Abstract
The protein composition of cerebrospinal fluid (CSF) in neural tube defects (NTDs) remains unknown. We investigated the protein composition of CSF from 9 infants with NTDs using isobaric tags for relative and absolute quantitation (iTRAQ). We identified 568 proteins in the CSF of infants with spina bifida, which is the most common type of NTD. Among these, 18 proteins were associated with neural tube closure in the CSF during human embryonic neurulation and 5 were involved in NTDs. Based on these results, an animal model was further utilized to investigate early serum biomarkers for NTDs. We found that the myristoylated alanine-rich C-kinase substrate, Kunitz-type protease inhibitor 2, and apolipoprotein B-100 protein levels were decreased in both embryos and the sera of pregnant Sprague-Dawley rats carrying embryos with NTDs. CSF proteins may be useful in the discovery of potential serum biomarkers for NTDs.
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0154-x) contains supplementary material, which is available to authorized users.
Keywords: Neural tube defects, Serum, Cerebrospinal fluid, Proteome, Biomarkers
Introduction
Neural tube defects (NTDs) result from a failure in neural tube closure between the 3rd and 4th weeks of human gestation; they include anencephaly, encephalocele, myelomeningocele, meningocele, and lipomeningocele [1]. The latter three defects are different types of spina bifida, the most common form of NTD. Maternal serum alpha-fetoprotein has been widely used as a biomarker for NTDs, screened after the 13th week of pregnancy [2]. Unfortunately, there are currently no biomarkers for early screening of NTDs.
Cerebrospinal fluid (CSF) is mainly produced in the ventricular choroid plexus, and drains into the cerebral venous blood. The components of CSF include glucose, amino acids, ions, steroids, fatty acids, several types of cells, and small molecular-weight proteins. The proteins in the CSF originate from the blood and brain. Brain-derived proteins account for ~20% of the total proteins in the CSF [3], so pathological changes in the brain may influence the protein composition of the CSF. Potential protein biomarkers in the CSF have been discovered for the diagnosis of Alzheimer’s and Parkinson’s diseases [4–7].
CSF finally drains into the blood. Since the composition of the CSF is less complex than that of the serum, it may be easier to find biomarkers in CSF, and then to search for biomarkers in serum based on the CSF analysis. However, the protein composition of the CSF of NTDs remains unknown. Our previous study suggested that the isobaric tags for relative and absolute quantitation (iTRAQ) method is a powerful tool for identifying new proteins in CSF [8]. Therefore, we collected CSF from infants with spina bifida and used this technology to investigate its protein composition. An animal model of NTDs was then further used to investigate early serum biomarkers based on our CSF results, in order to provide valuable data for understanding the occurrence of human NTDs and the identification of early indicators for NTDs.
Materials and Methods
Sample Collection
This study was approved by the Ethics Committee of the Medical School of Jinan University. Written consent was given by the parents/guardians of the infants involved. All cases were anonymous. The CSF of 9 hospitalized infants with spina bifida was collected from the Department of Pediatric Surgery, Guangzhou Children’s Hospital, Guangzhou, from September 2007 to October 2011 (Table S1). The infant age ranged from 2 days to 2 months. The CSF was stored at −80 °C until analysis.
Protein Digestion
From each of the 9 samples, 200 μL of CSF was mixed well in one tube. The mixture was equally separated by pipetting into 3 tubes as a triplicate for each sample. The protein content was subsequently determined using the Bradford assay. Protein (100 μg) from each sample was incorporated into STD buffer (4% SDS, 100 mmol/L DTT, and 150 mmol/L Tris-HCl), diluted with UA buffer (8 mol/L urea and 150 mmol/L Tris-HCl), and centrifuged for 15 min. Iodoacetamide (0.05 mol/L) was added to the UA buffer. After 10-min centrifugation, the filters were washed three times. DS buffer (50 mmol/L triethylammonium bicarbonate) was added to the filters. Finally, 2 μg trypsin (Promega Corp., Madison, WI) was added to each filter, and the samples were incubated overnight at 37 °C. The resulting peptides were collected by centrifugation.
iTRAQ Reagent Labeling and Fractionation by Strong Cation Exchange (SCX) Chromatography
iTRAQ labeling was performed according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Briefly, the peptide mixtures were labelled with iTRAQ reagent 113, 115, and 117. The peptide mixtures were subsequently pooled and dried by vacuum centrifugation. Prior to the LC-MS/MS analysis, the peptides were purified from the excess labeling reagent by strong cation exchange (SCX) chromatography using the AKTA Purifier system (GE Healthcare, Watertown, MA). The dried peptide mixture was reconstituted and acidified with buffer A (10 mmol/L KH2PO4 in 25% ACN) and loaded onto a polysulfoethyl column (PolyLC, Inc. Columbia, MD). The peptides were eluted with a 0%–10% buffer B gradient (500 mmol/L KCl and 10 mmol/L KH2PO4 in 25% ACN) for 2 min, 10%–20% buffer B for 25 min, 20%–45% buffer B for 5 min, and 50%–100% buffer B for 5 min. The fractions were collected every minute, and ultimately combined into 10 pools and desalted on C18 Cartridges (Sigma, St. Louis, MO). Each fraction was concentrated by centrifugation and reconstituted in 0.1% (v/v) trifluoroacetic acid.
Liquid Chromatography (LC)-Electrospray Ionization Tandem MS (MS/MS) Analysis by Q Exactive
For each fraction, a 10 μL volume was injected for nano LC-MS/MS analysis using a Q Exactive MS (Thermo Finnigan, San Jose, CA) equipped with an Easy nLC (Proxeon Biosystems, Roskilde, Denmark). The peptide mixture was loaded onto a C18 reversed-phase column packed in-house with RP-C18 5 μm resin in buffer A (0.1% formic acid) and separated with a linear gradient of buffer B (80% acetonitrile, 0.1% formic acid). MS data were acquired using a data-dependent top 10 method that dynamically selected the most abundant precursor ions from the survey scan for HCD fragmentation. The determination of the target value was based on the predictive Automatic Gain Control. The dynamic exclusion duration was 60 s. Survey scans were acquired at a resolution of 70,000 at m/z 200, and the resolution for the HCD spectra was set to 17,500 at m/z 200. The normalized collision energy was 30 eV, and the underfill ratio was defined as 0.1%.
Sequence Database Search and Data Analysis
The MS/MS spectra were searched using the MASCOT engine (version 2.2; Matrix Science, London, UK) against the non-redundant International Protein Index database (http://www.ebi.ac.uk/IPI/IPIhelp.htm) from the European Bioinformatics Institute.
The MASCOT search results for each SCX elution were further processed using the Identified Protein iTRAQ Statistic Builder (Version 3.3.2) provided by the Proteomics Tools Suite (https://github.com/shengqh/RCPA.Tools). All reported data are based on a 99% confidence for protein identification as determined by a false discovery rate (FDR) of ≤1%.
Animal Model of Neural Tube Defects
The animal model of NTDs was established with retinoic acid. Twenty-five pregnant Sprague-Dawley rats (from the Animal Center of Sun-Yat Sen University, Guangzhou, China) were randomly divided into control and drug groups. The drug group was fed a single dose of all trans-retinoic acid (70 mg/kg, Sigma-Aldrich Chemical, St. Louis, MO) dissolved in peanut oil via intragastric administration on E8 (embryonic day 8). The control group was gavage fed with peanut oil. Animals were deeply anaesthetized with pentobarbital sodium salt (50 mg/kg). Venous blood was drawn from the pregnant rats at E11. The serum was separated and stored at −80 °C. Embryos were collected at both E9 and E11, and stored at −80 °C. The pregnant rats that had embryos with or without NTDs were assessed on E15 (Fig. S1). Eight pregnant rats (8 of 15 total) had embryos with NTDs and their sera were chosen as the drug group.
Western Blotting
The rat embryos were lysed by RIPA lysis buffer (Cell Signaling, Danvers, MA), ultrasonicated, and centrifuged at 10,000×g for 10 min. The protein concentrations of the lysates and sera were determined by Bradford assays. Thirty micrograms of lysate or 30 µL of serum was loaded onto 10% SDS-PAGE and separated by gel electrophoresis. The proteins were subsequently transferred onto a polyvinylidene fluoride membrane. The membranes were incubated with primary antibodies against myristoylated alanine-rich C-kinase substrate (MARCKS, 1:10000, Abcam, Cambridge, MA), profilin 1 (PNF1, 1:10000, Abcam), kunitz-type protease inhibitor 2 (SPINT2, 1:1000, Abcam), and apolipoprotein B-100 (APOB, 1:200, Santa Cruz Biotechnology, Dallas, TX) overnight and subsequently incubated with anti-human IgG (1:5000, Bioworld, Louis Park, MN) for 1 h. The immune complexes were detected by ECL (Cell Signaling Technologies, Danvers, MD).
Enzyme-Linked Immunosorbent Assay (ELISA)
An ELISA kit (Shanghai Enzyme-linked Biotechnology Co., Ltd., Shanghai, China) was used to measure the thioredoxin 2 (Trx2) level. The procedure was performed following the manufacturer’s instructions. Each serum sample was run in duplicate. Briefly, the control, calibration, and serum samples were incubated with the sample dilution buffer for 30 min at 37 °C. An antibody-coated 96-well plate was washed 5 times and incubated with HRP-labeled solution for 30 min at 37 °C. After five washes, the plates were incubated with tetramethylbenzidine solution for 10 min. The absorbance in each well was determined using a microplate reader set to 450 nm. A standard curve was generated with the provided standards and used to calculate the Trx2 concentration (ng/mL) in each serum sample.
Statistical Analysis
The data are presented as the mean ± SD, and compared by one-way analysis of variance (ANOVA) followed by a Student-Newman-Keuls test with SPSS 11.0 software (IBM Corporation, New York). Statistical significance was defined as P < 0.05.
Results
Global Proteome Profile of CSF from Infants with Spina Bifida
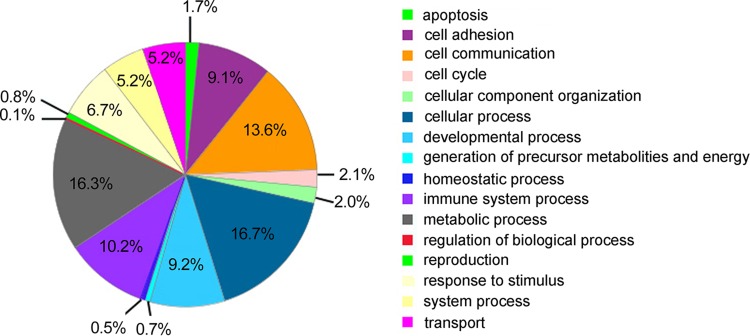
Five hundred sixty-eight proteins were identified from the CSF of infants with spina bifida at 1% FDR using iTRAQ analysis (Table S2). The theoretical isoelectric point values of these proteins ranged from 4.26 to 11.36. They varied from 9,368.06 to 572,016.68 Da in molecular weight (MW). The protein with the lowest MW was Ly-6/neurotoxin-like protein 1, while that with the highest MW was IgG Fc-binding protein. Using a protein analysis for evolutionary relationships (Los Angeles, CA; PANTHER, version 9.0) which were categorized based on the Gene Ontology (GO) biological process database, the proteins were grouped into 16 classes (Fig. 1). Most of the identified proteins belonged to the cellular process (16.7%), metabolic process (16.3%), cell communication (13.6%), immune system process (10.2%), developmental process (9.2%), and cell adhesion (9.1%) categories, and 21 were uncategorized.
Fig. 1.
GO biological annotation of the identified proteins in the CSF of infants with NTDs. The segments indicate the percentage of the corresponding GO term to the total number of annotated proteins.
CSF Proteins Involved in Neural Tube Closure during Human Embryonic Development
We searched the literature in the PubMed database and only identified one paper that investigated the genes involved in neural tube closure during human embryonic neurulation [9]. We then examined the CSF proteins encoded by these genes. We identified 18 CSF proteins with genes involved in neural tube closure (Table 1). Of these, ACTG1, GSN, and PFN1 are cytoskeletal proteins. SKP1 participates in the Wnt and TGF-β signaling pathways. CALM2 is a Ca2+-binding protein. ALCAM, CNTN1, CNTN2, HLA-A, NCAM1, NEO1, and VCAN are cell-adhesion molecules. NME1 and PKM2 regulate purine metabolism. LDHA and LDHB are involved in cysteine and methionine metabolism. IGF2, which is a major fetal growth factor, shares sequence homology with insulin. TPT1 is involved in Ca2+-binding and microtubule stabilization.
Table 1.
CSF proteins and genes associated with neural tube closure.
| Number | Protein ID | Gene name | Protein name |
|---|---|---|---|
| 1 | IPI00021440.1 | ACTG1 | Actin, cytoplasmic 2 |
| 2 | IPI00026314.1 | GSN | Isoform 1 of gelsolin |
| 3 | IPI00216691.5 | PFN1 | Profilin-1 |
| 4 | IPI00974176.1 | SKP1 | S-phase kinase-associated protein 1 |
| 5 | IPI00916600.1 | CALM2 | Calmodulin |
| 6 | IPI00944977.1 | ALCAM | CD166 antigen |
| 7 | IPI00029751.1 | CNTN1 | Isoform 1 of contactin-1 |
| 8 | IPI00024966.1 | CNTN2 | Contactin-2 |
| 9 | IPI00816779.1 | HLA-A; HLA-B; HLA-C | Leukocyte antigen |
| 10 | IPI00795918.1 | NCAM1 | Isoform 1 of neural cell adhesion molecule 1 |
| 11 | IPI00472011.1 | NEO1 | Neogenin isoform 2 precursor |
| 12 | IPI00009802.1 | VCAN | Isoform V0 of versican core protein |
| 13 | IPI00795292.1 | NME1;NME2 | Isoform 3 of nucleoside diphosphate kinase B |
| 14 | IPI01018161.1 | PKM2 | Pyruvate kinase isozymes M1/M2 isoform c |
| 15 | IPI00947127.1 | LDHA | L-lactate dehydrogenase A chain isoform 3 |
| 16 | IPI00219217.3 | LDHB | L-lactate dehydrogenase B chain |
| 17 | IPI00940065.1 | IGF2; INS-IGF2 | Isoform 2 of insulin-like growth factor II |
| 18 | IPI00009943.2 | TPT1 | Translationally controlled tumor protein |
CSF Proteins Involved in NTDs
Furthermore, we searched for genes that cause NTDs in the PubMed database and examined the CSF proteins encoded by these genes in our data. We identified five proteins with genes involved in NTDs: MARCKS, PFN1, TRX2, SPINT2, APOB (Table 2). Marcks, Pfn1, and Trx2 are involved in exencephaly in mice. Spint2 is linked to exencephaly and spina bifida in mice, whereas Apob is associated with exencephaly and hydrocephaly.
Table 2.
CSF proteins with genes involved in NTDs.
| Protein ID | Gene name | Protein name | NTD type | References |
|---|---|---|---|---|
| IPI00219301.7 | Marcks | Myristoylated alanine-rich C-kinase substrate | Exencephaly | [10] |
| IPI00216691.5 | Pfn1 | Profilin-1 | Exencephaly | [11] |
| IPI00552768.1 | Trx2 | Thioredoxin (mitochondrial) | Exencephaly | [12] |
| IPI00011662.1 | Spint2 | Kunitz-type protease inhibitor 2 | Exencephaly, spina bifida | [13] |
| IPI00022229.2 | Apob | Apolipoprotein B-100 | Exencephaly hydrocephaly | [14] |
CSF Proteins Involved in NTDs Identified in the Sera of Pregnant Rats
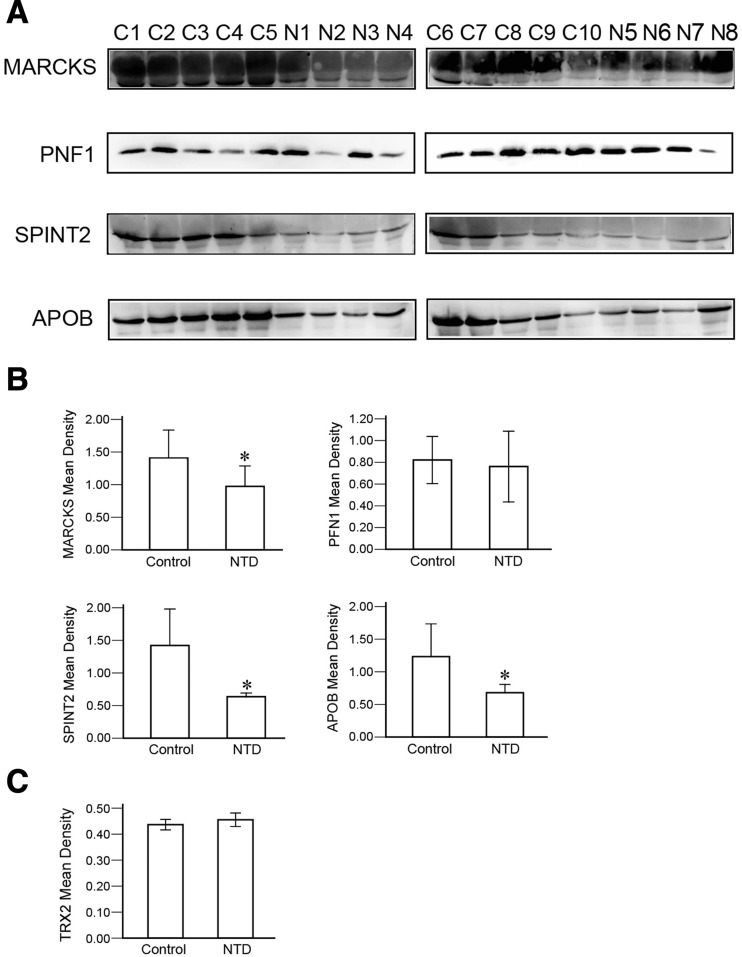
We identified the expression of CSF proteins involved in NTDs in maternal serum and further assessed their levels in the sera of pregnant rats that had embryos with NTDs at E11 (Fig. 2). The expression of MARCKS, SPINT2, and APOB in the NTD group was lower than in control (P < 0.05). However, no significant differences were identified in the serum levels of PNF1 or TRX2 between the control and NTD groups.
Fig. 2.
Changes in CSF proteins involved in NTDs in the serum of pregnant rats with NTDs. A Western blots of serum MARCKS, PNF1, SPINT2, and APOB levels in control and NTD groups. C, control group (n = 10); N, NTD group (n = 8). B Histograms of the quantitative analysis of the MARCKS, PNF1, SPINT2, and APOB expression using western blot (*P < 0.05 compared with the control group, ANOVA). C Quantitative analysis of the serum TRX2 levels in control and NTD groups using ELISA (ANOVA).
Serum Protein Alterations Identified in Rat Embryos
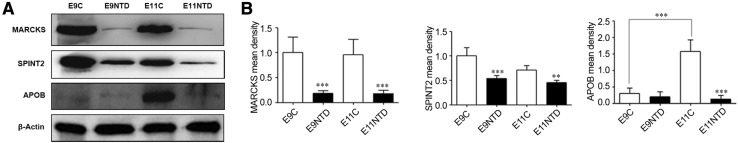
We further investigated whether the MARCKS, SPINT2, and APOB levels were changed in rat embryos. The expression of MARCKS and SPINT2 was lower in the NTD group at both E9 and E11 than in the control group (Fig. 3A). However, no differences in the expression of MARCKS and SPINT2 were found between E9 and E11 (Fig. 3B). The expression of APOB was low in both control and NTD groups at E9 (Fig. 3A). There was no difference in the expression of APOB between the control and the NTD group at E9 (Fig. 3B). However, the expression of APOB was significantly increased in the control group at E11 (Fig. 3A). Compared to the control group, the expression of APOB was still lower in the NTD group at E11 (Fig. 3A, B).
Fig. 3.
Changes of MARCKS, SPINT2 and APOB levels in the rat embryos with NTDs. A MARCKS, SPINT2 and APOB levels in the control and NTD groups assessed with western blot (n = 3). E9C and E11C represent E9 and E11 in the control group, respectively. E9NTD and E11NTD represent E9 and E11 in the NTD group, respectively. B Histogram of the quantitative analysis of western blot with ANOVA. **P < 0.01, ***P < 0.001 compared with the control group.
Discussion
To our knowledge, this investigation is the first proteomic study of the CSF of infants with NTDs. We identified 568 proteins from the CSF of infants with spina bifida. According to the GO biological processes, the proteins were classified into 16 categories: apoptosis, cell adhesion, cell communication, cell cycle, cell component organization, cellular process, developmental process, generation of precursor metabolites and energy, homeostatic process, immune system process, metabolic process, regulation of biological process, reproduction, response to stimulus, system process, and transport.
Neural tube closure is a multifactorial process that involves many molecular mechanisms. We searched the gene literature in the PubMed database for genes involved in human neural tube closure and identified 18 CSF proteins encoded by these genes in our study. Of these proteins, ACTG1, GSN, and PFN1 are cytoskeletal proteins associated with bending and closure of the neural folds [15]. It is worth noting that the 18 CSF proteins include the cell adhesion molecules ALCAM, CNTN1, CNTN2, HLA-A, NCAM1, NEO1, and VCAN. Cell adhesion molecules are critical for cell migration, cell-cell recognition, and adhesion during morphogenesis [16, 17]. The neural tube is formed by the contraction and fusion of the neural folds at the dorsal midline. Thus, the adhesion of apposed neural folds is necessary for neural tube closure [18].
Other CSF proteins involved in human neural tube closure include SKP1, CALM2, NME1, PKM2, LDHA, LDHB, IGF2, and TPT1. SKP1 is a component of the SCF (SKP1-CUL1-F-box protein) ubiquitin ligase complex which drives caudal neural tube closure and body axis elongation through the elimination of beta-catenin [19]. CALM2 is a Ca2+-binding protein, and contributes to neural tube closure by binding to nitric oxide synthase [20]. NME1 and PKM2 regulate purine metabolism. Impairment of purine synthesis leads to abnormal neural tube closure [21]. LDHA and LDHB are involved in methionine metabolism. Methionine is demethylated to form homocysteine, and high homocysteine levels in maternal blood have been associated with NTDs [22], so LDHA and LDHB are indirectly associated with neural tube development. IGF2 is a major fetal growth factor which is weakly expressed in the neural tube [23]. Hypermethylation of IGF2 DMR0 is associated with an increased risk of NTDs [24]. TPT1 is involved in Ca2+ binding and microtubule stabilization [25]. Krupp et al. reported that the TPT1 gene is differentially expressed during the development of neural tube tissues from human embryos.
Moreover, we searched for genes that cause NTDs in the PubMed database and examined the CSF proteins encoded by these genes, among which Marcks, Pfn1, Trx2, Spint2, and Apob are associated with NTDs. Specifically, Marcks, Pfn1, and Trx2 are involved in exencephaly in mice [10–12]. Spint2 is linked to exencephaly and spina bifida [13]. Apob is associated with exencephaly and hydrocephaly [14]. We also identified 5 proteins encoded by these genes in our CSF data: MARCKS, PFN1, TRX2, SPINT2, and APOB. Because it is not possible to collect CSF from normal infants, we had to establish an animal model of NTDs. Moreover, the CSF proteins were extracted from the blood and brain tissues. There is an intensive exchange of substances between the maternal and embryonic blood through the placenta. Therefore, we further assessed the expression of five proteins in the sera of the pregnant rats that had embryos with NTDs, expecting to reveal novel biomarkers as a predictor of NTDs. Our results demonstrated that the serum level of MARCKS, SPINT2, and APOB in the NTD group were significantly lower than the control. However, no change was identified in the serum PNF1 or TRX2 between the control and NTD groups.
MARCKS is a cellular substrate for protein kinase C, which plays an important role in the normal developmental processes of neurulation. MARCKS is highly expressed in the neural plate during chicken embryo development [26]. We also found that the MARCKS level was high in rat embryos at E9 and E11. However, the expression of MARCKS l was reduced in the rat embryos with NTDs at both E9 and E11. Similar to our results, a 25% high frequency of exencephaly has been demonstrated in MARCKS-deficient mice [10]. Moreover, our results showed that the serum MARCKS expression was lower in the rats pregnant with NTD embryos. These results suggest that MARCKS is important for development of the neural tube, and lower MARCKS expression may result in NTDs.
For the first time, we report that SPINT2 is present in the CSF. SPINT2 is an inhibitor of hepatocyte growth factor activation [27], which is essential to maintain neural tube closure. The gene for SPINT2 is abundant in the neuroepithelium of the cranial region and caudal extremity of the mouse embryo in the developing neural tube [13]. Ninety-six percent of the Spint2-deficient embryos demonstrated incomplete closure of the neural tube in the cranial region, showing as exencephaly [13]. One Spint2-deficient embryo exhibited an incomplete closure of the neural tube in the back region (spina bifida). Our results showed that SPINT2 expression was reduced in the rat embryos with NTDs, and the serum SPINT2 level was also decreased in the pregnant rats that had embryos with NTDs. Neural tube closure in the mouse begins at E8.5 and is completed at E10 [28]. We obtained rat embryos at E9 and E11, and the maternal blood of pregnant rats on E11. In addition, one important finding in our study is that SPINT2 expression was reduced in rat embryos with NTDs, and the sera of pregnant rats with NTD embryos, at the same time. Therefore, SPINT2 may be a promising candidate early biomarker of NTDs.
APOB, a major low-density lipoprotein, is involved in the transport and metabolism of cholesterol, lipids, and vitamin E in the blood. Mice with an Apob gene mutation are approximately 30% exencephalic, which have closed neural tubes [14]. Homozygous Apob mutant mice have significantly reduced plasma vitamin E concentrations. Vitamin E is an antioxidant essential for the development of the nervous system. Vitamin E deficiency in rats produces offspring with hydrocephalus and exencephalus [29, 30]. However, exencephalus and hydrocephalus have not been reported in patients with the Apob gene mutation causing familial hypobetalipoproteinemia [31, 32]. This difference between mice and humans may result from APOB levels, since hypobetalipoproteinemia patients still have plasma APOB concentrations that are 1/4 to 1/3 of normal; APOB is not absent from the plasma of patients. Interestingly, we found that the APOB level was very low in rat embryos at E9, and high at E11. Our results showed that APOB concentrations were greatly increased during neural tube closure, so APOB may contribute to this process. In addition, we found that the serum APOB level was significantly decreased in the rats pregnant with NTD embryos. Taken together, we speculate that low APOB expression may be associated with an increased risk of NTD.
PFN1, an actin-binding protein, is a high-affinity ligand for Mena [33]. Lanier reported that Mena-deficient heterozygous mice with a Pfn1 deletion have a failure of cephalic neural tube closure and develop with exencephaly or anencephaly [11]. These findings suggested that an interaction between Mena and PFN1 is required in neural tube closure. However, we did not find that the serum PFN1 level was significantly changed in the rats pregnant with NTD embryos. A previous study has shown that PFN1 occurs in many tissues, and is less abundant in brain [34]. So a change in the expression of PFN1 in the nervous system may have no effect on the serum PFN1 level.
Thioredoxin is a redox protein that has cytosolic (TRX1) and mitochondrial (TRX2) isoforms. Trx2-deficient embryos exhibit an open anterior neural tube [12] and massive apoptosis. Tanaka reported that Trx2-deficient cells show increased reactive oxygen species and apoptosis [35]. However, we did not find a significant change of the serum TRX2 level in the rats pregnant with NTD embryos which may be because that TRX2 in the nervous system may not be released into the CSF and serum as it is a mitochondrial protein.
A limitation of the present study is that the potential serum biomarkers (MARCKS, SPINT2, and APOB) of NTDs in the rats were not examined in human maternal serum. Given that there are genetic and environmental differences between animal models and human diseases, while many biomarkers found in animal models have been further confirmed in patients with a variety of different diseases including neurodegenerative, cancer, and inflammatory diseases [36–39], there are also a few reports that animal biomarkers are not or partly consistent with those in patients [40, 41]. Thus, in the future it is necessary to confirm the potential serum biomarkers (MARCKS, SPINT2, and APOB) in the serum of pregnant women carrying fetuses with NTDs.
Conclusions
We provide a proteomic profile of the CSF of infants with NTDs and analyze 18 CSF proteins that are involved in neural tube closure during human embryonic development. Furthermore, 5 CSF proteins are suggested to be associated with the occurrence of NTDs. Of the 5 CSF proteins, MARCKS, SPINT2, and APOB exhibited decreased levels in both sera and embryos of rats pregnant with NTD embryos. Because NTDs have multiple etiologies and mechanisms, the combination of several biomarkers may have high diagnostic accuracy for NTDs compared to using a single biomarker. Further research is needed to assess our results with MARCKS, SPINT2, and APOB proteins in human maternal serum and also to determine their potential role as biomarkers of NTDs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Development and Innovation Foundation of Jinan University, Guangdong Province, China (21611613) and the National Natural Science Foundation of China (81271280).
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12264-017-0154-x) contains supplementary material, which is available to authorized users.
Contributor Information
Lian Huang, Email: tlian@jnu.edu.cn.
Sanqiang Pan, Email: tpsq@jnu.edu.cn.
References
- 1.Au KS, Ashley-Koch A, Northrup H. Epidemiologic and genetic aspects of spina bifida and other neural tube defects. Dev Disabil Res Rev. 2010;16:6–15. doi: 10.1002/ddrr.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ZP, Li H, Hao LZ, Zhao ZT. The effectiveness of prenatal serum biomarker screening for neural tube defects in second trimester pregnant women: a meta-analysis. Prenat Diagn. 2009;29:960–965. doi: 10.1002/pd.2325. [DOI] [PubMed] [Google Scholar]
- 3.Thompson EJ. Cerebrospinal fluid. J Neurol Neurosurg Psychiatry. 1995;59:349–357. doi: 10.1136/jnnp.59.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- 5.Shi M, Bradner J, Hancock AM, Chung KA, Quinn JF, Peskind ER, et al. Cerebrospinal fluid biomarkers for Parkinson disease diagnosis and progression. Ann Neurol. 2011;69:570–580. doi: 10.1002/ana.22311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Zheng T, Zhang B. Exosomes in Parkinson’s disease. Neurosci Bull. 2017;33:331–338. doi: 10.1007/s12264-016-0092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sui X, Liu J, Yang X. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Neurosci Bull. 2014;30:233–242. doi: 10.1007/s12264-013-1412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Qian J, Borisov O, Pan S, Li Y, Liu T, et al. Optimized proteomic analysis of a mouse model of cerebellar dysfunction using amine-specific isobaric tags. Proteomics. 2006;6:4321–4334. doi: 10.1002/pmic.200600026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupp DR, Xu PT, Thomas S, Dellinger A, Etchevers HC, Vekemans M, et al. Transcriptome profiling of genes involved in neural tube closure during human embryonic development using long serial analysis of gene expression (long-SAGE). Birth Defects Res A Clin Mol Teratol 2012, 94: 683–692. [DOI] [PMC free article] [PubMed]
- 10.Stumpo DJ, Bock CB, Tuttle JS, Blackshear PJ. MARCKS deficiency in mice leads to abnormal brain development and perinatal death. Proc Natl Acad Sci U S A. 1995;92:944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lanier LM, Gates MA, Witke W, Menzies AS, Wehman AM, Macklis JD, et al. Mena is required for neurulation and commissure formation. Neuron. 1999;22:313–325. doi: 10.1016/S0896-6273(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 12.Nonn L, Williams RR, Erickson RP, Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136:2653–2663. doi: 10.1242/dev.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homanics GE, Maeda N, Traber MG, Kayden HJ, Dehart DB, Sulik KK. Exencephaly and hydrocephaly in mice with targeted modification of the apolipoprotein B (Apob) gene. Teratology. 1995;51:1–10. doi: 10.1002/tera.1420510102. [DOI] [PubMed] [Google Scholar]
- 15.Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev Dyn. 1999;215:273–283. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 16.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 17.Winther M, Walmod PS. Neural cell adhesion molecules belonging to the family of leucine-rich repeat proteins. Adv Neurobio. 2014;8:315–395. doi: 10.1007/978-1-4614-8090-7_14. [DOI] [PubMed] [Google Scholar]
- 18.Greene ND, Copp AJ. Development of the vertebrate central nervous system: formation of the neural tube. Prenat Diagn. 2009;29:303–311. doi: 10.1002/pd.2206. [DOI] [PubMed] [Google Scholar]
- 19.Su Y, Ishikawa S, Kojima M, Liu B. Eradication of pathogenic beta-catenin by Skp1/Cullin/F box ubiquitination machinery. Proc Natl Acad Sci U S A. 2003;100:12729–12734. doi: 10.1073/pnas.2133261100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nachmany A, Gold V, Tsur A, Arad D, Weil M. Neural tube closure depends on nitric oxide synthase activity. J Neurochem. 2006;96:247–253. doi: 10.1111/j.1471-4159.2005.03542.x. [DOI] [PubMed] [Google Scholar]
- 21.Christensen KE, Deng L, Leung KY, Arning E, Bottiglieri T, Malysheva OV, et al. A novel mouse model for genetic variation in 10-formyltetrahydrofolate synthetase exhibits disturbed purine synthesis with impacts on pregnancy and embryonic development. Hum Mol Genet. 2013;22:3705–3719. doi: 10.1093/hmg/ddt223. [DOI] [PubMed] [Google Scholar]
- 22.Mills JL, McPartlin JM, Kirke PN, Lee YJ, Conley MR, Weir DG, et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345:149–151. doi: 10.1016/S0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 23.Ralphs JR, Wylie L, Hill DJ. Distribution of insulin-like growth factor peptides in the developing chick embryo. Development. 1990;109:51–58. doi: 10.1242/dev.109.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Wang L, Shangguan S, Chang S, Wang Z, Lu X, et al. Altered methylation of IGF2 DMR0 is associated with neural tube defects. Mol Cell Biochem. 2013;380:33–42. doi: 10.1007/s11010-013-1655-1. [DOI] [PubMed] [Google Scholar]
- 25.Bazile F, Pascal A, Arnal I, Le Clainche C, Chesnel F, Kubiak JZ. Complex relationship between TCTP, microtubules and actin microfilaments regulates cell shape in normal and cancer cells. Carcinogenesis. 2009;30:555–565. doi: 10.1093/carcin/bgp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolessi FR, Arruti C. Apical accumulation of MARCKS in neural plate cells during neurulation in the chick embryo. BMC Dev Biol. 2001;1:7. doi: 10.1186/1471-213X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi T, Qin L, Shimomura T, Kondo J, Matsumoto K, Denda K, et al. Purification and cloning of hepatocyte growth factor activator inhibitor type 2, a Kunitz-type serine protease inhibitor. J Biol Chem. 1997;272:27558–27564. doi: 10.1074/jbc.272.44.27558. [DOI] [PubMed] [Google Scholar]
- 28.Copp AJ, Greene ND. Neural tube defects–disorders of neurulation and related embryonic processes. Wiley Interdiscip Rev Dev Biol. 2013;2:213–227. doi: 10.1002/wdev.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verma K, Wei KD. Disorders of the developing nervous system of vitamin E-deficient rats. Acta Anat (Basel) 1967;67:623–635. doi: 10.1159/000143009. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishna T. Vitamins and brain development. Physiol Res. 1999;48:175–187. [PubMed] [Google Scholar]
- 31.Burnett JR, Zhong S, Jiang ZG, Hooper AJ, Fisher EA, McLeod RS, et al. Missense mutations in APOB within the betaalpha1 domain of human APOB-100 result in impaired secretion of ApoB and ApoB-containing lipoproteins in familial hypobetalipoproteinemia. J Bio Chem. 2007;282:24270–24283. doi: 10.1074/jbc.M702442200. [DOI] [PubMed] [Google Scholar]
- 32.Gangloff A, Bergeron J, Couture P, Martins R, Hegele RA, Gagné C. A novel mutation of apolipoprotein B in a French Canadian family with homozygous hypobetalipoproteinemia. J Clin Lipidol. 2011;5:414–417. doi: 10.1016/j.jacl.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 33.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87:227–239. doi: 10.1016/S0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski DJ, Bruns GA. Human profilin. Molecular cloning, sequence comparison, and chromosomal analysis. J Biol Chem. 1988;263:5910–5915. [PubMed] [Google Scholar]
- 35.Tanaka T, Hosoi F, Yamaguchi-Iwai Y, Nakamura H, Masutani H, Ueda S, et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO. 2002;21:1695–1703. doi: 10.1093/emboj/21.7.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rose C, Peoc’h K, Chasseigneaux S, Paquet C, Dumurgier J, Bourasset F, et al. New highly sensitive rodent and human tests for soluble amyloid precursor protein alpha quantification: preclinical and clinical applications in Alzheimer’s disease. BMC Neurosci. 2012;13:84. doi: 10.1186/1471-2202-13-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shao S, Li Z, Gao W, Yu G, Liu D, Pan F. ADAM-12 as a diagnostic marker for the proliferation, migration and invasion in patients with small cell lung cancer. PLoS One. 2014;9:e85936. doi: 10.1371/journal.pone.0085936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Gao G, Cai J, Wang Y, Qu X, He L, et al. Annexin A2 is a discriminative serological candidate in early hepatocellular carcinoma. Carcinogenesis. 2013;34:595–604. doi: 10.1093/carcin/bgs372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Román ID, CanoMartínez D, Lobo MV, FernándezMoreno MD, Hernández-Breijo B, Sacristán S, et al. Infliximab therapy reverses the increase of allograft inflammatory factor-1 in serum and colonic mucosa of rats with inflammatory bowel disease. Biomarkers. 2017;22:133–144. doi: 10.1080/1354750X.2016.1252950. [DOI] [PubMed] [Google Scholar]
- 40.Oze I, Shimada S, Nagasaki H, Akiyama Y, Watanabe M, Yatabe Y, et al. Plasma microRNA-103, microRNA-107, and microRNA-194 levels are not biomarkers for human diffuse gastric cancer. J Cancer Res Clin Oncol. 2017;143:551–554. doi: 10.1007/s00432-016-2316-z. [DOI] [PubMed] [Google Scholar]
- 41.Tomasik J, Schultz TL, Kluge W, Yolken RH, Bahn S, Carruthers VB. Shared immune and repair markers during experimental toxoplasma chronic brain infection and schizophrenia. Schizophr Bull. 2016;42:386–395. doi: 10.1093/schbul/sbv134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.