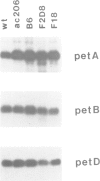
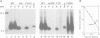Abstract
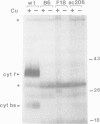
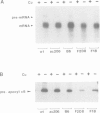
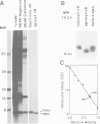
Cytochrome c6 functions in the thylakoid lumen to catalyze electron transfer from reduced cytochrome f of the cytochrome b6f complex to P700+ of photosystem I. The biogenesis of mature cyt c6 from cytosolically translated pre-apocytochrome c6 involves numerous post-translational modifications including the proteolytic removal of a transit sequence and the covalent attachment of heme to two cysteinyl thiols on the apoprotein. Here, we report on the characterization of a previously unrecognized class of non-allelic mutants of Chlamydomonas reinhardtii that are blocked at the conversion of apocyt c6 to holocyt c6. The mutants are acetate requiring since they are also deficient in cyt f, cyt b and the Rieske FeS protein. Pulse-chase studies indicate that heme attachment is not required for the two-step processing of pre-apocytochrome c6 to apocyt c6, but is required for the stability of the mature protein. This is in contrast to the biosynthesis of mitochondrial cyt c1 where heme attachment is required for the second processing step. We propose that the assembly of both holocytochrome c6 and the cytochrome b6f complex are dependent on common gene products, possibly those involved in heme delivery or metabolism. This is the first suggestion that multiple loci are involved in the biosynthesis of both plastidic c-type cytochromes.
Full text
PDF


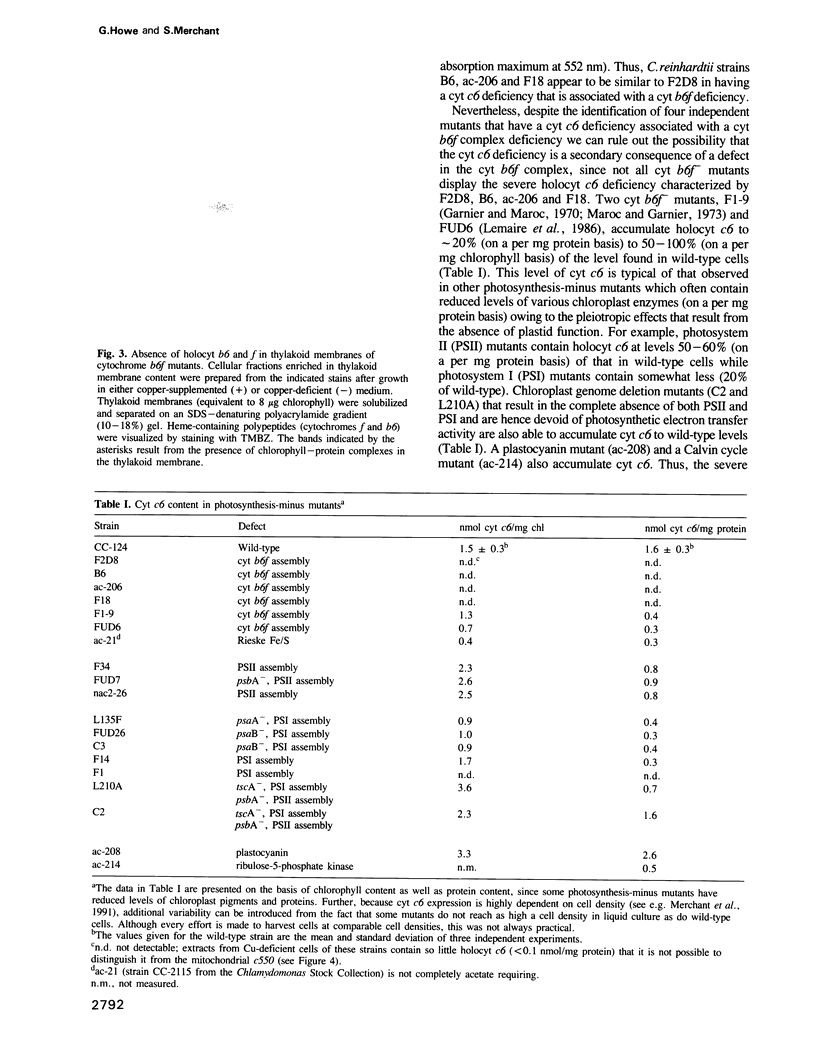
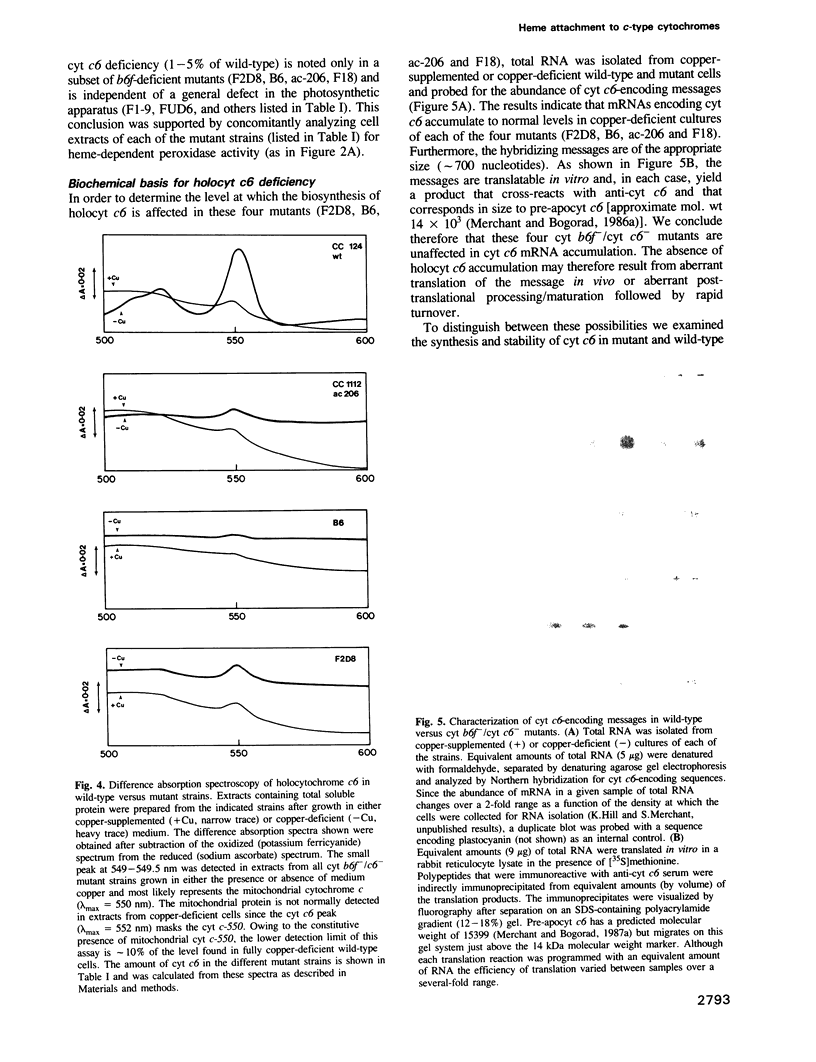







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alscher R., Patterson R., Jagendorf A. T. Activity of Thylakoid-bound Ribosomes in Pea Chloroplasts. Plant Physiol. 1978 Jul;62(1):88–93. doi: 10.1104/pp.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atteia A., de Vitry C., Pierre Y., Popot J. L. Identification of mitochondrial proteins in membrane preparations from Chlamydomonas reinhardtii. J Biol Chem. 1992 Jan 5;267(1):226–234. [PubMed] [Google Scholar]
- Bauerle C., Dorl J., Keegstra K. Kinetic analysis of the transport of thylakoid lumenal proteins in experiments using intact chloroplasts. J Biol Chem. 1991 Mar 25;266(9):5884–5890. [PubMed] [Google Scholar]
- Bauerle C., Keegstra K. Full-length plastocyanin precursor is translocated across isolated thylakoid membranes. J Biol Chem. 1991 Mar 25;266(9):5876–5883. [PubMed] [Google Scholar]
- Beckman D. L., Trawick D. R., Kranz R. G. Bacterial cytochromes c biogenesis. Genes Dev. 1992 Feb;6(2):268–283. doi: 10.1101/gad.6.2.268. [DOI] [PubMed] [Google Scholar]
- Bohner H., Böger P. Reciprocal formation of cytochrome c-553 and plastocyanin in Scenedesmus. FEBS Lett. 1978 Jan 15;85(2):337–339. doi: 10.1016/0014-5793(78)80486-4. [DOI] [PubMed] [Google Scholar]
- Bohner H., Merkle H., Kroneck P., Böger P. High variability of the electron carrier plastocyanin in microalgae. Eur J Biochem. 1980 Apr;105(3):603–609. doi: 10.1111/j.1432-1033.1980.tb04538.x. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brandner J. P., Stabb E. V., Temme R., Donohue T. J. Regions of Rhodobacter sphaeroides cytochrome c2 required for export, heme attachment, and function. J Bacteriol. 1991 Jul;173(13):3958–3965. doi: 10.1128/jb.173.13.3958-3965.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellone M. D., Wu M. A., Tzagoloff A. Assembly of the mitochondrial membrane system. Analysis of structural mutants of the yeast coenzyme QH2-cytochrome c reductase complex. J Biol Chem. 1988 Oct 5;263(28):14323–14333. [PubMed] [Google Scholar]
- Dumont M. D., Mathews A. J., Nall B. T., Baim S. B., Eustice D. C., Sherman F. Differential stability of two apo-isocytochromes c in the yeast Saccharomyces cerevisiae. J Biol Chem. 1990 Feb 15;265(5):2733–2739. [PubMed] [Google Scholar]
- Dumont M. E., Ernst J. F., Sherman F. Coupling of heme attachment to import of cytochrome c into yeast mitochondria. Studies with heme lyase-deficient mitochondria and altered apocytochromes c. J Biol Chem. 1988 Nov 5;263(31):15928–15937. [PubMed] [Google Scholar]
- Fisher W. R., Taniuchi H., Anfinsen C. B. On the role of heme in the formation of the structure of cytochrome c. J Biol Chem. 1973 May 10;248(9):3188–3195. [PubMed] [Google Scholar]
- Franzén L. G., Frank G., Zuber H., Rochaix J. D. Isolation and characterization of cDNA clones encoding photosystem I subunits with molecular masses 11.0, 10.0 and 8.4 kDa from Chlamydomonas reinhardtii. Mol Gen Genet. 1989 Oct;219(1-2):137–144. doi: 10.1007/BF00261169. [DOI] [PubMed] [Google Scholar]
- Garnier J., Maroc J. Recherche de plusieurs transporteurs d'électrons, notamment des cytochromes b-559 et c-553, chez trois mutants non photosynthétiques de Chlamydomonas reinhardi. Biochim Biophys Acta. 1970;205(2):205–219. doi: 10.1016/0005-2728(70)90251-3. [DOI] [PubMed] [Google Scholar]
- Gerhus E., Steinrücke P., Ludwig B. Paracoccus denitrificans cytochrome c1 gene replacement mutants. J Bacteriol. 1990 May;172(5):2392–2400. doi: 10.1128/jb.172.5.2392-2400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi VI. Electron Transport in Mutant Strains Lacking Either Cytochrome 553 or Plastocyanin. Plant Physiol. 1966 Dec;41(10):1648–1656. doi: 10.1104/pp.41.10.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Photosynthetic Electron Transport Chain of Chlamydomonas reinhardi. IV. Purification and Properties of Plastocyanin. Plant Physiol. 1966 Dec;41(10):1637–1642. doi: 10.1104/pp.41.10.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartl F. U., Neupert W. Protein sorting to mitochondria: evolutionary conservations of folding and assembly. Science. 1990 Feb 23;247(4945):930–938. doi: 10.1126/science.2406905. [DOI] [PubMed] [Google Scholar]
- Hauska G., Hurt E., Gabellini N., Lockau W. Comparative aspects of quinol-cytochrome c/plastocyanin oxidoreductases. Biochim Biophys Acta. 1983 Jul 15;726(2):97–133. doi: 10.1016/0304-4173(83)90002-2. [DOI] [PubMed] [Google Scholar]
- Kirwin P. M., Elderfield P. D., Williams R. S., Robinson C. Transport of proteins into chloroplasts. Organization, orientation, and lateral distribution of the plastocyanin processing peptidase in the thylakoid network. J Biol Chem. 1988 Dec 5;263(34):18128–18132. [PubMed] [Google Scholar]
- Konishi K., Van Doren S. R., Kramer D. M., Crofts A. R., Gennis R. B. Preparation and characterization of the water-soluble heme-binding domain of cytochrome c1 from the Rhodobacter sphaeroides bc1 complex. J Biol Chem. 1991 Aug 5;266(22):14270–14276. [PubMed] [Google Scholar]
- Kranz R. G. Isolation of mutants and genes involved in cytochromes c biosynthesis in Rhodobacter capsulatus. J Bacteriol. 1989 Jan;171(1):456–464. doi: 10.1128/jb.171.1.456-464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li H. M., Theg S. M., Bauerle C. M., Keegstra K. Metal-ion-center assembly of ferredoxin and plastocyanin in isolated chloroplasts. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6748–6752. doi: 10.1073/pnas.87.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroc J., Garnier J. Recherche du cytochrome b-563 et du P700 chez trois mutants non photosynthetiques de Chlamydomonas reinhardti. Biochim Biophys Acta. 1973 Feb 22;292(2):477–490. doi: 10.1016/0005-2728(73)90052-2. [DOI] [PubMed] [Google Scholar]
- Mathews F. S. The structure, function and evolution of cytochromes. Prog Biophys Mol Biol. 1985;45(1):1–56. doi: 10.1016/0079-6107(85)90004-5. [DOI] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Metal ion regulated gene expression: use of a plastocyanin-less mutant of Chlamydomonas reinhardtii to study the Cu(II)-dependent expression of cytochrome c-552. EMBO J. 1987 Sep;6(9):2531–2535. doi: 10.1002/j.1460-2075.1987.tb02540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Rapid degradation of apoplastocyanin in Cu(II)-deficient cells of Chlamydomonas reinhardtii. J Biol Chem. 1986 Dec 5;261(34):15850–15853. [PubMed] [Google Scholar]
- Merchant S., Bogorad L. Regulation by copper of the expression of plastocyanin and cytochrome c552 in Chlamydomonas reinhardi. Mol Cell Biol. 1986 Feb;6(2):462–469. doi: 10.1128/mcb.6.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Bogorad L. The Cu(II)-repressible plastidic cytochrome c. Cloning and sequence of a complementary DNA for the pre-apoprotein. J Biol Chem. 1987 Jul 5;262(19):9062–9067. [PubMed] [Google Scholar]
- Merchant S., Hill K., Howe G. Dynamic interplay between two copper-titrating components in the transcriptional regulation of cyt c6. EMBO J. 1991 Jun;10(6):1383–1389. doi: 10.1002/j.1460-2075.1991.tb07658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S., Hill K., Kim J. H., Thompson J., Zaitlin D., Bogorad L. Isolation and characterization of a complementary DNA clone for an algal pre-apoplastocyanin. J Biol Chem. 1990 Jul 25;265(21):12372–12379. [PubMed] [Google Scholar]
- Nargang F. E., Drygas M. E., Kwong P. L., Nicholson D. W., Neupert W. A mutant of Neurospora crassa deficient in cytochrome c heme lyase activity cannot import cytochrome c into mitochondria. J Biol Chem. 1988 Jul 5;263(19):9388–9394. [PubMed] [Google Scholar]
- Nicholson D. W., Köhler H., Neupert W. Import of cytochrome c into mitochondria. Cytochrome c heme lyase. Eur J Biochem. 1987 Apr 1;164(1):147–157. doi: 10.1111/j.1432-1033.1987.tb11006.x. [DOI] [PubMed] [Google Scholar]
- Nicholson D. W., Neupert W. Import of cytochrome c into mitochondria: reduction of heme, mediated by NADH and flavin nucleotides, is obligatory for its covalent linkage to apocytochrome c. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4340–4344. doi: 10.1073/pnas.86.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson D. W., Stuart R. A., Neupert W. Biogenesis of cytochrome c1. Role of cytochrome c1 heme lyase and of the two proteolytic processing steps during import into mitochondria. J Biol Chem. 1989 Jun 15;264(17):10156–10168. [PubMed] [Google Scholar]
- Ohashi A., Gibson J., Gregor I., Schatz G. Import of proteins into mitochondria. The precursor of cytochrome c1 is processed in two steps, one of them heme-dependent. J Biol Chem. 1982 Nov 10;257(21):13042–13047. [PubMed] [Google Scholar]
- Page M. D., Ferguson S. J. A bacterial c-type cytochrome can be translocated to the periplasm as an apo form; the biosynthesis of cytochrome cd1 (nitrite reductase) from Paracoccus denitrificans. Mol Microbiol. 1989 May;3(5):653–661. doi: 10.1111/j.1365-2958.1989.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Page M. D., Ferguson S. J. Apo forms of cytochrome c550 and cytochrome cd1 are translocated to the periplasm of Paracoccus denitrificans in the absence of haem incorporation caused either mutation or inhibition of haem synthesis. Mol Microbiol. 1990 Jul;4(7):1181–1192. doi: 10.1111/j.1365-2958.1990.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Pakrasi H. B., De Ciechi P., Whitmarsh J. Site directed mutagenesis of the heme axial ligands of cytochrome b559 affects the stability of the photosystem II complex. EMBO J. 1991 Jul;10(7):1619–1627. doi: 10.1002/j.1460-2075.1991.tb07684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramseier T. M., Winteler H. V., Hennecke H. Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem. 1991 Apr 25;266(12):7793–7803. [PubMed] [Google Scholar]
- Schmidt G. W., Matlin K. S., Chua N. H. A rapid procedure for selective enrichment of photosynthetic electron transport mutants. Proc Natl Acad Sci U S A. 1977 Feb;74(2):610–614. doi: 10.1073/pnas.74.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Shochat S., Adir N., Gal A., Inoue Y., Mets L., Ohad I. Photoinactivation of photosystem II and degradation of the D 1 protein are reduced in a cytochrome b6/f-less mutant of Chlamydomonas reinhardtii. Z Naturforsch C. 1990 May;45(5):395–401. doi: 10.1515/znc-1990-0514. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbeek P., Hageman J., de Boer D., Pilon R., Smeekens S. Import of proteins into the chloroplast lumen. J Cell Sci Suppl. 1989;11:199–223. doi: 10.1242/jcs.1989.supplement_11.16. [DOI] [PubMed] [Google Scholar]
- Wildner G. F., Hauska G. Localization of the reaction site of cytochrome 552 in chloroplasts from Euglena gracilis. Effects of a specific antibody on endogenous, external and reincorporated cytochrome 552 in chloroplast membranes. Arch Biochem Biophys. 1974 Sep;164(1):136–144. doi: 10.1016/0003-9861(74)90015-0. [DOI] [PubMed] [Google Scholar]
- Willey D. L., Auffret A. D., Gray J. C. Structure and topology of cytochrome f in pea chloroplast membranes. Cell. 1984 Feb;36(2):555–562. doi: 10.1016/0092-8674(84)90248-4. [DOI] [PubMed] [Google Scholar]
- Wood P. M. Interchangeable copper and iron proteins in algal photosynthesis. Studies on plastocyanin and cytochrome c-552 in Chlamydomonas. Eur J Biochem. 1978 Jun 1;87(1):9–19. doi: 10.1111/j.1432-1033.1978.tb12346.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]