Abstract
Human bocavirus type-1 (HBoV1) has a high tropism for the apical membrane of human airway epithelia. The packaging of a recombinant adeno-associated virus 2 (rAAV2) genome into HBoV1 capsid produces a chimeric vector (rAAV2/HBoV1) that also efficiently transduces human airway epithelia. As such, this vector is attractive for use in gene therapies to treat lung diseases such as cystic fibrosis. However, preclinical development of rAAV2/HBoV1 vectors has been hindered by the fact that humans are the only known host for HBoV1 infection. This study reports that rAAV2/HBoV1 vector is capable of efficiently transducing the lungs of both newborn (3- to 7-day-old) and juvenile (29-day-old) ferrets, predominantly in the distal airways. Analyses of in vivo, ex vivo, and in vitro models of the ferret proximal airway demonstrate that infection of this particular region is less effective than it is in humans. Studies of vector binding and endocytosis in polarized ferret proximal airway epithelial cultures revealed that a lack of effective vector endocytosis is the main cause of inefficient transduction in vitro. While transgene expression declined proportionally with growth of the ferrets following infection at 7 days of age, reinfection of ferrets with rAAV2/HBoV1 at 29 days gave rise to approximately 5-fold higher levels of transduction than observed in naive infected 29-day-old animals. The findings presented here lay the foundation for clinical development of HBoV1 capsid-based vectors for lung gene therapy in cystic fibrosis using ferret models.
Keywords: : rAAV2/HBoV1 vector, airway transduction, newborn and juvenile ferret
Introduction
Cystic Fibrosis (CF) is a lethal autosomal-recessive disorder that affects a population of >30,000 in the United States alone and of 70,000 worldwide.1 CF affects multiple organs, and lung disease caused by chronic infection and inflammation is the primary cause of morbidity and mortality.2 The genetic basis of CF is a defect in a single gene that encodes cystic fibrosis transmembrane conductance regulator (CFTR), a chloride and bicarbonate channel that is expressed on the cell surface of many epithelia, including the airways.3,4 With the exception of recent small molecule modulators for certain CFTR mutations, current treatments for CF lung disease are limited to symptomatic therapies and alleviate only certain pulmonary complications.5 Gene therapy is an attractive mutation agnostic approach for treating this disorder, and clinical trials attempting to introduce a normal copy of the CFTR gene to airway cells in CF patients were initiated in the mid-1990s.6 In spite of the successes of gene therapy in treating some other genetic diseases, in the case of CF, significant challenges have been met, and no trial to date has achieved the desired outcomes.7,8
A requirement for the success of gene therapy is an efficient vector. Among the current available viral vectors, recombinant adeno-associated virus (rAAV) is the most promising one that has been widely used for delivering therapeutic genes in various clinical applications.9,10 However, clinical trials of rAAV for CF lung disease have failed to demonstrate efficacy, despite its favorable safety profile and genome persistence in human airway epithelia.11,12 Studies evaluating the transduction biology of rAAV in polarized human airway epithelia cultured at an air-liquid interface (HAE-ALI) have shed light on mechanisms that limit transduction of the human airway by rAAV-based vectors. Specifically, although most rAAV serotypes enter airway epithelia apically, these cells are equipped with mechanisms that prevent the virus from trafficking to the nucleus for productive transduction.13–15 In addition, an inherent disadvantage of rAAV in CF gene therapy is its small 4.679 kb genome size. This precludes packaging the large CFTR gene (of 4.43 kb coding sequence) in conjunction with an effective promoter, and thus requires the use of a CFTR minigene and minimal promoter.16–18 As such, gene therapy for CF lung disease would benefit from innovation in the development of vectors that are efficient for human airway transduction.
The human airway has evolved defense mechanisms and luminal barriers that inhibit infection by most of the commonly used viral vectors. Ideally, a vector for airway gene transfer would be derived from a respiratory virus whose native host is the human airway. Although respiratory syncytial virus19 and parainfluenza virus20 are candidates, their inherent complexity poses challenges for the construction of recombinant viral vectors and the production of virus. Human bocavirus 1 (HBoV1) is a recently identified parvovirus that naturally infects the human respiratory tracts.21 Evidence suggests that HBoV1 is pathogenic and leads to acute lower and upper respiratory infections in infants and young children.22–24 Recombinant HBoV1 is a potentially attractive vector for transduction of the airway, given that HBoV1 is one of the most common viruses in human respiratory secretions24 and reinfection can occur during childhood despite seroconversion.25 HBoV1 is an autonomous human parvovirus, belonging to species Primate bocaparvovirus of the genus Bocaparvovirus of the Parvoviridae family.26 To date, HBoV1 and parvovirus B19,27 which causes rash, arthropathy, anemia, and fetal death, are the only parvoviruses known to be pathogenic in humans. In contrast, as a relative to HBoV1, AAV is a replication-deficient, non-pathogenic parvovirus. Although AAV and bocavirus are both single-stranded DNA viruses, HBoV1 has a genome size of 5,543 nt, and as such is 18.5% (863 nt) larger than that of AAV2 (4,679 nt).
Since a full-length sequence of HBoV1 genome (including terminal palindromic sequences at two termini) was cloned and identified, a cell culture system for its production from proviral plasmid-transfected HEK293 cells has been established.28 Furthermore, the virology of HBoV1 has been studied in HAE-ALI cultures,29–34 revealing an extremely high tropism for the apical membrane of the HAE, as well as efficient replication.35 These findings, together with the advantages of the size of the HBoV1 genome, led to the development of HBoV1-based vectors for CF gene therapy. To eliminate the safety concerns related to HBoV1 as a human pathogen, a chimeric vector (rAAV2/HBoV1) was engineered, packaging a rAAV2 genome into the HBoV1 capsid.36 This chimeric parvoviral vector has increased efficacy in apical transduction of HAE-ALI, resulting in fivefold higher levels in transgene expression than the best-performing rAAV vector (rAAV2/1). Importantly, the capsid of HBoV1 accommodates an oversized (5.5 kb) rAAV2 genome, overcoming the size limitations of conventional rAAV for delivery of the full-length CFTR cDNA. A rAAV2/HBoV1 vector (AV2/HBc.CBAhCFTR) has been successfully used to deliver a large rAAV genome harboring a 5.2 kb cassette that encodes a strong CMV-β-actin promoter, the full-length CFTR cDNA coding sequence, and a short synthetic polyadenylation signal. This vector is capable of partially correcting CFTR-mediated chloride transport across CF HAE-ALI cultures following apical infection.36
Historically, a lack of appropriate CF animal models has been an obstacle for validating approaches to CF gene therapy. This was also a major concern for preclinical testing of rAAV2/HBoV1, given that the only known host of HBoV1 is human. The recent development of two larger animal models of CF, in the ferret and pig, has opened new avenues for such testing.37,38 Importantly, CF in these models closely mimics the human disease phenotype, with animals spontaneously developing lung infections.39,40 Thus, these models are useful for evaluating the therapeutic effect of CFTR gene transfer to CF airways. The current study tested transduction of the rAAV2/HBoV1 vector in ferret airway epithelial models, including in vitro ALI cultures and ex vivo tracheal xenografts, as well as in the airways of newborn and juvenile ferrets (in vivo). It was found that the ferret airway is permissive to gene transfer mediated by HBoV1 capsid, suggesting that the ferret is a suitable animal model for preclinical studies evaluating the potential of rAAV2/HBoV1-mediated gene therapy to the CF lung.
Materials and Methods
Chemicals and reagents
The proteasome inhibitor doxorubicin was from Sigma–Aldrich (St. Louis, MO), and N-acetyl-L-leucine-L-leucine-L-norleucine (LLnL) was from Boston Biochem (Cambridge, MA). Proteinase K was from Roche (Indianapolis, IN). DNase I was from MP Biochemicals (Santa Ana, CA). Ultraser G (USG) was from Pall Corporation (Port Washington, NY). The firefly luciferase detection kit, Luciferase Assay System, and VivoGlo Luciferin substrate for live imaging were from Promega (Madison, WI). The gaussia luciferase detection kit, BioLux® Gaussia Luciferase Assay Kit, was from New England BioLabs, Inc. (Ipswich, MA). TaqMan primer and probe sets were synthesized by IDT (Coralville, IA).
Production of recombinant virus
rAAV2/HBoV1 vectors, AV2/HBc.fluc, AV2/HBc.gLuc, and AV2/HBc.mCherry-fluc were generated by pseudotyping of the rAAV genome into HBoV1 capsid, as described previously.36 In brief, HEK293 cells were transfected with a rAAV proviral plasmid harboring one of the various reporter, together with three helper plasmids: pHBoV1KUm630, pAd4.1, and pAV-Rep2. At 72 h post transfection, cell pellets were collected, and the cells were lysed in hypotonic buffer (10 mM of Tris-HCl, pH 8.0). Following digestion with DNase I, virus was purified from clarified cell lysate through two rounds of CsCl ultracentrifugation, and the titers was determined as DNase I-resistant particles (DRP) by TaqMan polymerase chain reaction (PCR). The virus-containing fractions, which ranged in density from 1.43 to 1.40 g/mL, were pooled and dialyzed against phosphate-buffered saline (PBS) prior to use.
Cell culture and in vitro infection conditions
HEK293 cells, which were used for vector production, were cultured as monolayers in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and penicillin-streptomycin, and maintained in a 37°C incubator at 5% CO2. Human airway epithelial cultures grown at an air-liquid interface (HAE-ALI) and primary airway cells were obtained from the In Vitro Models and Cell Culture Core of the University of Iowa. Research was conducted on these human tissue cultures from the Core that has been verified as compliant with the University of Iowa policies, including IRB/IEC approval. The primary epithelial cells, which were obtained from normal human lung donors, were seeded onto transwell inserts (Corning, Tewksbury, MA) and differentiated in USG medium (2% Ultraser G in DMEM/F12 medium supplemented with antibiotics) at an ALI for 3 weeks prior to use, as described previously.41
Ferret airway epithelial cultures grown at an ALI (FAE-ALI) were generated from primary epithelial cells obtained from adult ferret trachea using methods previously described.42,43 The ferret cells were seeded onto transwell inserts and differentiated in PneumaCult ALI medium (StemCell Technologies, Vancouver, Canada) at an ALI for 3 weeks prior to use. For apical infections of ALI cultures, 7.5 × 109 DRP of rAAV2/HBoV1 vector in a 50 μL inoculum was applied to the upper chamber of the transwell insert, and the epithelia were exposed to the virus for 16 h. Infections of polarized HAE-ALI or FAE ALI cultures were performed in the presence of proteasome inhibitors (2.5 μM of doxorubicin and 20 μM of LLnL were added to the medium in the basolateral chamber). After the 16 h infection period, the inoculum in the upper chamber and the medium in the basolateral chamber were removed. The cultures were then briefly washed with a small amount culture medium and fed fresh medium in the absence of proteasome inhibitors. With approximately 7.5 × 105 cells present on each insert, the multiplicity of infection (MOI) was around 10,000 DRP/cell.
Animal care and viral infection
All animal experimentation was performed according to protocols approved by the Institutional Animal Care and Use Committees of the University of Iowa.
Infection of xenografts
Human and ferret tracheal xenografts were generated as subcutaneous implants in athymic mice, and were used as an ex vivo model of a vascularized airway for virus infection. As previously described,44 human tracheal xenografts were generated by seeding the denuded rat tracheas with primary human bronchial airway cells; ferret tracheal xenografts were generated directly from around a 1 cm segment of 2-day-old ferret trachea.45,46 The denuded rat tracheas or segments of fresh ferret trachea were cannulated with flexible plastic tubing, and the assembled tracheal cassettes were transplanted subcutaneously into athymic mice. Four weeks post transplantation, each fully differentiated human or ferret xenograft (n = 4 each) was infected with a 100 μL inoculum consisting of 4.5 × 1010 DRP of AV2/HBc.fLuc, 4.5 × 1010 DRP of AV2/HBc.gLuc, and 7.4 μg of doxorubicin (final concentration 125 μM). The inoculum was applied to the grafted lumen for 4 h and then removed by irrigation of the xenografts with 1 mL of Ham's F-12 medium, followed by an air flush. At each test time point tested, the xenografts were irrigated with 0.5 mL of Ham's F-12 medium and then flushed with air. These luminal washes were saved for gLuc assays.
In vivo infection of ferrets
Ferret airways were infected with rAAV2/HBoV1 by intra-tracheal injection. In brief, under anesthesia (by inhalation of a mixture of isofluorane and oxygen), surgery was conducted to expose the trachea (∼1 cm incision), and the vector inoculum was directly injected into the tracheal lumen under a dissecting microscope. The incision was sutured closed after infection. rAAV2/HBoV1 vectors were diluted with saline and included doxorubicin (final concentration 250 μM). The volume administered and the virus load varied depending on the age of the ferret (values are indicated in the figure legends). Mock infections were performed by intra-tracheal injection of the same volume of saline lacking virus but containing doxorubicin at the same concentration.
Measurement of luciferase reporter expression
Quantification of fLuc activity in live animals was performed using the Xenogen IVIS Biophotonic Imaging system (Xenogen, Alameda, CA), according to the manufacturer's instructions. Ferret kits were anesthetized by inhalation of a mixture of isofluorane and oxygen, and were injected intraperitoneally with VivoGlo Luciferin substrate at a dose of 150 mg/kg body weight. Images were taken 10 min after injection, and signals were quantified using the Living Image 2.51 software (Xenogen). For the end-point assay, cells or tissues were lysed with passive lysis buffer or reporter buffer (Promega), and firefly luciferase activity in cell/tissue lysates was determined using the Luciferase Assay System (Promega). Gaussia luciferase in the cell culture medium, serum, and tissue lysates was determined using the BioLux® Gaussia Luciferase Assay Kit (New England BioLabs, Inc.). All the enzymatic assays for luciferase activities were conducted using a 20/20 luminometer equipped with an automatic injector (Turner Biosystems, Sunnyvale, CA). Tissues harvested from animals were snap frozen in liquid nitrogen and pulverized while frozen. The powder was then mixed thoroughly, and a fraction of material was removed for assays of levels of luciferase or vector DNA.
Analysis of bound and internalized viral genomes
After apical rAAV2/HBoV1 infection of ALI cultures at one of two temperatures (4°C or 37°C) for 1 h, the transwells were washed with 40 mL of cold PBS in 50 mL conical tubes three times to remove unbound virus. In the 37°C condition, transwells were returned to the incubator for an additional period, while in the 4°C condition, transwells were processed after washing. The membrane supporting the cells was then excised from the insert and submerged in 200 μL of digestion buffer containing 50 mM of KCl, 2.5 mM of MgCl2, 10 mM of Tris pH 8.0, 0.5% NP40, 0.5% Tween-20, and 400 μg/mL of proteinase K. After digestion at 56°C for 45 min and heat inactivation at 95°C for 15 min, 1 μL (1/200) of the digestion mixture was used for TaqMan PCR.
Quantitative analysis of rAAV genome by TaqMan PCR
TaqMan real-time PCR was used to quantify the DRP titer in the viral stocks and the number of viral genomes in lysates from AAV2/HBoV1-infected cells or tissues. The PCR primers used to generate a 73 bp amplicon from the fLuc cDNA were 5′-TTTTTGAAGCGAAGGTTGTGG-3′ (forward) and 5′-CACACACAGTTCGCCTCTTTG-3′ (reverse). Those used to generate a 130 bp amplicon from gLuc cDNA were 5′-CGACATTCCTGAGATTCCTGG-3′ (forward) and 5′-TTGAGCAGGTCAGAACACTG-3′ (reverse). Those used to generate a 85 bp amplicon from mCherry cDNA were 5′-CCTGAAGGGCGAGATCAAGC-3′ (forward) and 5′-CTTGGCCTTGTAGGTGGTCTTG-3′ (reverse). The Taqman probe for fLuc was 5′-ATCTGGATACCGGGAAAACGCTGGGCGTTAAT-3′; that for gLuc was 5′-TGGAGCAGTTCATCGCACAGGT-3′; and that for mCherry was 5′-CTGAAGCTGAAGGACGGCGG-3′. The probes were tagged with 6-carboxy fluorescein (FAM) at the 5′-end as the reporter, and with Dark Hole Quencher 1 (BHQ1) at the 3′-end as the quencher. The PCR reaction was performed and analyzed using the Bio-Rad My IQ™ Real-Time PCR detection system and software (Bio-Rad, Hercules, CA).
Results
rAAV2/HBoV1 transduction of ferret versus human airway epithelium using in vitro and ex vivo models
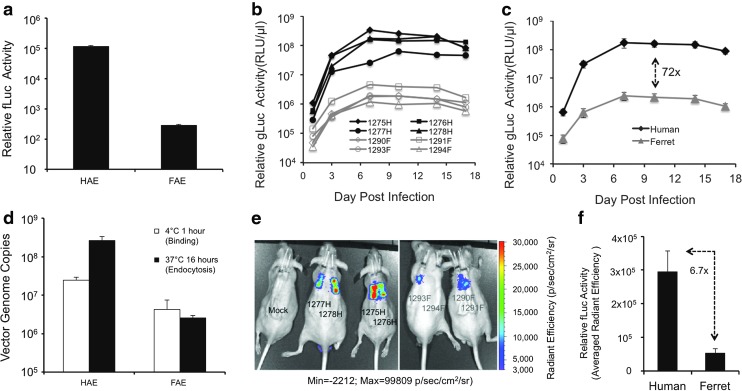
Although humans are thought to be the only host for wild-type HBoV1 infection, given that transduction with recombinant vectors does not require functional components of the lytic viral cycle, it was hypothesized that the rAAV2/HBoV1 chimeric vector would be capable of transducing the ferret airway epithelium. This would require only that ferret airway epithelial cells harbor the receptor and co-receptor typically used by HBoV1. To test this possibility, first rAAV2/HBoV1 transduction was evaluated in an in vitro model of the proximal airway epithelium. Specifically, ferret airway epithelial ALI cultures (FAE-ALI) similar to the HAE-ALI cultures were generated by differentiating primary proximal airway cells at an ALI.42 Comparison of the transduction (i.e., transgene expression) of a rAAV2/HBoV1 vector (AV2/HBc.fLuc) in FAE-ALI versus HAE-ALI revealed that transgene firefly luciferase (fLuc) expression in FAE-ALI cultures was around 1,500-fold lower than that in their HAE-ALI counterparts (Fig. 1a). Reasoning that receptors and/or co-receptors required for HBoV1 binding may be absent in the ferret proximal airway epithelium, the extent of vector binding at 4°C (1 h exposure), as well as that of endocytosis at 37°C (16-hour exposure), was compared in FAE-ALI versus HAE-ALI cultures. Results from the 1 h 4°C binding studies demonstrated an approximately 10-fold reduction in the level of cell-associated vector genomes (Fig. 1b), suggesting the presence of receptor(s) for HBoV1 capsid on surface of FAE-ALI but with less abundance and/or lower affinity than those of HAE-ALI. For the endocytosis assays, the same amount of vector as for the binding studies was applied to the ALI cultures at 37°C for 1 h incubation. Next, the cultures were washed to remove the inoculum and sent back to normal culture condition at 37°C for a further 15 h in medium only. Quantitation of cell-associated vector genomes at 16 h post infection demonstrated that the level of cell-associated vector genomes in FAE-ALI was around 100-fold lower than in HAE-ALI cultures (Fig. 1b). Notably, the number of cell-associated vector genomes in HAE-ALI cultures maintained at 37°C 16 h (associated with endocytosis) was 10-fold higher than those measured following exposure at 4°C for 1 h (associated with binding only), indicating an effective entry to HAE-ALI of the bound vector during the infection period. By contrast, there was no difference in the amount of vector genomes in FAE-ALI cultures at 37°C versus 4°C (Fig. 1b). These results suggest that the ∼1,500-fold lower level of rAAV2/HBoV1 transduction of FAE-ALI cultures, as compared to HAE-ALI, is most likely caused by inefficient viral endocytosis by the ferret epithelium.
Figure 1.
Recombinant adeno-associated virus 2 (rAAV2)/human bocavirus type-1 (HBoV1) transduces ferret proximal airway epithelium in vitro and ex vivo. (a) Firefly luciferase (fLuc) activity in human airway epithelia cultured at an air-liquid interface (HAE-ALI) and ferret airway epithelial cultures grown at an ALI (FAE-ALI) derived from primary human and ferret proximal airway cells, following apical infection with AV2/HBc.fLuc, at 5 days post infection. Data represent the mean (±standard error of the mean [SEM]; n = 3) relative luciferase activity in 5 μL cell lysates (1/40 of each infection). (b) Binding and endocytosis of rAAV2/HBoV1 in HAE and FAE cultures. A 50 μL inoculum of 7.5 × 109 DNase I-resistant particles (DRP) of AV2/HBc.fLuc was apically applied to FAE-ALI and HAE-ALI cultures and removed after incubation at 4°C or 37°C for 1 h. After extensive washing of unbound vector, cultures incubated at 4°C were lysed for the binding assays, whereas those incubated at 37°C were returned to 37°C for another 15 h prior to lysis for endocytosis assays. Bound (white column) or/and internalized (black column) viral genomes were quantitated by TaqMan real-time polymerase chain reaction (PCR). Data represent the mean (±SEM; n = 3) of the viral genome copies (per well) for each condition. (c–f) gLuc and fLuc expression in human and ferret tracheal xenografts co-infected with AV2/HBc.fLuc and AV2/HBc.gLuc (4.5 × 1010 DRP of each vector). (c) gLuc expression in luminal washes from individual human (H) and ferret (F) xenografts over time. (d) Averaged (mean ± SEM; n = 4) gLuc expression in luminal washes from human and ferret xenografts over time. (e) Biophotonic images of live mice harboring xenografts at 10 days post infection, detecting fLuc. (f) Average radiant efficiencies (mean ± SEM; n = 4) of bioluminescence for infected human and ferret grafts shown in (e).
Previous studies of HBoV1 infection and rAAV2/HBoV1 transduction in in vitro human airway cell cultures have demonstrated that HBoV1 preferentially infects well-differentiated airway epithelia, and that the efficiency of infection varies depending on the extent of differentiation.28 Tenfold higher transduction was also observed using rAAV2/HBoV1 in HAE-ALI cultures derived from primary airway cells versus in the immortalized human airway cell line CuFi8.36 Reasoning that the extent of differentiation of FAE-ALI cultures may affect the findings, the study was extended to an ex vivo tracheal xenograft model in which subcutaneous implantation of human44, 47 and ferret46 proximal airway epithelia into athymic mice produces better differentiated epithelia than the ALI cultures. Both the human and ferret xenografts were infected with equal amounts of a mixture of two rAAV2/HBoV1 vectors, one carrying the secreted Gaussia luciferase (gLuc) and the other carrying intracellular fLuc. This dual expression allowed transgene expression to be monitored in both airway secretions (gLuc; via enzymatic assay) and in the airways in situ (fLuc; bioluminescence monitoring following injection of firefly luciferase substrate). Whereas rAAV2/HBoV1 transduced ferret airway xenografts, its efficiency was 10- to 100-fold lower than in human airway xenografts (Fig. 1c–f). However, the kinetics of expression of the secreted gLuc reporter in ferret xenografts was similar to that in human xenografts (Fig. 1c), with onset detectable as early as 3 days post infection and the plateau occurring at 7–10 days post infection. At 10 days post infection, the average level of gLuc expression in ferret xenografts was 72-fold lower than in human xenografts (Fig. 1d). Analysis of fLuc expression at this time point by monitoring of bioluminescence showed that fLuc expression varied greatly among infected samples but was absent in uninfected xenografts (Fig. 1e). The average radiant efficiency in the infected ferret xenografts was 6.7-fold lower than that in the infected human xenografts (Fig. 1f). Although the fLuc data were quite variable, in all human xenografts the bioluminescence was stronger than that of any ferret xenograft. Of note, in the case of one ferret xenograft (1294F), its fLuc expression was too low to be displayed through the bioluminescence in the static view of live imaging, but analysis of the image using analytic quantitation software (Living Image v2.51) revealed the level to be above that in the non-infected grafts. Consistently, enzymatic measurement of gLuc expression revealed that the 1294F signal was the lowest among the four ferret grafts. Although it remains unclear that the different degrees of transduction efficiency indicated by gLuc and fLuc assays were due to the transduction potencies of the different vector preps or the detection sensitivity of the reporters, the general trends confirmed the in vitro findings indicating that the human proximal airway epithelium is more highly transduced by rAAV2/HBoV1 than is the ferret proximal airway epithelium. However, the fact that the difference in transduction between HAE-ALI and FAE-ALI cultures (∼1,500-fold) was much greater than that between human and ferret xenografts (7- to 70-fold) suggests that the extent of differentiation in FAE-ALI cultures may have exacerbated the difference in tropism between ferret and human tissue.
rAAV2/HBoV1 transduction of the ferret lung
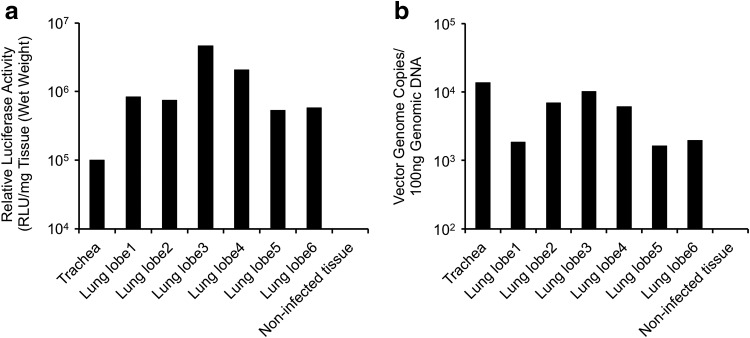
Although the efficiency of rAAV2/HBoV1 transduction of ex vivo ferret tracheal xenografts was lower than that of human bronchial xenografts, it is clear that ferret proximal airway epithelial cells possess the components that are required for HBoV1 capsid-mediated transduction, although their abundance would be less and/or affinity would be lower. The studies were extended to evaluate rAAV2/HBoV1 transduction of more distal airways of the ferret lung in vivo. As an initial test of whether ferret lung is permissive for rAAV2/HBoV1 transduction, a pilot study was performed in a single 3-day-old ferret kit, infecting it with the rAAV2/HBoV1 vector AV2/HBc.fLuc (∼3.6 × 1012 DRP/kg body weight) by transtracheal instillation. This experiment was designed to evaluate how effectively vector was distributed throughout the various lobes of the lung following tracheal delivery. At 7 days post infection, the trachea and individual lobes of the lung were evaluated for both luciferase expression and number of copies of the vector genome (Fig. 2). An effective transduction of rAAV2/HBoV1 in newborn ferret airway was justified by the transgene expression (Fig. 2a) and vector distribution (Fig. 2b) throughout all lobes of the ferret lung, suggesting that larger studies in ferrets were warranted.
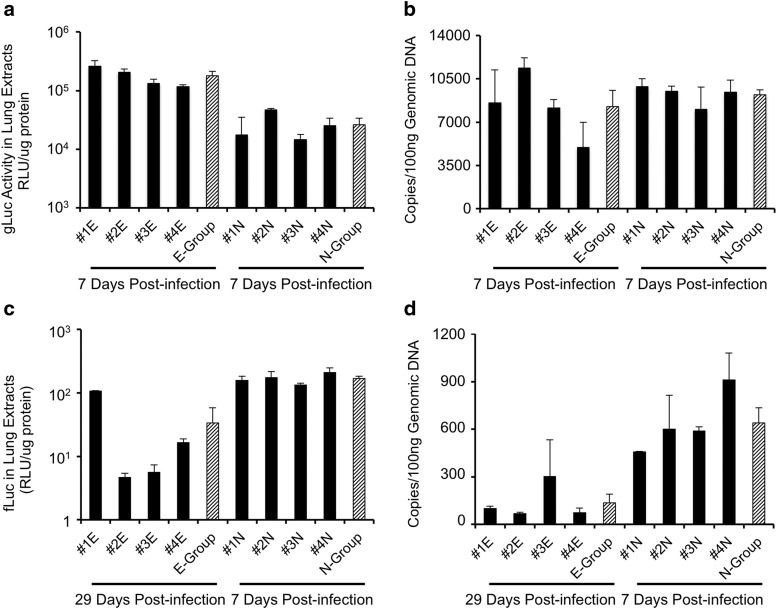
Figure 2.
Pilot test evaluating the distribution of rAAV2/HBoV1 transduction in various lung lobes and trachea of newborn ferret. Relative transduction at 8 days post infection, as determined by (a) expression of fLuc and (b) number of vector genome copies in individual lung lobes and trachea. Data are from one 3-day-old ferret kit infected with 5 × 1010 DRP of AV2/HBc.fLuc.
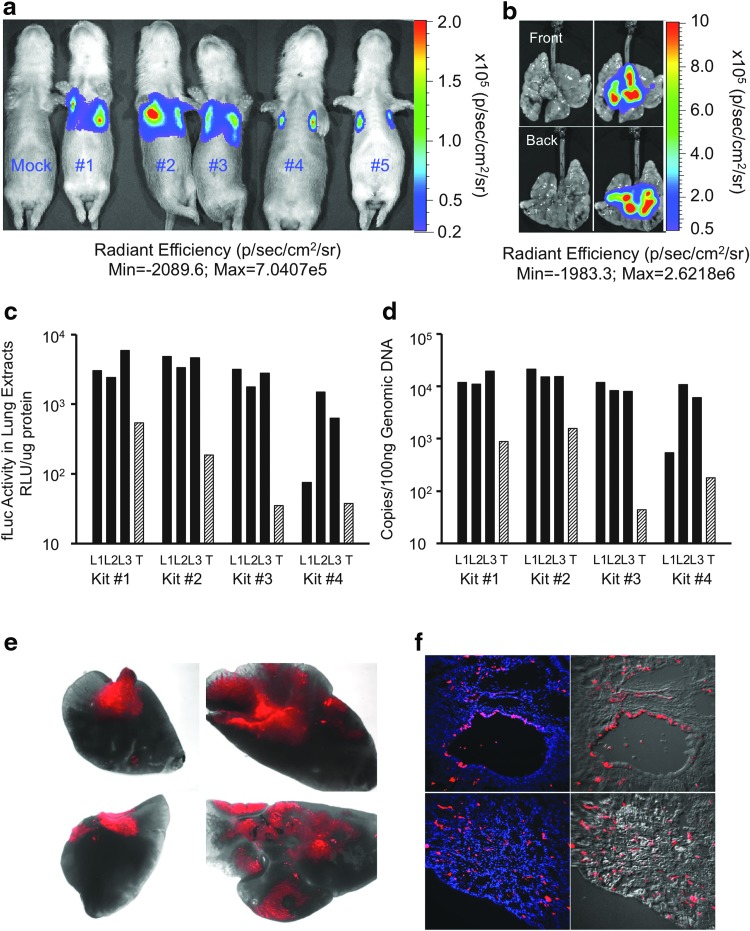
Next, five 5-day-old neonatal ferrets were infected with a dual reporter vector, AV2/HBc.mCherry-fLuc, using the same dose of vector (7.5 × 1010 DRP, ∼3.6 × 1012 DRP/kg body weight) and route of delivery. Transgene fLuc expression was assayed at 10 days post infection, first by monitoring of bioluminescence in live animals and then by enzymatic method following tissue harvesting. Analyses of the transduction also included the quantitation of number of vector genomes in tissue and localization of mCherry expression in tissue sections. Bioluminescence assays demonstrated that transgene expression was confined to the chest of infected animals, but also that variability among animals was high (Fig. 3a). To assess whether poor distribution of luciferin might limit detection in vivo, the lung of the animal with the lowest expression (Kit #5) was inflated, submerged in luciferin substrate, and reimaged. The level of bioluminescence in this excised lung was significantly greater than that seen in vivo (Fig. 3b), and was most strongly localized to intralobar regions of the lung. Thus, the bioluminescence data were interpreted as qualitative, and the study sought to evaluate transduction more thoroughly in each lobe by more quantitative lysate luciferase assays. The remaining animals were allowed to recover following imaging to allow for clearance of the luciferin prior to harvesting the lung.
Figure 3.
rAAV2/HBoV1 vector transduction to the airway of 5-day-old ferret kits. Five-day-old ferret kits (n = 5) were infected with 7.5 × 1010 DRP of AV2/HBc.mCherry-fluc and evaluated at 10 days post infection. (a–b) Biophotonic images acquired with the IVIS system, color bar set at the same scale of radiant efficiency applies to all the pseudocolored images. (a) Luminescent images visualized the firefly luciferase reporter expression in live kits. (b) Front and back views of whole lung cassette of Kit #5 in (a). Left: photographic image displaying the tissue only. Right: Luminescent pseudocolored image overlaid on photographic image. (c) Quantitation of fLuc activity in tissue extracts prepared from three entire lung lobes (L) and half of trachea (T), of Kits #1–4 in (a). (d) Quantitation of vector genomes, using genomic DNA prepared from the tissues used in (c). (e) mCherry expression in representative lung lobes, shown as merge of bright-field and fluorescence (red) taken on a fluorescent dissecting microscope. (f) mCherry expression in sections of lung tissue (red), with nuclei stained with DAPI (blue). Left: fluorescence only, showing both mCherry and DAPI. Right: Merge of red fluorescent channel with phase contrast image. Images were taken at 10 × magnification.
The tracheas and lungs of the remaining kits (Kit #1–4 and mock infected) were divided for two sets of experiments: (1) three of the lung lobes and half the trachea from each animal were snap frozen and pulverized for analysis of fLuc activity and vector DNA, and (2) the remaining lung lobes and trachea were embedded for cryosectioning for localization of mCherry expression. fLuc expression was detectable in all the tissue lysates of the tested lung lobes and tracheas of Kits #1–4 (Fig. 3c). Although the biophotonic imaging was not able to demonstrate the fLuc expression in the tracheas of all four kits, expression was detectable in the tracheal tissue extracts, albeit at lower levels and more varied than in the lung extracts among the four kits. The number of vector genomes in these samples roughly correlated with fLuc expression (Fig. 3c and d), suggesting that post-endocytic processing of the virus was likely not responsible for differences in transduction between the trachea and intralobar airways. Analysis of mCherry reporter expression in the intact lobes of the lungs under fluorescence dissecting microscope was patchy (Fig. 3e), suggesting that the vector delivery was unevenly distributed. Further analysis at the cellular level in sections demonstrated widespread mCherry expression in the bronchiolar airways and the surrounding lung alveolar airspaces (Fig. 3f).
rAAV2/HBoV1 transduction of the ferret lung is not adversely affected by vector exposure early in life
Given that CF ferrets develop lung infections starting early in life48,49 and the rAAV2/HBoV1 genomes from dose-limited neonatal infection will be diluted as the animal grows, evaluating rAAV2/HBoV1 for gene therapy in the neonatal CF ferret lung will require repetitive dosing. Furthermore, although the data to this point suggested that rAAV2/HBoV1 transduces the neonatal airway, it remained unclear whether this would be the case in the fully mature airways of the juvenile ferret. In the ferret airway, ciliogenesis and the development of submucosal glands occur during the first 3 weeks of life.50 rAAV1 transduction of the airway declines over this period due to the presence of inhibitory factors in airway fluid that may be produced by the submucosal glands.43 Thus, it was critical to evaluate rAAV2/HBoV1 for the ability to infect the juvenile ferret lung, as well as to determine whether prior exposure to rAAV2/HBoV1 inhibits transduction of repetitively administered vector.
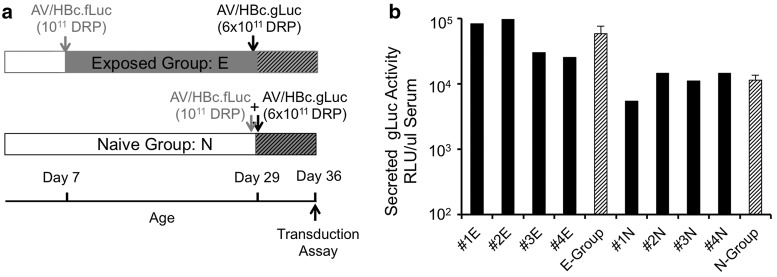
An experiment to evaluate transduction by a vector harboring gLuc reporter (AV2/HBc.gLuc) at 29 day of age was designed in animals that were either naïve to rAAV2/HBoV1 exposure or had been infected with a rAAV2/HBoV1 vector harboring the fLuc reporter (AV2/HBc.fLuc) at 7 days old (Fig. 4a). The animals in the naïve group were also co-infected with AV2/HBc.fLuc vector at the same dose that was given to the 7-day-old kits of the exposed group. All kits were sacrificed for transduction assays at the age of 36 days (Fig. 4a). Since equal amounts of AV2/HBc.gLuc were administered to all kits at 29 days of age, first gLuc expression was compared in the serum of the naïve versus previously exposed groups. Whereas gLuc activity was detectable in the sera of all the infected kits, the activity in the exposed group (E) was significantly higher (5.5-fold; p < 0.04) than that in the naïve group (N; Fig. 4b).
Figure 4.
rAAV2/HBoV1 transduction of 29-day-old ferrets with or without previous exposure to rAAV2/HBoV1. (a) Schematic outline of the infection protocol. Arrows indicate the ages at which kits were infected and sacrificed. Ferrets in exposed group (E; n = 4) were infected with 1011 DRP of AV2/HBc.fLuc at 7 days of age (gray arrow), and with 6 × 1011 DRP of AV2/HBc.gLuc at 29 days of age (black arrow). Ferrets in naïve group (N; n = 4) were not infected at 7 days of age but were co-infected with 1011 DRP of AV2/HBc.fLuc and 6 × 1011 DRP of AV2/HBc.gLuc at 29 days of age. Kits of both groups were sacrificed at 36 days of age, after serum was collected for assessment of secreted gLuc. (b) Comparison of gLuc expression in serum, for individual kits (black columns) and as averages for the two test groups (striped columns, mean ± SEM).
Transduction in lung tissues of the two groups was also compared using assays for fLuc and gLuc expression in lysates. Measurements of the gLuc activity in extracts from lung tissue demonstrated that expression was significantly higher (6.9-fold; p < 0.004) in the exposed versus naïve group (Fig. 5a), consistent with the data from the plasma measurements. However, TaqMan PCR-based analysis of the number of vector genomes for AV2/HBc.gLuc in these lung samples did not reveal significant differences between the two groups (Fig. 5b; p = 0.51). This suggests that post-entry pathways that influence transduction may be altered by previous exposure to the virus. However, given that the measurements were made shortly after infection (7 days), possible presence of a large amount of transcriptionally silent genomes in both groups might impact the interpretation of these findings.
Figure 5.
rAAV2/HBoV1-mediated transgene expression and vector genomes in naïve and previously exposed ferret lungs. Transgene expression and vector genomes in extracts of lung lobes for each ferret in the exposed (E) and naïve (N) groups shown in Fig. 5, with the time post infection and transgene indicated. Data are the averages of measurements from two randomly selected lung lobes from individual ferrets (mean ± range, black columns), and the averages for each group (mean ± SEM, striped column). (a) gLuc expression. (b) Number of copies of the AV2/HBc.gLuc genome. (c) fLuc expression. (d) Number of copies of AV2/HBc.fLuc genome. Relative gLuc and fLuc activity was normalized to the protein content of the tissue extracts. Genomic DNA (100 ng) was used for TaqMan PCR, and copies of vector genome were quantified using gLuc or fLuc cDNA standard curves.
Insights into viral persistence during growth of the neonatal lung were provided by evaluation of fLuc expression and the number of vector genomes in the two groups at 36 days of age. In lung tissues from the exposed group (29 days post infection with AV2/HBc.fLuc), the average levels of fLuc activity (Fig. 5c) and number of vector genome copies (Fig. 5d) were around 5.0- and 4.7-fold lower, respectively, than in the naïve group (7 days post infection with AV2/HBc.fLuc). In addition, although variation in the level of fLuc expression in kits of the naive group was marginal, it varied substantially in animals in the exposed group. In the exposed group, one kit in particular (#1E; Fig. 5c) maintained 6.5- to 23-fold higher expression than the others of the same group. Similarly, at 10 days post infection, the outcome of fLuc expression from the kits infected at 5 days old was also largely varied. Thus, the variability in gene expression from the rAAV2/HBoV1 infection to neonatal lungs appears larger than that to the fully mature juvenile lungs. It was reasoned that the difference in fLuc activities between the two groups could relate to the amount of growth the animals experience between the time of infection and the measurement of outcome. During the first month of life, the ferrets gained a significant amount of weight, starting out at an average of about 5–8 g at birth and ending at 241 g by 36 days of age. During the 29-day period from the time of their first infection, the kits in the exposed group gained an average of 186 g (+760%) body weight; in contrast, during the 7 days from the time of their infection, the kits of the naïve group gained only 58 g (+37%). Given that the relative luciferase activities were normalized to amount of protein in the tissue lysates using for comparison, the approximately fivefold lower fLuc expression at 29 days post infection most likely relates to the expansion of cell/tissue mass in the lung, reflected by the ∼7.6-fold weight gain during this period.
Relationship between growth and transgene expression following infection with rAAV2/HBoV1 vector
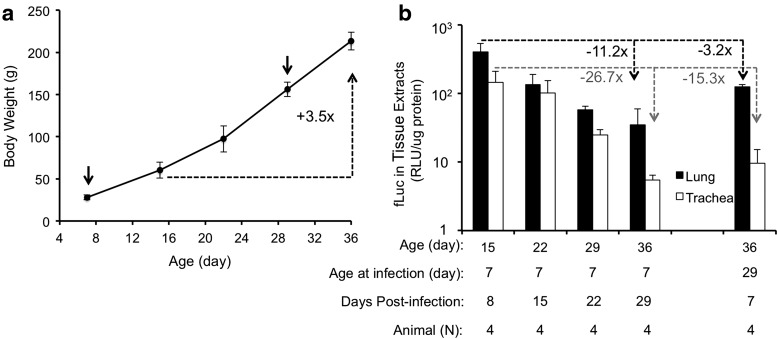
To evaluate the relationship between growth and the persistence of transgene expression more fully, experiments were designed to evaluate transgene expression in the lung at various ages following AV2/HBc.fLuc infection of 7-day-old animals. Initial attempts to monitor fLuc expression continually in the exposed group by biophotonic imaging were unsuccessful because maturation of the animals (including thickening of the skin and hair) led to inadequate sensitivity of the IVIS instrument. To study the persistence of transgene expression during the neonatal period more accurately, a time course of enzymatic luciferase endpoint assays was performed for separate sets of animals. The dose of fLuc vector was the same as used for the exposed group in the studies described in Fig. 5. In total, sixteen 7-day-old ferret kits were infected with AV2/HBc.fLuc vector, and transduction was evaluated at days 8, 15, 22, and 29 post infection. At each time point, four kits were sacrificed, and their body weights were recorded. fLuc activities were measured in the tissue homogenates from lungs (average of the measurements from six lung lobes for each kit) and tracheas. It was found that as the weight of the animals increased (Fig. 6a), the average fLuc activity decreased (Fig. 6b). Over the 3-week period of the study (8–29 days post infection), transgene expression decreased 11.2-fold in the lung and 26.7-fold in the trachea. Growth of the lung, as inferred by weight gain (60–214 g over the 3-week period), is likely partly responsible for the decrease. However, this increase in weight only represents a 3.5-fold difference and does not account for the 11.2- to 26.7-fold reduction in transgene expression. Thus, the duration of transgene expression could be influenced by additional factors, perhaps the shedding of transduced cells and/or inactivation of the promoter.
Figure 6.
Duration of rAAV2/HBoV1-mediated transgene expression in the ferret lung. Experiments were performed in ferrets infected with 1011 DRP of AV2/HBc.fLuc at the age of 7 or 29 days. (a) Growth curve for the rAAV2/HBoV1-infected ferrets. Values represent the mean ± SEM (with n for each age given in panel (b)) of the body weight at the age at which the ferrets were infected (arrows, days 7 and 29 of age) and/or sacrificed (days 8, 15, 22, and 29 post infection). All groups of kits but one were infected at 7 days of age; only one group was infected at 29 days of age. (b) Averaged fLuc activities (mean ± SEM, n = 4 for each condition) in tissue extracts (lung and trachea) of the infected ferrets. Relative fLuc activity was normalized to protein content of the tissue extract.
Discussion
While rAAV2/HBoV1 represents a potentially promising vector for gene therapy to the CF lung, there is a critical need for a suitable animal model in which to perform preclinical studies. Given that a CF ferret model exists, the ability of rAAV2/HBoV1 to transduce ferret airway epithelial cells was tested using three model systems: an in vitro model of polarized FAE-ALI cultures, an ex vivo model of tracheal xenografts, and an in vivo model of infection of lungs of newborn and juvenile ferret. Results demonstrated that rAAV2/HBoV1 transduction in the ferret in vitro and ex vivo airway model systems were much poorer than that in HAE-ALI and human tracheal xenografts. Given that this species difference was much less in tracheal xenografts compared to polarized ALI cultures, the extent of differentiation may influence the HBoV1 tropism for proximal ferret airway epithelia. Data from the analyses of vector binding and endocytosis suggest that the species discrepancies in the case of the ALI cultures can be accounted for by inefficient endocytosis of HBoV1 capsids by in vitro polarized ferret proximal airway epithelia. This implies that the receptor and/or co-receptor for HBoV1 is poorly expressed on the apical surface of in vitro cultured ferret proximal airway epithelia. Together with the in vivo tests demonstrating poor transduction of the ferret trachea compared to the distal airways, it is concluded that HBoV1 capsids do not efficiently transduce the ferret tracheal epithelium.
While the in vivo cellular site of lytic HBoV1 infections in human is unknown, HBoV1 is thought to cause both upper and lower respiratory infections.23,24 HBoV1 clinical presents with bronchiolitis and pneumonia,51 suggesting that distal lung may be the primary site of infection; this is consistent with the location of rAAV2/HBoV1 transduction in the ferret lung. rAAV2/HBoV1 vector preferentially transduced the lower respiratory tract in the neonatal ferret lung, with transgene expression predominantly localized to intralobar regions. Histologic examination revealed high levels of transgene expression (mCherry fluorescent protein) in the bronchiolar airways as well as the alveolar regions of the lung. Furthermore, mature lungs of juvenile ferrets (1 month old) were also permissive to rAAV2/HBoV1 transduction.
Gene therapy to the lung with episomally persistent parvoviral vectors will likely require repeat administration. However, the adaptive immune system is known to prevent repetitive viral infection.52 In this context, repeated rAAV administration to mouse lung has given variable results. Re-administration of rAAV5 or rAAV6 vectors to the mouse lung is prevented by neutralizing antibodies,53,54 but an effective second administration with rAAV6 can be achieved by transient immunosuppression during the first dose.54 By contrast, re-administration of rAAV9 is effective in the mouse lung.55 Analysis of rAAV2/HBoV1 indicates that repetitive vector administration during the first month of life is feasible in the ferret. Of particular interest in these studies was the observed five- to sevenfold enhancement in rAAV2/HBoV1 transduction following a second round of virus administration. Notably, although HBoV1 lung infections in children 1–2 years of age lead to seroconversion, these children can nevertheless contract HBoV1 infection years later, and cases of secondary HBoV1 infection occurring in older children and adults have also been reported.25,56,57 It is currently unclear if the neonatal adaptive immune response, which may be different from that in adults, plays a role in the ability of HBoV1 to infect children repetitively. The present studies of repeated rAAV2/HBoV1 infection in neonatal and juvenile ferrets were modeled after data describing both primary and second HBoV1 infections in infants and toddlers, respectively. The findings in the ferret studies also support data of secondary infections in infants and toddlers. Based on extrapolations between humans and ferrets, a 1-month-old ferret would be the approximate age equivalent of a 1-year-old human.58 Thus, the studies in ferrets represent the earliest window for HBoV1 secondary infection in humans, for which around 22% occur by 1 year of age and ∼42% occur at ages ≥6 years.25 Since nearly 100% of humans are seroposititve for HBoV1 antibodies by 6 years of age,59 it remains unclear if primary and secondary infections can similarly occur in naïve adults. Similarly, it is unclear if re-administration of rAAV2/HBoV1 will be effective when first exposure to rAAV2/HBoV1 occurs in older ferrets. The observation of enhanced secondary transduction in the exposed group is interesting and warrants further investigation.
These observations are reminiscent of findings reported in Mucopolysaccharidosis type I (MPS I) dogs, which have interesting implications for circumventing unwanted immune responses to gene therapy. In this study, the dogs were infected twice with rAAV vectors: with a rAAV8 vector on postnatal day 1 or 7, and then with rAAV9 at 1 month of age. rAAV8 and rAAV9 served as vectors for the same canine α-I-iduronidase (IDUA) expression cassette, and the IDUA activity in cerebrospinal fluid from the exposed group of dogs was 3- to 100-fold greater than that in the naïve animals.60 These findings suggest that the postnatal period may represent a developmental window during which tolerance to a viral vector can be induced, and that neonatal gene transfer might be an effective approach for preventing immune responses to a transgene product and/or vector proteins following re-administration at older ages.61,62 Although further experimentation is required to determine the mechanisms by which prior exposure to the HBoV1 capsid can enhance transduction from the same vector later in life, investigation on the repetitive infection of rAAV2/HBoV1 vector with the ferret model may also provide useful clues for the immunogenicity of HBoV1 capsid proteins and development of HBoV1 vaccine.
Consistent with proteasome inhibitor augmentation of HAE-ALI cultures following apical infection with rAAV1, 2, and 514,63,64 or rAAV2/HBoV1,36 proteasome inhibition is required for effective in vivo transduction of the ferret airway by rAAV143 and facilitated effective in vivo rAAV2/HBoV1 transduction in the present study. Although in vitro studies have revealed that the application of proteasome inhibitors (including doxorubicin) increases nuclear accumulation of rAAV in HAE-ALI cultures,63 a detailed understanding of how doxorubicin augments transduction by parvoviral vectors (rAAV and rAAV2/HBoV1) in airway epithelial cells in vivo remains unclear. Doxorubicin is a pharmaceutical agent clinically used in cancer chemotherapy. As a multifunctional molecule, doxorubicin inhibits the activities of DNA topoisomerase II65 and the 20S core proteasome,66 and can also affect cell proliferation and the immune response.67 Any of these cellular effects could facilitate rAAV2/HBoV1 transduction to the ferret airway in vivo and the ability to repeat administer vector. Indeed, studies in mice have shown that the proteasome inhibitor bortezomib can enhance rAAV2 transduction of mouse liver while also reducing MHC I presentation of AAV2 capsid peptides.68 Proteasome inhibition has also been shown to induce immune tolerance in human and mouse models,69,70 and thus it is possible that the administration of doxorubicin with vector to 7-day-old ferrets might play a critical role in preventing immune responses to the second infection at 1 month of age. The dose doxorubicin required to facilitate rAAV2/HBoV1 transduction maximally in vivo and the consequences on the ferret immune system are remaining major questions that will assist in translating the rAAV2/HBoV1 vector system to repeat dosing in CF ferrets.
While interpreting the persistence of transgene expression in the lungs of growing animals has caveats, given that lung size may not correlate directly with body weight, the present findings suggest that actively replicating cells in the growing lung may more rapidly dispose of rAAV2 genomes. Over the 3-week period during which transgene expression was monitored (8–29 days post infection), transgene expression decreased 11.2- and 26.7-fold in the lung and trachea, respectively, whereas body weight increased only 3.5-fold. This suggests that when gene therapy is applied in rapidly growing children, more frequent dosing will be required than in adults. Similar experiments in fully grown ferrets will be required to confirm this hypothesis. However, it will first be necessary to improve rAAV2/HBoV1 packaging systems, so that vector can be generated in quantities sufficient for those studies.
Postnatal development of the ferret lung involves the formation of ciliated cells, goblet cells, and submucosal glands. These developmental changes alter the extracellular milieu of the airways, and it was previously shown that this negatively affects rAAV2/1 transduction of the ferret lung.43 That study demonstrated that the airways of the newborn ferret are highly transduced by rAAV2/1, but that by 18 days of age, an inhibitory factor in the airway fluid significantly reduces transduction, and by adulthood it is undetectable. It was hypothesized that submucosal glands may be responsible for the secretion of this inhibitor. By contrast, rAAV2/HBoV1 efficiently transduces the lungs of both 7- and 29-day-old ferrets, suggesting that the HBoV1 capsid is relatively unaffected by lung development and the change in airway secretions.
In summary, this study demonstrates that ferret airways are permissive to rAAV2/HBoV1 transduction in vivo, with rAAV2/HBoV1 effectively transducing ferret lungs from at least 3 to 29 days of age. Additionally, during this period repetitive vector administration was successful, without a reduction in transduction efficiency. Collectively, this work lays a foundation for using the ferret to study the transduction biology of HBoV1 in the airway, and for using the CF ferret as a preclinical model to develop rAAV2/HBoV1-mediated gene therapies for the CF lung.
Acknowledgments
This work was supported by grants from the National Institutes of Health (HL051670 and HL123482 to J.F.E.; AI070723, AI105543, and AI112803 to J.Q.), Cystic Fibrosis Foundation (YAN15XX0 to Z.Y.), the University of Iowa Center for Gene Therapy (DK54759), and the Roy J. Carver Chair in Molecular Medicine (to J.F.E.).
Author Disclosure
Z.Y., J.Q., and J.F.E. have sponsored research with Pfizer, Inc. and receive patent royalties related to the vector system described in this manuscript. The remaining authors have no conflicts of interest to declare.
References
- 1.O'Sullivan BP, Freedman SD. Cystic fibrosis. Lancet 2009;373:1891–1904 [DOI] [PubMed] [Google Scholar]
- 2.Rowe SM, Clancy JP. Advances in cystic fibrosis therapies. Curr Opin Pediatr 2006;18:604–613 [DOI] [PubMed] [Google Scholar]
- 3.Riordan JR, Rommens JM, Kerem B, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245:1066–1073 [DOI] [PubMed] [Google Scholar]
- 4.Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science 1992;256:774–779 [DOI] [PubMed] [Google Scholar]
- 5.van Gool K, Norman R, Delatycki MB, et al. Understanding the costs of care for cystic fibrosis: an analysis by age and health state. Value Health 2013;16:345–355 [DOI] [PubMed] [Google Scholar]
- 6.Driskell RA, Engelhardt JF. Current status of gene therapy for inherited lung diseases. Annu Rev Physiol 2003;65:585–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prickett M, Jain M. Gene therapy in cystic fibrosis. Transl Res 2013;161:255–264 [DOI] [PubMed] [Google Scholar]
- 8.Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther 2015;26:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther 2005;16:541–550 [DOI] [PubMed] [Google Scholar]
- 10.Samulski RJ, Muzyczka N. AAV-mediated gene therapy for research and therapeutic purposes. Annu Rev Virol 2014;1:427–451 [DOI] [PubMed] [Google Scholar]
- 11.Aitken ML, Moss RB, Waltz DA, et al. A Phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 2001;12:1907–1916 [DOI] [PubMed] [Google Scholar]
- 12.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled Phase 2B trial. Hum Gene Ther 2007;18:726–732 [DOI] [PubMed] [Google Scholar]
- 13.Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest 2000;105:1573–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan Z, Zak R, Zhang Y. et al. Distinct classes of proteasome-modulating agents cooperatively augment recombinant adeno-associated virus type 2 and type 5-mediated transduction from the apical surfaces of human airway epithelia. J Virol 2004;78:2863–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding W, Zhang L, Yan Z, et al. Intracellular trafficking of adeno-associated viral vectors. Gene Ther 2005;12:873–880 [DOI] [PubMed] [Google Scholar]
- 16.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther 1996;7:2101–2112 [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Wang D, Fischer H, et al. Efficient expression of CFTR function with adeno-associated virus vectors that carry shortened CFTR genes. Proc Natl Acad Sci U S A 1998;95:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan Z, Sun X, Feng Z, et al. Optimization of recombinant adeno-associated virus-mediated expression for large transgenes, using a synthetic promoter and tandem array enhancers. Hum Gene Ther 2015;26:334–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwilas AR, Yednak MA, Zhang L, et al. Respiratory syncytial virus engineered to express the cystic fibrosis transmembrane conductance regulator corrects the bioelectric phenotype of human cystic fibrosis airway epithelium in vitro. J Virol 2010;84:7770–7781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari S, Griesenbach U, Iida A, et al. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther 2007;14:1371–1379 [DOI] [PubMed] [Google Scholar]
- 21.Allander T, Tammi MT, Eriksson M, et al. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci U S A 2005;102:12891–12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allander T, Jartti T, Gupta S, et al. Human bocavirus and acute wheezing in children. Clin Infect Dis 2007;44:904–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu J, Soderlund-Venermo M, Young NS. Human parvoviruses. Clin Microbiol Rev 2017;30:43–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jartti T, Hedman K, Jartti L, et al. Human bocavirus-the first 5 years. Rev Med Virol 2012;22:46–64 [DOI] [PubMed] [Google Scholar]
- 25.Meriluoto M, Hedman L, Tanner L, et al. Association of human bocavirus 1 infection with respiratory disease in childhood follow-up study, Finland. Emerg Infect Dis 2012;18:264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotmore SF, Agbandje-McKenna M, Chiorini JA, et al. The family Parvoviridae. Arch Virol 2014;159:1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young NS, Brown KE. Parvovirus B19. N Engl J Med 2004;350:586–597 [DOI] [PubMed] [Google Scholar]
- 28.Huang Q, Deng X, Yan Z, et al. Establishment of a reverse genetics system for studying human bocavirus in human airway epithelia. PLoS Pathog 2012;8:e1002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Deng X, Zou W, et al. Identification and functional analysis of novel nonstructural proteins of human bocavirus 1. J Virol 2015;89:10097–10109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng X, Yan Z, Cheng F, et al. Replication of an autonomous human parvovirus in non-dividing human airway epithelium is facilitated through the DNA damage and repair pathways. PLoS Pathog 2016;12:e1005399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen W, Deng X, Zou W, et al. Analysis of cis and trans requirements for DNA replication at the right-end hairpin of the human bocavirus 1 genome. J Virol 2016;90:7761–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou W, Cheng F, Shen W, et al. Nonstructural protein NP1 of human bocavirus 1 plays a critical role in the expression of viral capsid proteins. J Virol 2016;90:4658–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng X, Xu P, Zou W, et al. DNA Damage Signaling Is Required for Replication of Human Bocavirus 1 DNA in Dividing HEK293 Cells. J Virol 2017;91:e01831-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Z, Shen W, Cheng F, et al. Parvovirus expresses a small noncoding RNA that plays an essential role in virus replication. J Virol 2017;91: e02375-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng X, Yan Z, Luo Y, et al. In vitro modeling of human bocavirus 1 infection of polarized primary human airway epithelia. J Virol 2013;87:4097–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Z, Keiser NW, Song Y, et al. A novel chimeric adenoassociated virus 2/human bocavirus 1 parvovirus vector efficiently transduces human airway epithelia. Mol Ther 2013;21:2181–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rogers CS, Hao Y, Rokhlina T, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus-mediated gene targeting and somatic cell nuclear transfer. J Clin Invest 2008;118:1571–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X, Yan Z, Yi Y, et al. Adeno-associated virus-targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest 2008;118:1578–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keiser NW, Engelhardt JF. New animal models of cystic fibrosis: what are they teaching us? Curr Opin Pulm Med 2011;17:478–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan Z, Stewart ZA, Sinn PL, et al. Ferret and pig models of cystic fibrosis: prospects and promise for gene therapy. Hum Gene Ther Clin Dev 2015;26:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karp PH, Moninger TO, Weber SP, et al. An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 2002;188:115–137 [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Luo M, Zhang L, et al. Bioelectric properties of chloride channels in human, pig, ferret, and mouse airway epithelia. Am J Respir Cell Mol Biol 2007;36:313–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan Z, Sun X, Evans IA, et al. Postentry processing of recombinant adeno-associated virus type 1 and transduction of the ferret lung are altered by a factor in airway secretions. Hum Gene Ther 2013;24:786–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keiser NW, Engelhardt JF. Gene delivery to the airway. Curr Protoc Hum Genet 2013;Chapter 13:Unit 13 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Zhang Y, Amberson A, et al. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol 2001;24:195–202 [DOI] [PubMed] [Google Scholar]
- 46.Dajani R, Zhang Y, Taft PJ, et al. Lysozyme secretion by submucosal glands protects the airway from bacterial infection. Am J Respir Cell Mol Biol 2005;32:548–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engelhardt JF, Schlossberg H, Yankaskas JR, et al. Progenitor cells of the adult human airway involved in submucosal gland development. Development 1995;121:2031–2046 [DOI] [PubMed] [Google Scholar]
- 48.Sun X, Olivier AK, Liang B, et al. Lung phenotype of juvenile and adult cystic fibrosis transmembrane conductance regulator-knockout ferrets. Am J Respir Cell Mol Biol 2014;50:502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun X, Sui H, Fisher JT, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest 2010;120:3149–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keiser NW, Birket SE, Evans IA, et al. Defective innate immunity and hyperinflammation in newborn cystic fibrosis transmembrane conductance regulator-knockout ferret lungs. Am J Respir Cell Mol Biol 2015;52:683–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Don M, Soderlund-Venermo M, Valent F, et al. Serologically verified human bocavirus pneumonia in children. Pediatr Pulmonol 2010;45:120–126 [DOI] [PubMed] [Google Scholar]
- 52.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther 2010;17:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sumner-Jones SG, Gill DR, Hyde SC. Lack of repeat transduction by recombinant adeno-associated virus type 5/5 vectors in the mouse airway. J Virol 2007;81:12360–12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halbert CL, Standaert TA, Wilson CB, et al. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J Virol 1998;72:9795–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci U S A 2006;103:12993–12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jula A, Waris M, Kantola K, et al. Primary and secondary human bocavirus 1 infections in a family, Finland. Emerg Infect Dis 2013;19:1328–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghietto LM, Camara A, Camara J, et al. High frequency of human bocavirus 1 DNA in infants and adults with lower acute respiratory infection. J Med Microbiol 2012;61:548–551 [DOI] [PubMed] [Google Scholar]
- 58.Yi Y, Sun X, Gibson-Corley K, et al. A transient metabolic recovery from early life glucose intolerance in cystic fibrosis ferrets occurs during pancreatic remodeling. Endocrinology 2016;157:1852–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao B, Yu X, Wang C, et al. High human bocavirus viral load is associated with disease severity in children under five years of age. PLoS One 2013;8:e62318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hinderer C, Bell P, Louboutin JP, et al. Neonatal systemic AAV induces tolerance to CNS gene therapy in MPS I dogs and nonhuman primates. Mol Ther 2015;23:1298–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan X, Ang A, Pollock-Barziv SM, et al. Donor-specific B-cell tolerance after ABO-incompatible infant heart transplantation. Nat Med 2004;10:1227–1233 [DOI] [PubMed] [Google Scholar]
- 62.Adkins B. T-cell function in newborn mice and humans. Immunol Today 1999;20:330–335 [DOI] [PubMed] [Google Scholar]
- 63.Yan Z, Lei-Butters DC, Liu X, et al. Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia. J Biol Chem 2006;281:29684–29692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan Z, Zak R, Luxton GW, et al. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol 2002;76:2043–2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer 2009;9:338–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fekete MR, McBride WH, Pajonk F. Anthracyclines, proteasome activity and multi-drug-resistance. BMC Cancer 2005;5:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eckert R, Gruner S, Volk HD, et al. Studies on the immunomodulatory effects of anthracycline antibiotics in mice: effects on immune responses and graft immunogenicity. Immunobiology 1989;179:445–455 [DOI] [PubMed] [Google Scholar]
- 68.Finn JD, Hui D, Downey HD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther 2010;18:135–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berges C, Haberstock H, Fuchs D, et al. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology 2008;124:234–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo H, Wu Y, Qi S, et al. A proteasome inhibitor effectively prevents mouse heart allograft rejection. Transplantation 2001;72:196–202 [DOI] [PubMed] [Google Scholar]