipsychotics on brain structure is controversial, treatment may influence structural connectivity. However, in the current study, no correlation between rich-club organization and antipsychotic dose was found, and we also found impaired feeder edge connectivity in the never-medicated parents of patients. Together, we may infer that medication usage had no significant influence on our results. Third, we cannot exclude the potential effect of special life-experiences, emotional state, and the stress of the special role of the unaffected parents, such as the stress of living with chronic schizophrenic patients. It is necessary to assess the life experiences and emotional state of unaffected parents in future work.
In summary, the present study provides further evidence that connectome abnormalities, especially impaired feeder connectivity, are associated with a familial risk, possibly reflecting a predisposition for schizophrenia. Furthermore, the connectivity pattern showed potential discriminative ability.
Acknowledgements
This work was supported by grants from the National Basic Research Program of China (2011CB707805), the National Natural Science Foundation of China (81370032, 91232305, 81361120395, and 91432304), and the International Science and Technology Cooperation Program of China (2010DFB30820).
Contributor Information
Hao Yan, Email: hao_y@bjmu.edu.cn.
Dai Zhang, Email: daizhang@bjmu.edu.cn.
References
- 1.Mueser KT, McGurk SR. Schizophrenia. Lancet. 2004;363:2063–2072. doi: 10.1016/S0140-6736(04)16458-1. [DOI] [PubMed] [Google Scholar]
- 2.Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res. 2014;158:156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Bienvenu OJ, Davydow DS, Kendler KS. Psychiatric ‘diseases’ versus behavioral disorders and degree of genetic influence. Psychol Med. 2011;41:33–40. doi: 10.1017/S003329171000084X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 5.van Haren NE, Rijsdijk F, Schnack HG, Picchioni MM, Toulopoulou T, Weisbrod M, et al. The genetic and environmental determinants of the association between brain abnormalities and schizophrenia: the schizophrenia twins and relatives consortium. Biol Psychiatry. 2012;71:915–921. doi: 10.1016/j.biopsych.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler D, Walton E, Naylor M, Roessner V, Lim KO, Charles Schulz S, et al. Brain structure and function correlates of cognitive subtypes in schizophrenia. Psychiatry Res. 2015;234:74–83. doi: 10.1016/j.pscychresns.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Towlson EK, Vertes PE, Ahnert SE, Schafer WR, Bullmore ET. The rich-club of the C. elegans neuronal connectome. J Neurosci. 2013;33:6380–6387. doi: 10.1523/JNEUROSCI.3784-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 9.van den Heuvel MP, Sporns O, Collin G, Scheewe T, Mandl RC, Cahn W, et al. Abnormal rich-club organization and functional brain dynamics in schizophrenia. JAMA Psychiatry. 2013;70:783–792. doi: 10.1001/jamapsychiatry.2013.1328. [DOI] [PubMed] [Google Scholar]
- 10.Zamora-Lopez G, Zhou C, Kurths J. Graph analysis of cortical networks reveals complex anatomical communication substrate. Chaos. 2009;19:015117. doi: 10.1063/1.3089559. [DOI] [PubMed] [Google Scholar]
- 11.Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. doi: 10.1016/j.neuroimage.2011.12.090. [DOI] [PubMed] [Google Scholar]
- 13.Whitford TJ, Kubicki M, Shenton ME. Diffusion tensor imaging, structural connectivity, and schizophrenia. Schizophr Res Treatment. 2011;2011:709523. doi: 10.1155/2011/709523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Heuvel MP, Sporns O. Rich-club organization of the human connectome. J Neurosci. 2011;31:15775–15786. doi: 10.1523/JNEUROSCI.3539-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ottet M-C, Schaer M, Debbané M, Cammoun L, Thiran JP, Eliez S. Graph theory reveals dysconnected hubs in 22q11DS and altered nodal efficiency in patients with hallucinations. Front Hum Neurosci 2013, 7: 402. [DOI] [PMC free article] [PubMed]
- 17.Zhang Y, Lin L, Lin CP, Zhou Y, Chou KH, Lo CY, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141:109–118. doi: 10.1016/j.schres.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Su TP, Zhou Y, Chou KH, Chen IY, Jiang T, et al. Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage. 2012;59:1085–1093. doi: 10.1016/j.neuroimage.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 19.Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 20.Kubicki M, Westin CF, McCarley RW, Shenton ME. The application of DTI to investigate white matter abnormalities in schizophrenia. Ann N Y Acad Sci. 2005;1064:134–148. doi: 10.1196/annals.1340.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubicki M, Shenton ME, Maciejewski PK, Pelavin PE, Hawley KJ, Ballinger T, et al. Decreased axial diffusivity within language connections: a possible biomarker of schizophrenia risk. Schizophr Res. 2013;148:67–73. doi: 10.1016/j.schres.2013.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skudlarski P, Schretlen DJ, Thaker GK, Stevens MC, Keshavan MS, Sweeney JA, et al. Diffusion tensor imaging white matter endophenotypes in patients with schizophrenia or psychotic bipolar disorder and their relatives. Am J Psychiatry. 2013;170:886–898. doi: 10.1176/appi.ajp.2013.12111448. [DOI] [PubMed] [Google Scholar]
- 23.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li M, Li T. Altered topological properties in the heritable schizophrenic brain. Neurosci Bull. 2015;31:515–516. doi: 10.1007/s12264-015-1554-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP. Impaired rich-club connectivity in unaffected siblings of schizophrenic patients. Schizophr Bull. 2014;40:438–448. doi: 10.1093/schbul/sbt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yan H, Tian L, Wang Q, Zhao Q, Yue W, Yan J, et al. Compromised small-world efficiency of structural brain networks in schizophrenic patients and their unaffected parents. Neurosci Bull. 2015;31:275–287. doi: 10.1007/s12264-014-1518-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Shekhar S, Benedetto S. The ICD-10 classification of mental and behavioural disorders. World Health Organization, 1993.
- 29.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/JCP.v64n0607. [DOI] [PubMed] [Google Scholar]
- 30.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 31.Strauss E, Sherman EMS, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary (3rd. ed). Cogn Behav Neurol 1998, 12: 70–71.
- 32.Shallice T. Specific impairments of planning. Philos Trans R Soc Lond B Biol Sci. 1982;298:199–209. doi: 10.1098/rstb.1982.0082. [DOI] [PubMed] [Google Scholar]
- 33.Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 34.Cui Z, Zhong S, Xu P, He Y, Gong G. PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci. 2013;7:42. doi: 10.3389/fnhum.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang J, Wang X, Xia M, Liao X, Evans A, He Y. GRETNA: a graph theoretical network analysis toolbox for imaging connectomics. Front Hum Neurosci. 2015;9:386. doi: 10.3389/fnhum.2015.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xia M, Wang J, He Y. BrainNet Viewer: a network visualization tool for human brain connectomics. PLoS One. 2013;8:e68910. doi: 10.1371/journal.pone.0068910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006;27:861–874. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 38.Yu Q, Sui J, Liu J, Plis SM, Kiehl KA, Pearlson G, et al. Disrupted correlation between low frequency power and connectivity strength of resting state brain networks in schizophrenia. Schizophr Res. 2013;143:165–171. doi: 10.1016/j.schres.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. doi: 10.1007/s11065-014-9248-7. [DOI] [PubMed] [Google Scholar]
- 40.Gong G, He Y, Concha L, Lebel C, Gross DW, Evans AC, et al. Mapping anatomical connectivity patterns of human cerebral cortex using in vivo diffusion tensor imaging tractography. Cereb Cortex. 2009;19:524–536. doi: 10.1093/cercor/bhn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi F, Yap PT, Gao W, Lin W, Gilmore JH, Shen D. Altered structural connectivity in neonates at genetic risk for schizophrenia: a combined study using morphological and white matter networks. Neuroimage. 2012;62:1622–1633. doi: 10.1016/j.neuroimage.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubinov M, Bullmore E. Schizophrenia and abnormal brain network hubs. Dialogues Clin Neurosci. 2013;15:339–349. doi: 10.31887/DCNS.2013.15.3/mrubinov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao Y, Yan Q, Liu H, Xu L, Xue Z, Song X, et al. Schizophrenic patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res. 2009;114:128–135. doi: 10.1016/j.schres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Camchong J, Lim KO, Sponheim SR, Macdonald AW. Frontal white matter integrity as an endophenotype for schizophrenia: diffusion tensor imaging in monozygotic twins and patients’ nonpsychotic relatives. Front Hum Neurosci. 2009;3:35. doi: 10.3389/neuro.09.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knochel C, O’Dwyer L, Alves G, Reinke B, Magerkurth J, Rotarska-Jagiela A, et al. Association between white matter fiber integrity and subclinical psychotic symptoms in schizophrenic patients and unaffected relatives. Schizophr Res. 2012;140:129–135. doi: 10.1016/j.schres.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 48.Knochel C, Oertel-Knochel V, Schonmeyer R, Rotarska-Jagiela A, van de Ven V, Prvulovic D, et al. Interhemispheric hypoconnectivity in schizophrenia: fiber integrity and volume differences of the corpus callosum in patients and unaffected relatives. Neuroimage. 2012;59:926–934. doi: 10.1016/j.neuroimage.2011.07.088. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt A, Crossley NA, Harrisberger F, Smieskova R, Lenz C, Riecher-Rossler A, et al. Structural network disorganization in subjects at clinical high risk for psychosis. Schizophr Bull. 2017;43:583–591. doi: 10.1093/schbul/sbw110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardno AG, Gottesman II. Twin studies of schizophrenia: from bow-and-arrow concordances to star wars Mx and functional genomics. Am J Med Genet. 2000;97:12–17. doi: 10.1002/(SICI)1096-8628(200021)97:1<12::AID-AJMG3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 51.Kendler KS. The genetics of schizophrenia: chromosomal deletions, attentional disturbances, and spectrum boundaries. Am J Psychiatry. 2003;160:1549–1553. doi: 10.1176/appi.ajp.160.9.1549. [DOI] [PubMed] [Google Scholar]
- 52.Diwadkar VA, Wadehra S, Pruitt P, Keshavan MS, Rajan U, Zajac-Benitez C, et al. Disordered corticolimbic interactions during affective processing in children and adolescents at risk for schizophrenia revealed by functional magnetic resonance imaging and dynamic causal modeling. Arch Gen Psychiatry. 2012;69:231–242. doi: 10.1001/archgenpsychiatry.2011.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glahn DC, Winkler AM, Kochunov P, Almasy L, Duggirala R, Carless MA, et al. Genetic control over the resting brain. Proc Natl Acad Sci U S A. 2010;107:1223–1228. doi: 10.1073/pnas.0909969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fornito A, Zalesky A, Bassett DS, Meunier D, Ellison-Wright I, Yucel M, et al. Genetic influences on cost-efficient organization of human cortical functional networks. J Neurosci. 2011;31:3261–3270. doi: 10.1523/JNEUROSCI.4858-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Heuvel MP, van Soelen IL, Stam CJ, Kahn RS, Boomsma DI, Hulshoff Pol HE. Genetic control of functional brain network efficiency in children. Eur Neuropsychopharmacol. 2013;23:19–23. doi: 10.1016/j.euroneuro.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 56.Hoptman MJ, Nierenberg J, Bertisch HC, Catalano D, Ardekani BA, Branch CA, et al. A DTI study of white matter microstructure in individuals at high genetic risk for schizophrenia. Schizophr Res. 2008;106:115–124. doi: 10.1016/j.schres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 57.van den Heuvel MP, Sporns O. An anatomical substrate for integration among functional networks in human cortex. J Neurosci. 2013;33:14489–14500. doi: 10.1523/JNEUROSCI.2128-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lencz T, Knowles E, Davies G, Guha S, Liewald DC, Starr JM, et al. Molecular genetic evidence for overlap between general cognitive ability and risk for schizophrenia: a report from the Cognitive Genomics consorTium (COGENT) Mol Psychiatry. 2014;19:168–174. doi: 10.1038/mp.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeo RA, Ryman SG, van den Heuvel MP, de Reus MA, Jung RE, Pommy J, et al. Graph metrics of structural brain networks in individuals with schizophrenia and healthy controls: group differences, relationships with intelligence, and genetics. J Int Neuropsychol Soc. 2016;22:240–249. doi: 10.1017/S1355617715000867. [DOI] [PubMed] [Google Scholar]
- 60.Li K, Liu L, Yin Q, Dun W, Xu X, Liu J, et al. Abnormal rich-club organization and impaired correlation between structural and functional connectivity in migraine sufferers. Brain Imaging Behav. 2017;11:526–540. doi: 10.1007/s11682-016-9533-6. [DOI] [PubMed] [Google Scholar]