Abstract
Soft tissue reconstruction to restore volume to damaged or deficient tissue beneath the skin remains a challenging endeavor. Current techniques are centered around autologous fat transfer, or the use of synthetic substitutes, however, a great deal of scientific inquiry has been made into both the molecular mechanisms involved in, and limitations of, de novo adipogenesis, that is, the formation of new adipose tissue from precursor cells. To best comprehend these mechanisms, an understanding of defined markers for adipogenic differentiation, and knowledge of both commercially available and primary cell lines that enable in vitro and in vivo studies is necessary. We review the growth factors, proteins, cytokines, drugs, and molecular pathways that have shown promise in enhancing adipogenesis and vasculogenesis, in addition to the multitude of scaffolds that act as delivery vehicles to support these processes. While progress continues on these fronts, equally important is how researchers are optimizing clinically employed strategies such as autologous fat transfer through cell-based intervention, and the potential to augment this approach through isolation of preferentially adipogenic or angiogenic precursor subpopulations, which exists on the horizon. This review will highlight the novel molecular and synthetic modifications currently being studied for inducing adipose tissue regeneration on a cellular level, which will expand our arsenal of techniques for approaching soft tissue reconstruction.
Keywords: : adipose, mesenchymal stem cells, growth factors, adult stem cells, adipose tissue engineering
Introduction
A variety of both acquired and congenital conditions, including infection, trauma, and tumor resection, can result in contour irregularity and defects of the soft tissue. Correction of these defects offers patients significant psychological and social benefits. Contemporary techniques revolve around the transfer of mature adipose tissue, however, the practice of autologous fat transfer is still fraught with unpredictable outcomes; graft retention rates can fall 10% to 90% over time due to necrosis of the graft and formation of cysts and granulomas.1,2 Understanding the cellular and molecular processes of de novo adipose tissue formation will help in the design of implantable matrices, guide the use of supplemental factors, and help to develop strategies that exploit adipogenic pathways.
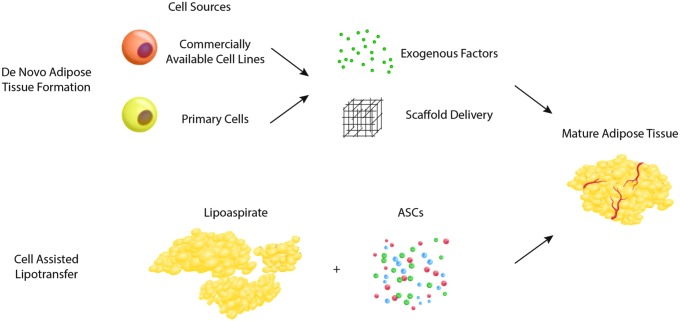
Adipose tissue must not be thought of as an inert cellular mass, rather a sophisticated and dynamic set of heterogeneous populations capable of generating and responding to hormones, creating vasculature, storing energy, and converting dormant precursor cells to mature cells upon stimuli. Engineering fat tissue in vivo can be done naturally through manipulating the resident preadipocyte population. The adipocyte precursor cells are a population that may diapedese and travel from different tissues, or exist dormant in situ ready to convert to mature adipocytes under the correct microenvironmental conditions.3 As such, harnessing de novo mechanisms provides a valuable target for natural adipose tissue regeneration. Furthermore, enhanced knowledge of how specific precursor cells function may help to refine contemporary strategies already in use clinically to address soft tissue deficit (Fig. 1). While the studies referenced in this review largely cover both human and rodent fat models, it is understood through genome wide maps of histone modifications/chromatin state maps that the molecular mechanisms that govern adipogenesis are largely conserved across mice and humans.4
FIG. 1.
Strategies for cell-based adipose tissue engineering include de novo adipogenesis (top), combining cellular building blocks with exogenous growth factors and delivery scaffolds, and cell-assisted lipotransfer (bottom), where lipoaspirate is enriched with supplemental adipose-derived stromal cells (ASCs), to produce mature adipose tissue.
De novo Adipose Tissue Formation
Guided differentiation of cell populations into adipocytes is the basis for de novo adipose tissue engineering. The cellular changes associated with acquisition of an adipogenic cell fate has been well studied and a multitude of markers for this process have been defined. Terminal differentiation of preadipocytes into triacylglyceride-containing adipocytes is dependent on glycerol-3-phosphate dehydrogenase (GPDH). The presence and activation of this enzyme leads to accumulation of intracellular lipid droplets,5,6 allowing use of GPDH as a marker for adipogenesis to assess tissue engineering in vitro. Other commonly used markers of successful differentiation into adipocytes are perosixome proliferator-activated receptor gamma (PPAR-γ), lipoprotein lipase (LPL), and fatty acid binding protein (FABP)-4.7 Paralleling cell commitment along an adipocyte fate is the ability of cells to create triglycerides de novo. Adipocytes generally absorb fully formed triglycerides from the microenvironment using LPL uptake, but are also able to form fatty acids from nonlipid precursor material. In LPL knockout mice, adipocytes still retain the capacity for accumulation of triglycerides through de novo lipid formation.8 This is reflected in the fact that palmiteoleate, which comprises less than 4% of all dietary intake, is still the second most abundant monounsaturated fat in the body, and it serves in a positive feedback manner on neoadipogenesis.9,10
Cell sources for adipose tissue engineering
A variety of cell lines with the ability to differentiate into fat have been employed to study the process of adipogenesis, many of which are commercially available.11 Preadipocyte lines used for investigations include 3T3-F442A, 3T3-L1, and Ob17 murine lines, and each of these have the benefit of being homogeneous, well defined, and capable of extended culture.11,12 These cell lines differentiate spontaneously into adipocytes in vitro in the presence of serum when growth arrest is maintained.13–15 In vivo adipogenesis has also been demonstrated through subcutaneous implantation of 3T3-F442A and 3T3-L1 adipose precursor cells into immunocompromised mice.16,17 Within this natural adipose niche, preadipocytes alone are capable of early maturation into adipose tissue without addition of exogenous inductive signals, forming fat pads comprised of both adipocytes and microvessels within 2 weeks.16 However, addition of fibroblast growth factor (FGF)-2 and Matrigel to 3T3 preadipocytes was found to further potentiate maturation of adipose tissue, doubling triglyceride content and GPDH activity.16,18 With this approach, formation of engineered fat pads in mice comprised of mature adipocytes has been observed even in ear cartilage or in muscle recipient sites.18
Importantly, cellular behavior of these preadipocyte cell lines may not necessarily reflect true in vivo cellular behavior, and therefore primary cells have also been extensively studied for de novo adipose tissue engineering. Mesenchymal stem cells (MSCs) isolated from mature adult tissue represent the most commonly investigated primary cells, and their ability to undergo adipogenesis has been well documented.19–23 Adult stem cells derived from bone marrow (bMSCs) are among the best studied cellular building blocks for adipose regenerative strategies. Both rat and human bMSCs have been demonstrated to undergo adipogenic differentiation when cultured ex vivo with fibroblast growth factor-2 (FGF-2) on polylactide-co-glycolide or gelatin scaffolds, offering promising results for creation of in vivo constructs.24,25 Additionally, Choi et al. described the ability to generate new adipose tissue by pretreating rabbit bMSCs with adipogenic medium before implantation in immunocompromised mice.26 Finally, Alhadlaq and Mao employed human bMSCs encapsulated in a poly(ethylene glycol) dacrylate scaffold and found that subcutaneous implantation into SCID mice after a period of in vitro adipogenic differentiation could generate de novo lipid-containing tissue, as demonstrated by gene analysis and Oil Red O staining.27,28 While various scaffolds and growth factors have been employed in these studies, which are discussed in more detail below, they nonetheless highlight the potential for use of MSCs in adipose tissue engineering.
Similar to bMSCs, adipose-derived stromal cells (ASCs) have also been well studied as a potential cellular source for adipose regenerative strategies. Owing to their relative abundance and ease of harvest compared to bMSCs, ASCs represent an attractive alternative for de novo fat tissue engineering.29 Furthermore, the use of ASCs naturally found within the stromal vascular fraction (SVF) of adipose tissue to engineer mature adipose tissue may be a more rational strategy, particularly given their well-described capacity to undergo adipogenic differentiation.20,22,23,30,31 Studies have also suggested these cells may serve as a reservoir for the generation of new adipocytes.32–35 Reports have shown human ASCs to be capable of in vitro self-assembly in the presence of serum, ascorbic acid, and rosiglitazone, forming dense adipocyte-containing cell sheets with an organized extracellular matrix.36 In vivo adipose tissue constructs have also been generated using ASCs predifferentiated with adipogenic medium.37,38 Mouse-derived ASCs cultured in this setting and then injected into a subcutaneous pocket of immunocompromised mice were shown to generate mature lipid containing cells.37 This was similarly described with human ASCs, where subcutaneous implantation into nude mice demonstrated formation of mature adipose tissue of human origin.38 These findings thus reveal that ASCs may not only function as progenitor cells for natural adipose tissue homeostasis, but can also induce de novo adipogenesis, providing a promising approach to generate autologous adipose substitutes for a wide range of soft tissue defects.
Growth Factors and Cytokines Supporting Adipogenesis
Many studies have also involved the use of added factors and proteins to enhance the process of adipogenesis. These have included FGF-2, insulin and insulin-like growth factor (IGF)-1, matrix metalloproteinases (MMPs), glucocorticoids such as dexamethasone, thyroid hormone, epidermal growth factor (EGF), transforming growth factor (TGF)-β, platelet-derived growth factor (PDGF), and tumor necrosis factor alpha (TNF-α). While the breadth of these factors precludes an in-depth discussion of each within the scope of this review, the cellular physiology of healthy fat points to many of these potential growth factors and cytokines as promising strategies for adipose tissue engineering.
FGF-2 is naturally expressed in adipose tissue, and local production by adipocytes may regulate both subcutaneous and omental fat tissue mass.39 In combination with Matrigel, exogenous FGF-2 has proven to be a potent proadipogenic growth factor. When injected subcutaneously in mice with gelatin microspheres and Matrigel, FGF-2 has been shown to induce de novo formation of adipose tissue accompanied by microvasculature.40 Similar studies by Kimura et al. demonstrated FGF-2 injection to result in significantly greater novel adipose tissue volume generated in mice when compared to Matrigel implantation alone.41 Further detailed investigations with electron microscopy have suggested FGF-2 with Matrigel to promote a primary fibrotic response, with subsequent digestion of the scaffold stimulating adipogenic differentiation and accumulation of lipid droplets by local cells.42 These findings thus demonstrate the clinical potential for using FGF-2 to guide both adipogenesis and angiogenesis to generate stable soft tissue constructs.
Nearly 75% of IGF-1 found in fat is made locally in mature adipose tissue, specifically, by resident adipose tissue macrophages.43,44 IGF-1 has a significant impact on homeostatic regulation of fat tissue, and can promote the conversion of existing preadipocytes into mature adipocytes. IGF-1 has also been shown to be involved in glucocorticoid-mediated stimulation of adipocyte differentiation through small G protein Dexras1.45 Soluble IGF-1 bound to poly(lactic-co-glycolic) acid (PLGA)/PEG microspheres and injected subcutaneously in the deep muscular fascia of the abdominal muscle wall has been found to induce de novo adipocyte formation from within nonadipocyte cell depots 4 weeks after injection,46 making this growth factor a potential target for use in adipose tissue regenerative strategies.
MMPs are expressed in human adipocytes, and have been shown to drive preadipocyte differentiation into mature adipocytes.47 Congruently, TIMP-1 (tissue inhibitors of metalloproteinases-1), when supplied to adipose tissue, leads to a decrease in the formation of healthy, unilocular adipocytes in vivo, indicating a significant role of MMPs in native adipose tissue engineering. Captopril and batimastat are MMP inhibitors used to treat cancer via angiogenesis inhibition. Treatment of preadipocytes with these compounds markedly decreases lipogenesis and differentiation when compared to an untreated preadipocytes.47 Similarly, CP-544439, a pharmacological inhibitor of MMP-13, has also been shown to reduce adipocyte differentiation.48 In contrast, several gene expression studies have found multiple MMPs (e.g., MMP-2 and MMP-9) to be differentially upregulated during adipocyte differentiation,47,49,50 and their role as key regulators of adipocyte maturation may make them viable therapeutics to promote adipose tissue growth.
PDGF has been identified as an important factor in promoting formation of excess adipose tissue in the pathogenesis of Grave's ophthalmopathy, and inhibition of PDGF has been actively investigated to block adipogenesis in these patients.51 Treatment of fibroblasts in vitro with PDGF-BB induces the formation of adipose tissue, which highlights a potential for this growth factor in adipose tissue engineering strategies.51 Ting et al. also found use of PDGF-BB, in concert with other growth factors, to significantly increase creation of adipose tissue within subcutaneously implanted silicone chambers in immunocompromised mice.52 Interestingly, once preadipocytes are differentiated using PDGF, PDGF receptors on the cell surface have been found to downregulate. This may indicate a role for PDGF in the acquisition of an adipogenic fate, but this may not be as important for subsequent long-term adipose tissue maintenance.53 Other projects cite the importance of PDGF in adipose tissue growth, as hypertrophy of adipocytes is correlated directly with duration of PDGFR-α expression.54
Like several other growth factors, TNF-α has also been found to be an important regulator of adipogenesis. Specifically, studies have shown TNF-α to downregulate a series of genes responsible for de novo fat formation in white adipose tissue.55 Upregulation of TNF-α is also associated with adipocyte dysfunction and insulin resistance in the diabetic state.56 Controlling the inhibitory effects of TNF-α can be achieved using thiazolidinediones (TZD), a commonly used class antidiabetic drugs used in type II diabetes. TZD treated adipocytes in vitro showed rescue of adipocyte wasting induced by TNF-α.57 Furthermore, treatment of mice with rosiglitazone, a member of the TZD class of drugs, has been found to cause robust expression of proadipogenic transcripts including PPAR-γ, and this has been associated with higher bone marrow adiposity and ectopic adipogenesis.58
Finally, recent studies have identified the WNT family of autocrine and paracrine growth factors to be critical for adult adipose tissue maintenance and remodeling, and several reports have implicated WNT signaling in homeostatic regulation of adipogenesis.59,60 Wnt10b has been shown to have high expression in preadipocytes, which declines rapidly after induction of differentiation,61,62 and overexpression can block adipogenesis in mesenchymal stem cells.62 Disruption of WNT signaling with recombinant secreted frizzled-related proteins, or through constitutive expression of Axin, has been shown to result in spontaneous adipocyte differentiation.59,61,62 Additionally, studies have also shown Wnt10b anti-sera to promote adipocyte differentiation.63 These findings thus support manipulation of WNT signaling to enhance de novo adipogenesis strategies.
Importantly, although studies have shown manipulation of growth factors to be successful in enhancing adipose regeneration, well-known limitations of this approach include the high cost of production, and the requirement for supraphysiological concentration for efficacy, which can result in unwanted adverse effects in patients. These considerations have resulted in significant financial and regulatory hurdles that have limited the translational potential of growth factors for use in adipose tissue engineering.
Scaffolds for Adipose Regenerative Strategies
While multiple growth factors and cytokines have been evaluated to guide undifferentiated cells toward adipocyte lineage, there are still unaddressed concerns regarding tissue shape, volume, vascularity, and survival. Synthetic scaffolds, decellularized tissues, and use of more specific cell populations may provide ways of circumventing these issues. Scaffolds are three-dimensional (3D) constructs that can deliver live preadipocytes or supportive cells, growth factors, cytokines, or small molecules to promote formation of mature adipose tissue. Scaffolds can increase cellular viability and adhesion, guide cells toward de novo adipogenesis or vasculogenesis, and be used as a model to study complex cell–cell interactions. Scaffolds have been fabricated using a variety of different substrates, including decellularized adipose tissue (DAT) matrix, hydrogels, silk composites, and synthetically made “3D printed” scaffolds. They can exist as powders, sheets, injectable hydrogels, nanoparticles, beads, and macroporous foams, and encompass an array of different delivery methods.
Synthetic scaffolds
Biodegradable synthetic polymers are among the most widely investigated scaffolding materials for adipose tissue engineering due to the ability to modify their physical properties, scalability of production, and the fact that many are already FDA approved for clinical use (e.g., sutures, mesh). Poly(α-hydroxyacids) have been fabricated to mimic the physical architecture of natural collagen, and studies have shown their ability to enhance cell adhesion and guide differentiation. Rat preadipocytes have been seeded on PLGA, and when implanted into the dorsa of rats, early differentiated adipocytes could be detected by 5 weeks.64 Furthermore, PLGA modifications have been described with creation of PLGA-polyethylene glycol microspheres and PLGA (75:25) foam to serve as drug delivery vehicles for insulin, IGF-1, or FGF-2 to enhance de novo adipogenesis.24,65 However, as PLGA is degradable, some studies have noted degeneration of engineered adipose tissue over time and partially attribute this to resorption of the scaffold.66 Alternative nondegradable polymeric scaffolds have also been investigated, with fibronectin-coated polytetrafluoroethylene mesh examined. This scaffold has been shown to support both rat and human ASC attachment and differentiation into adipocytes for soft-tissue reconstruction purposes.67 These findings thus demonstrate that a variety of synthetic polymeric scaffolds may be potentially employed for adipose tissue engineering, each with their associated strengths. However, the number of available options also hints at their inherent limitations, as synthetic scaffolds can induce fibrous encapsulation, be extruded, or can result in a large inflammatory response, with associated cyst formation and overlying skin ulceration.68
Decellularized adipose tissue
The utility of DAT matrix as a substrate for tissue engineering revolves around its ability to closely mimic the architecture, mechanical, and biochemical properties of native adipose extracellular matrix. Thus, DAT scaffolds have been applied widely for studying adipogenesis.69–72 DAT matrices are typically derived through a decellularization process converting donor adipose tissue (e.g., subcutaneous abdominal wall adipose tissue) into acellular scaffolds using multiple freeze-thaw cycles, lyophilization, and ethanol sterilization.73 DAT scaffolds loaded with ASCs have shown improved volume retention in vivo as compared to hyaluronic acid fillers, and studies have revealed durable adipogenesis and neovascularization at 1 month.74 DAT scaffolds have also been delivered with various growth factors and cytokines. For instance, heparinized DAT loaded with FGF-2 was noted to significantly promote adipogenesis and neovascularization after 6 and 12 weeks, as compared to DAT scaffolds without FGF-2.75 Similarly, pretreatment of DAT with fibronectin has shown greater retention of ASCs in vivo in a rat hernia repair model,76 and this strategy may be similarly applied for adipose tissue regeneration.
Recent studies have now further modified DAT scaffolds to enhance their adipocyte-inductive ability and deliverability. Both DAT amalgamated with injectable hydrogel and enzyme-solubilized DAT to generate a microporous foam-beads have been designed for minimally invasive delivery.74,77 Similarly, interconnected networks of porous DAT beads fused through controlled freeze-thawing and lyophilization have generated bead foams capable of enhancing cell–cell interactions of seeded ASCs, which may further promote their capacity to undergo adipogenesis. Furthermore, DAT particles of smaller size, seeded with higher densities of ASCs have been found to potentially support more adipogenesis compared to larger DAT particles.78 These injectable DAT formulations have been found to be well tolerated in animal studies, with subcutaneous injection of these scaffolds in rats promoting a strong angiogenic and adipogenic response. Finally, DAT scaffolds have also been noted to have a proadipogenic effect on resident cells at the implant site. In terms of promoting native cell populations to create fat, DAT scaffolds seeded with ASCs have been found to enhance adipogenesis and angiogenesis by host-derived cells in rats. Han et al. found that the combination of ASCs with a natural adipose extracellular matrix could provide an inductive environment for adipose regeneration by infiltrating host cell populations.79
Though DAT scaffolds hold promise for adipose tissue engineering, as a decellularized material, it may still contain epitopes that may elicit an undesired immune response. The process of decellularization can also strip away more than just cells from the extracellular matrix, leaving a suboptimal environment to repopulate with functional cells. Finally, total removal of all cellular material is also difficult to ensure in the context of industrial mass production. Despite this, other forms of decellularized matrices are already in use clinically, and adoption of DAT scaffolds for soft tissue reconstruction may be forthcoming.
Hydrogels
Similar to DAT, hydrogels have been actively studied as a cellular delivery vehicle for adipose tissue regenerative strategies. However, a number factors need to be considered when creating a hydrogel for stabilizing cellular building blocks in soft tissue reconstruction, particularly, the ability of the hydrogel to bind and retain cell populations. For instance, PEG hydrogels loaded with YIGSR (a laminin-derived binding protein) can increase preadipocyte cell attachment to the hydrogel, and differentiation into mature unilocular adipocytes.80 Various other hydrogel/scaffold amalgams have been investigated to allow maximal cell attachment, viability, and differentiation. These have included collagen/alginate microspheres, fibrous-structures atelocollagen scaffolds, and honeycomb atelocollagen scaffolds. Hydrogels can also be converted to a foam-like state with hierarchical porosity and high water capacity. Examples of such foams are poly(amidoamine) oligomer macroporous foam (OPAAF). These foams contain high concentrations of RGD sites, meant to encourage integrin-mediated preadipocyte/mesenchymal cell binding.72,81,82 These scaffolds containing RGD-integrin sequences have been found to promote enhanced in vitro adipogenesis by SVF cells.83
Hydrogel scaffold modifications have also revolved around manipulation of 3D cellular interactions among seeded cells to promote adipogenesis. For example, macro-molecular crowding created by use of high molecular weight sucrous polymers to engender high volume cellular occupancy has been described to increase rates of microenvironmental biochemical reactions that can favor adipogenic differentiation through greater deposition of extracellular matrix proteins such as collagen type IV and perlecan.84 Hydrogel scaffolds that also allow growth of multipotent cells in the geometry of “small circular islands” have also been shown to enhance expression of adipogenic markers when compared to cells cultured in random geometries.85 Finally, sliding hydrogels with mobile molecular ligands allowing for reorganization of stem cells within the microenvironmental niche by flexible ligand cross-linkage can help to support more efficient differentiation along an adipogenic lineage.86
Scaffold mechanobiology
Apart from varying the actual hydrogel material, other important factors in designing these scaffolds include stabilization and stiffness of the gel. The degree of cross-linking is one variable that can be manipulated to control hydrogel stiffness. Interestingly, ASCs seeded on noncross-linked hyaluronic acid have been found to exhibit increased formation of adipocyte cellular spheroids, indicating some noncross-linked hydrogels to favor adipogenesis.87,88 Hydrogel stabilization using dexamethasone has been actively investigated, and is a noncytotoxic method for introducing a cell-laden gel to engineer adipose tissue.89 Furthermore, studies with DAT scaffolds and hydrogels evaluating stiffness have revealed that gels with stiffness most similar to adipose tissue (2 kPa) significantly increase adipogenic marker expression by seeded cells in the absence of any additional exogenous biochemical aid.90 Hyaluronic acid hydrogels with elastic moduli that also restrict cell-mediated degradation exhibit low cell spreading and low traction, favoring adipogenesis over osteogenesis, and switching permissive hydrogels to those with less traction caused a switch from osteogenesis to adipogenesis.91
3D printed scaffolds
Emerging technology has now led to the development of 3D printed DAT matrix constructs, which can be seeded with cells for tissue regenerative purposes. Such an approach allows the fabrication of customizable anatomically relevant tissue constructs comprised of suitable matrix materials and living cells. Pati et al. have demonstrated the utility of this method through their development of a DAT matrix bioink, which can be precisely printed into flexible 3D structures encapsulating ASCs.92,93 With this technique, ASCs have shown high cell viability and expression of standard adipogenic genes.92 Also of importance, this DAT matrix bioink was found to support positive tissue infiltration with minimal chronic inflammation and to promote mature adipose tissue formation when implanted subcutaneously in mice.92 Studies have also employed 3D printing with various hydrogel scaffolds, and the conformation and mechanical properties of 3D printed hydrogel scaffolds have been shown to be important for promoting adipogenesis.94 Nonetheless while the microstructure of 3D printed scaffolds is important for differentiation, translation to larger, clinically relevant macroscopic structures remains challenging. At the present time, application of this strategy to reconstruct smaller facial deformities may thus be more feasible than for larger soft tissue deficits as seen after cancer resection.
Emerging Developments in Autologous Fat Transfer
While efforts continue to refine strategies for de novo adipose tissue engineering with studies on adipocyte precursors, growth factor supplements, and development of scaffolds, studies have also focused on contemporary approaches to reconstruct soft tissue defects with transfer of autologous fat. Recent investigations have also begun to evaluate how this approach may be improved through the incorporation of progenitor cells, namely ASCs. Autologous fat grafting has emerged over the past two and half decades as popular means to address tissue deficits secondary to trauma, cancer resection, or congenital malformations. However, clinical outcomes with fat grafting alone have varied widely, and reported retention rates remain inconsistent.95–100
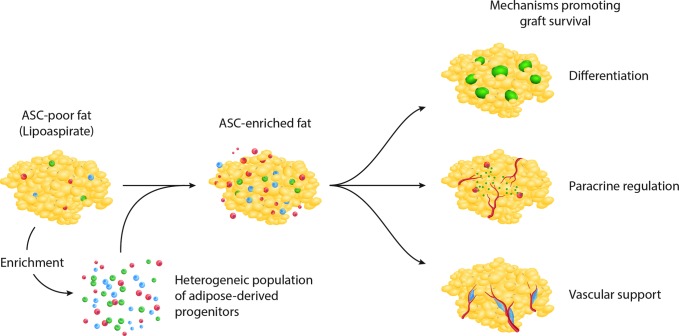
To address variability in fat graft retention, enrichment of fat before injection with ASCs has been promoted (cell-assisted lipotransfer [CAL]).101,102 This approach was developed based on the relative paucity of ASCs found in lipoaspirate relative to excised whole fat, which may contribute to long-term atrophy of fat grafts.78,103 Both preclinical and clinical studies have reported enhanced fat graft retention when enriched with ASCs,78,101,102,104 and investigations have demonstrated as little as 5 × 104 supplemental ASCs per milliliter of transferred fat to be necessary to enhance outcomes.105 How cellular supplementation promotes graft survival, however, remains actively investigated, and as discussed above, the known capacity for ASC adipogenesis along with their well-described angiogenic function have been proposed for their role in CAL (Fig. 2).
FIG. 2.
Proposed roles supplemental ASCs play in cell-assisted lipotransfer include contribution to adipose tissue through adipogenic differentiation, paracrine regulation of angiogenesis, and direct vascular support.
Autologous fat is transferred under ischemic conditions, predisposing adipocytes to cell death. Early studies, however, have suggested a process of dynamic remodeling of adipose tissue after nonvascularized grafting, with resident ASCs participating in adipocyte regeneration.32,35 This was similarly suggested by findings of CD34+/Ki67+ proliferating ASCs associated with perilipin-positive small new adipocytes after fat grafting in mice.33 In concert with these findings, Fu et al. supplemented wild-type mouse inguinal fat pad adipose tissue with green fluorescence protein (GFP)-positive ASCs and noted development of labeled adipocytes by immunofluorescence 7 days after implantation.106 However, fluorescence signal intensity was also noted to fall drastically after 14 days, raising questions regarding the durability of these new adipocytes.
Considering these findings, a number of other studies have instead suggested supplemental ASCs to enhance autologous fat graft retention through promotion of early revascularization. This is supported by the Garza et al. who noted only transient retention of GFP-labeled human ASCs in a mouse model of human fat grafting.107 Single cell gene analysis of retrieved ASCs primarily demonstrated upregulation of proangiogenic factors in conjunction with low of expression for markers of adipogenic differentiation. Furthermore, Lu et al. observed enhanced fat graft outcomes when supplementation was performed with ASCs induced to overexpress vascular endothelial growth factor instead of untransfected ASCs, findings that are also in agreement with a paracrine role promoting graft revascularization. It has even been suggested that supplemental ASCs may improve vascularity through direct incorporation into new blood vessels, although a robust contribution has yet to be demonstrated.106,108,109
With an improved understanding of how ASCs may be used to enhance contemporary soft tissue reconstruction strategies, emerging studies have now begun to evaluate how this may be optimized. It is well known that ASCs, isolated from the SVF, are a heterogeneous population and functionally distinct subpopulations can be defined through cell surface marker profiles.110 This has already been well demonstrated in bone where a multitude of markers have been defined, which successfully enrich for cells with enhanced osteogenic potential (e.g., CD105, CD90, bone morphogenetic protein receptor [BMPR]-IB).111–113 In similar fashion, isolation of proadipogenic cell populations may also help to facilitate improved soft tissue reconstructive strategies. Gierloff and colleagues noted CD29-negative rat ASCs to have enhanced in vitro adipogenic potential114 compared to other fractions. Expression of BMPR-IA was also recently found to identify a subpopulation of human ASCs with a preferential adipogenic fate.31 Transplanting GFP-positive ASCs enriched for BMPR-IA into the inguinal fat pads of immunocompromised mice, Zielins et al. noted significantly greater formation of labeled, matured adipocytes after 4 weeks.31 Finally, CD24 and CD271 have both been employed to identify murine ASCs with enhanced in vitro and in vivo adipogenic capacity. However, it is prudent to recognize that the low frequency of these populations may blunt their translational application.115,116 Nonetheless, these studies have potential implications for future strategies involving de novo adipogenesis, as previously discussed, and for enhancing current fat grafting outcomes through enrichment with specific ASC subpopulations.
Identification of angiogenic ASC subpopulations may also enhance current approaches to soft tissue reconstruction, as supplementation of autologous adipose tissue with these functionally distinct ASCs may promote earlier revascularization of ischemic grafts. Importantly, studies in diabetes have revealed depletion of specific ASCs with a highly angiogenic profile. Distribution analysis of ASCs from diabetic or wild-type mice demonstrated loss of specific ASCs characterized by a transcriptional profile with high expression of vascular-related genes such as angiopoietin-1, stromal cell-derived factor-1, and MMP-3.117 Analogous findings have also been reported with aging, where age-related depletion of a subpopulation of ASCs characterized by a provascular transcriptional profile has been observed.118 These data suggest that subpopulations of ASCs exist, which may be primed to support angiogenesis and depletion in the diabetic and aged state may correlate with impairment of vascular potential. In more recent studies, preliminary findings by our own group using single cell transcriptional analysis and linear regression have identified CD146 and CD248 as candidate cell surface markers for isolation of proangiogenic human ASCs. Ongoing studies will determine whether these subpopulations of ASCs may be incorporated into CAL strategies to further enhance reconstructive approaches to soft tissue defects.
Conclusion
The creation of adipose tissue can be optimized through multiple different methods. Choosing to supply cellular populations to enhance de novo adipogenesis in early animal studies has certainly been proven to be a promising approach, but obtaining these populations may require ex vivo expansion and/or flow cytometric isolation, which raises certain regulatory hurdles. Supplying growth factors or small molecule agonists/antagonists in concert with carefully designed scaffolds to augment proadipogenic pathways represents another promising avenue to enhance cell-based approaches to adipose tissue engineering. Finally, with enhanced understanding of progenitor cell function, contemporary techniques to address soft tissue reconstruction may be further refined to yield improved outcomes in the treatment of patients with deficient soft tissue.
Acknowledgments
M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-05, R56 DE025597, the Oak Foundation, the Gunn/Olivier Fund, and the Hagey Laboratory for Pediatric Regenerative Medicine. D.C.W. was supported by NIH grant K08 DE024269, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award.
Disclosure Statement
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this article.
References
- 1.Yoshimura K., and Coleman S.R. Complications of fat grafting: how they occur and how to find, avoid, and treat them. Clin Plast Surg 42, 383, ix, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Yu N.Z., Huang J.Z., Zhang H., Wang Y., Wang X.J., Zhao R., et al. A systemic review of autologous fat grafting survival rate and related severe complications. Chin Med J 128, 1245, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maumus M., Peyrafitte J.A., D'Angelo R., Fournier-Wirth C., Bouloumie A., Casteilla L., et al. Native human adipose stromal cells: localization, morphology and phenotype. Int J Obes (2005) 35, 1141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mikkelsen T.S., Xu Z., Zhang X., Wang L., Gimble J.M., Lander E.S., et al. Comparative epigenomic analysis of murine and human adipogenesis. Cell 143, 156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ailhaud G. Extracellular factors, signalling pathways and differentiation of adipose precursor cells. Curr Opin Cell Biol 2, 1043, 1990 [DOI] [PubMed] [Google Scholar]
- 6.Ye J., Gao Z., Yin J., and He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 293, E1118, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Schiller Z.A., Schiele N.R., Sims J.K., Lee K., and Kuo C.K. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem Cell Res Ther 4, 79, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartelt A., Weigelt C., Cherradi M.L., Niemeier A., Tödter K., Heeren J., et al. Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim Biophys Acta 1831, 934, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Hodson L., and Karpe F. Is there something special about palmitoleate? Curr Opin Clin Nutr Metab Care 16, 225, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Cao H., Gerhold K., Mayers J.R., Wiest M.M., Watkins S.M., and Hotamisligil G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 134, 933, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green H., and Kehinde O. Spontaneous heritable changes leading to increased adipose conversion in 3T3 cells. Cell 7, 105, 1976 [DOI] [PubMed] [Google Scholar]
- 12.Green H., and Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell 3, 127, 1974 [DOI] [PubMed] [Google Scholar]
- 13.Kuri-Harcuch W., and Green H. Adipose conversion of 3T3 cells depends on a serum factor. Proc Natl Acad Sci U S A 75, 6107, 1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn L., and Woodhouse K.A. Adipose tissue engineering with cells in engineered matrices. Organogenesis 4, 228, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischbach C., Seufert J., Staiger H., Hacker M., Neubauer M., Gopferich A., et al. Three-dimensional in vitro model of adipogenesis: comparison of culture conditions. Tissue Eng 10, 215, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Kawaguchi N., Toriyama K., Nicodemou-Lena E., Inou K., Torii S., and Kitagawa Y. Reconstituted basement membrane potentiates in vivo adipogenesis of 3T3-F442A cells. Cytotechnology 31, 215, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischbach C., Spruss T., Weiser B., Neubauer M., Becker C., Hacker M., et al. Generation of mature fat pads in vitro and in vivo utilizing 3-D long-term culture of 3T3-L1 preadipocytes. Exp Cell Res 300, 54, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi N., Toriyama K., Nicodemou-Lena E., Inou K., Torii S., and Kitagawa Y. De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci U S A 95, 1062, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. Multilineage potential of adult human mesenchymal stem cells. Science 284, 143, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7, 211, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Lin T.M., Tsai J.L., Lin S.D., Lai C.S., and Chang C.C. Accelerated growth and prolonged lifespan of adipose tissue-derived human mesenchymal stem cells in a medium using reduced calcium and antioxidants. Stem Cells Dev 14, 92, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Guilak F., Lott K.E., Awad H.A., Cao Q., Hicok K.C., Fermor B., et al. Clonal analysis of the differentiation potential of human adipose-derived adult stem cells. J Cell Physiol 206, 229, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Zuk P.A., Zhu M., Ashjian P., De Ugarte D.A., Huang J.I., Mizuno H., et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13, 4279, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neubauer M., Hacker M., Bauer-Kreisel P., Weiser B., Fischbach C., Schulz M.B., et al. Adipose tissue engineering based on mesenchymal stem cells and basic fibroblast growth factor in vitro. Tissue Eng 11, 1840, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Hong L., Peptan I., Clark P., and Mao J.J. Ex vivo adipose tissue engineering by human marrow stromal cell seeded gelatin sponge. Ann Biomed Eng 33, 511, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Choi Y.S., Park S.N., and Suh H. Adipose tissue engineering using mesenchymal stem cells attached to injectable PLGA spheres. Biomaterials 26, 5855, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Alhadlaq A., and Mao J.J. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am 87, 936, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Alhadlaq A., Tang M., and Mao J.J. Engineered adipose tissue from human mesenchymal stem cells maintains predefined shape and dimension: implications in soft tissue augmentation and reconstruction. Tissue Eng 11, 556, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Gomillion C.T., and Burg K.J. Stem cells and adipose tissue engineering. Biomaterials 27, 6052, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Lin G., Garcia M., Ning H., Banie L., Guo Y.L., Lue T.F., et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev 17, 1053, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zielins E.R., Paik K., Ransom R.C., Brett E.A., Blackshear C.P., Luan A., et al. Enrichment of adipose-derived stromal cells for BMPR1A facilitates enhanced adipogenesis. Tissue Eng Part A 22, 214, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eto H., Kato H., Suga H., Aoi N., Doi K., Kuno S., et al. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast Reconstr Surg 129, 1081, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Kato H., Mineda K., Eto H., Doi K., Kuno S., Kinoshita K., et al. Degeneration, regeneration, and cicatrization after fat grafting: dynamic total tissue remodeling during the first 3 months. Plast Reconstr Surg 133, 303e, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Suga H., Eto H., Aoi N., Kato H., Araki J., Doi K., et al. Adipose tissue remodeling under ischemia: death of adipocytes and activation of stem/progenitor cells. Plast Reconstr Surg 126, 1911, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Doi K., Ogata F., Eto H., Kato H., Kuno S., Kinoshita K., et al. Differential contributions of graft-derived and host-derived cells in tissue regeneration/remodeling after fat grafting. Plast Reconstr Surg 135, 1607, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Vallee M., Cote J.F., and Fradette J. Adipose-tissue engineering: taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol Biol (Paris) 57, 309, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Mizuno H., Itoi Y., Kawahara S., Ogawa R., Akaishi S., and Hyakusoku H. In vivo adipose tissue regeneration by adipose-derived stromal cells isolated from GFP transgenic mice. Cells Tissues Organs 187, 177, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Tsuji W., Inamoto T., Yamashiro H., Ueno T., Kato H., Kimura Y., et al. Adipogenesis induced by human adipose tissue-derived stem cells. Tissue Eng Part A 15, 83, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Gabrielsson B.G., Johansson J.M., Jennische E., Jernas M., Itoh Y., Peltonen M., et al. Depot-specific expression of fibroblast growth factors in human adipose tissue. Obes Res 10, 608, 2002 [DOI] [PubMed] [Google Scholar]
- 40.Tabata Y., Miyao M., Inamoto T., Ishii T., Hirano Y., Yamaoki Y., et al. De novo formation of adipose tissue by controlled release of basic fibroblast growth factor. Tissue Eng 6, 279, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Kimura Y., Ozeki M., Inamoto T., and Tabata Y. Time course of de novo adipogenesis in matrigel by gelatin microspheres incorporating basic fibroblast growth factor. Tissue Eng 8, 603, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Toriyama K., Kawaguchi N., Kitoh J., Tajima R., Inou K., Kitagawa Y., et al. Endogenous adipocyte precursor cells for regenerative soft-tissue engineering. Tissue Eng 8, 157, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Kloting N., Koch L., Wunderlich T., Kern M., Ruschke K., Krone W., et al. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes 57, 2074, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang H.R., Kim H.J., Xu X., and Ferrante A.W., Jr. Macrophage and adipocyte IGF1 maintain adipose tissue homeostasis during metabolic stresses. Obesity (Silver Spring) 24, 172, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H.J., Cha J.Y., Seok J.W., Choi Y., Yoon B.K., Choi H., et al. Dexras1 links glucocorticoids to insulin-like growth factor-1 signaling in adipogenesis. Sci Rep 6, 28648, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuksel E., Weinfeld A.B., Cleek R., Waugh J.M., Jensen J., Boutros S., et al. De novo adipose tissue generation through long-term, local delivery of insulin and insulin-like growth factor-1 by PLGA/PEG microspheres in an in vivo rat model: a novel concept and capability. Plast Reconstr Surg 105, 1721, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Bouloumie A., Sengenes C., Portolan G., Galitzky J., and Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes 50, 2080, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Shih C.L., and Ajuwon K.M. Inhibition of MMP-13 prevents diet-induced obesity in mice and suppresses adipogenesis in 3T3-L1 preadipocytes. Mol Biol Rep 42, 1225, 2015 [DOI] [PubMed] [Google Scholar]
- 49.Chavey C., Mari B., Monthouel M.N., Bonnafous S., Anglard P., Van Obberghen E., et al. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem 278, 11888, 2003 [DOI] [PubMed] [Google Scholar]
- 50.Croissandeau G., Chretien M., and Mbikay M. Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem J 364(Pt 3), 739, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virakul S., Dalm V.A., Paridaens D., van den Bosch W.A., Mulder M.T., Hirankarn N., et al. Platelet-derived growth factor-BB enhances adipogenesis in orbital fibroblasts. Invest Ophthalmol Vis Sci 56, 5457, 2015 [DOI] [PubMed] [Google Scholar]
- 52.Ting A.C., Craft R.O., Palmer J.A., Gerrand Y.W., Penington A.J., Morrison W.A., et al. The adipogenic potential of various extracellular matrices under the influence of an angiogenic growth factor combination in a mouse tissue engineering chamber. Acta Biomater 10, 1907, 2014 [DOI] [PubMed] [Google Scholar]
- 53.Vaziri C., and Faller D.V. Down-regulation of platelet-derived growth factor receptor expression during terminal differentiation of 3T3-L1 pre-adipocyte fibroblasts. J Biol Chem 271, 13642, 1996 [DOI] [PubMed] [Google Scholar]
- 54.Bluher M., Patti M.E., Gesta S., Kahn B.B., and Kahn C.R. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem 279, 31891, 2004 [DOI] [PubMed] [Google Scholar]
- 55.Anghel S.I., and Wahli W. Fat poetry: a kingdom for PPAR[gamma]. Cell Res 17, 486, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Ruan H., Miles P.D., Ladd C.M., Ross K., Golub T.R., Olefsky J.M., et al. Profiling gene transcription in vivo reveals adipose tissue as an immediate target of tumor necrosis factor-alpha: implications for insulin resistance. Diabetes 51, 3176, 2002 [DOI] [PubMed] [Google Scholar]
- 57.Ohsumi J., Sakakibara S., Yamaguchi J., Miyadai K., Yoshioka S., Fujiwara T., et al. Troglitazone prevents the inhibitory effects of inflammatory cytokines on insulin-induced adipocyte differentiation in 3T3-L1 cells. Endocrinology 135, 2279, 1994 [DOI] [PubMed] [Google Scholar]
- 58.Youm Y.H., Yang H., Amin R., Smith S.R., Leff T., and Dixit V.D. Thiazolidinedione treatment and constitutive-PPARgamma activation induces ectopic adipogenesis and promotes age-related thymic involution. Aging Cell 9, 478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christodoulides C., Lagathu C., Sethi J.K., and Vidal-Puig A. Adipogenesis and WNT signalling. Trends Endocrinol Metab 20, 16, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Laudes M. Role of WNT signalling in the determination of human mesenchymal stem cells into preadipocytes. J Mol Endocrinol 46, R65, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Ross S.E., Hemati N., Longo K.A., Bennett C.N., Lucas P.C., Erickson R.L., et al. Inhibition of adipogenesis by Wnt signaling. Science 289, 950, 2000 [DOI] [PubMed] [Google Scholar]
- 62.Bennett C.N., Ross S.E., Longo K.A., Bajnok L., Hemati N., Johnson K.W., et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem 277, 30998, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Krishnan V., Bryant H.U., and Macdougald O.A. Regulation of bone mass by Wnt signaling. J Clin Invest 116, 1202, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patrick C.W., Jr., Chauvin P.B., Hobley J., and Reece G.P. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering. Tissue Eng 5, 139, 1999 [DOI] [PubMed] [Google Scholar]
- 65.Shenaq S.M., and Yuksel E. New research in breast reconstruction: adipose tissue engineering. Clin Plast Surg 29, 111, vi, 2002 [DOI] [PubMed] [Google Scholar]
- 66.Patrick C.W. Breast tissue engineering. Annu Rev Biomed Eng 6, 109, 2004 [DOI] [PubMed] [Google Scholar]
- 67.Kral J.G., and Crandall D.L. Development of a human adipocyte synthetic polymer scaffold. Plast Reconstr Surg 104, 1732, 1999 [DOI] [PubMed] [Google Scholar]
- 68.O'Brien F.J. Biomaterials & scaffolds for tissue engineering. Mater Today 14, 88, 2011 [Google Scholar]
- 69.Turner A.E., Yu C., Bianco J., Watkins J.F., and Flynn L.E. The performance of decellularized adipose tissue microcarriers as an inductive substrate for human adipose-derived stem cells. Biomaterials 33, 4490, 2012 [DOI] [PubMed] [Google Scholar]
- 70.Choi Y.C., Choi J.S., Kim B.S., Kim J.D., Yoon H.I., and Cho Y.W. Decellularized extracellular matrix derived from porcine adipose tissue as a xenogeneic biomaterial for tissue engineering. Tissue Eng Part C Methods 18, 866, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu I., Nahas Z., Kimmerling K.A., Rosson G.D., and Elisseeff J.H. An injectable adipose matrix for soft-tissue reconstruction. Plast Reconstr Surg 129, 1247, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Flynn L.E. The use of decellularized adipose tissue to provide an inductive microenvironment for the adipogenic differentiation of human adipose-derived stem cells. Biomaterials 31, 4715, 2010 [DOI] [PubMed] [Google Scholar]
- 73.Dunne L.W., Huang Z., Meng W., Fan X., Zhang N., Zhang Q., et al. Human decellularized adipose tissue scaffold as a model for breast cancer cell growth and drug treatments. Biomaterials 35, 4940, 2014 [DOI] [PubMed] [Google Scholar]
- 74.Young D.A., Ibrahim D.O., Hu D., and Christman K.L. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater 7, 1040, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S., Lu Q., Cao T., and Toh W.S. Adipose tissue and extracellular matrix development by injectable decellularized adipose matrix loaded with basic fibroblast growth factor. Plast Reconstr Surg 137, 1171, 2016 [DOI] [PubMed] [Google Scholar]
- 76.Klinger A., Kawata M., Villalobos M., Jones R.B., Pike S., Wu N., et al. Living scaffolds: surgical repair using scaffolds seeded with human adipose-derived stem cells. Hernia 20, 161, 2016 [DOI] [PubMed] [Google Scholar]
- 77.Yu C., Bianco J., Brown C., Fuetterer L., Watkins J.F., Samani A., et al. Porous decellularized adipose tissue foams for soft tissue regeneration. Biomaterials 34, 3290, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Matsumoto D., Sato K., Gonda K., Takaki Y., Shigeura T., Sato T., et al. Cell-assisted lipotransfer: supportive use of human adipose-derived cells for soft tissue augmentation with lipoinjection. Tissue Eng 12, 3375, 2006 [DOI] [PubMed] [Google Scholar]
- 79.Han T.T., Toutounji S., Amsden B.G., and Flynn L.E. Adipose-derived stromal cells mediate in vivo adipogenesis, angiogenesis and inflammation in decellularized adipose tissue bioscaffolds. Biomaterials 72, 125, 2015 [DOI] [PubMed] [Google Scholar]
- 80.Brandl F.P., Seitz A.K., Teßmar J.K.V., Blunk T., and Göpferich A.M. Enzymatically degradable poly(ethylene glycol) based hydrogels for adipose tissue engineering. Biomaterials 31, 3957, 2010 [DOI] [PubMed] [Google Scholar]
- 81.Luan A., Duscher D., Whittam A.J., Paik K.J., Zielins E.R., Brett E.A., et al. Cell-assisted lipotransfer improves volume retention in irradiated recipient sites and rescues radiation-induced skin changes. Stem Cells 34, 668, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Adam Young D., Bajaj V., and Christman K.L. Award winner for outstanding research in the PhD category, 2014 Society for Biomaterials annual meeting and exposition, Denver, Colorado, April 16–19, 2014: decellularized adipose matrix hydrogels stimulate in vivo neovascularization and adipose formation. J Biomed Mater Res Part A 102, 1641, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Doi K., Tanaka S., Iida H., Eto H., Kato H., Aoi N., et al. Stromal vascular fraction isolated from lipo-aspirates using an automated processing system: bench and bed analysis. J Tissue Eng Regen Med 7, 864, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Ang X.M., Lee M.H., Blocki A., Chen C., Ong L.L., Asada H.H., et al. Macromolecular crowding amplifies adipogenesis of human bone marrow-derived mesenchymal stem cells by enhancing the pro-adipogenic microenvironment. Tissue Eng Part A 20, 966, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee J., Abdeen A.A., Zhang D., and Kilian K.A. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials 34, 8140, 2013 [DOI] [PubMed] [Google Scholar]
- 86.Tong X., and Yang F. Sliding hydrogels with mobile molecular ligands and crosslinks as 3D stem cell niche. Adv Mater 28, 7257, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mineda K., Feng J., Ishimine H., Takada H., Doi K., Kuno S., et al. Therapeutic potential of human adipose-derived stem/stromal cell microspheroids prepared by three-dimensional culture in non-cross-linked hyaluronic acid gel. Stem Cells Transl Med 4, 1511, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Korurer E., Kenar H., Doger E., and Karaoz E. Production of a composite hyaluronic acid/gelatin blood plasma gel for hydrogel-based adipose tissue engineering applications. J Biomed Mater Res Part A 102, 2220, 2014 [DOI] [PubMed] [Google Scholar]
- 89.Fan M., Ma Y., Zhang Z., Mao J., Tan H., and Hu X. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels-Alder chemistry for adipose tissue engineering. Mater Sci Eng C Mater Biol Appl 56, 311, 2015 [DOI] [PubMed] [Google Scholar]
- 90.Young D.A., Choi Y.S., Engler A.J., and Christman K.L. Stimulation of adipogenesis of adult adipose-derived stem cells using substrates that mimic the stiffness of adipose tissue. Biomaterials 34, 8581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., and Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 12, 458, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pati F., Ha D.H., Jang J., Han H.H., Rhie J.W., and Cho D.W. Biomimetic 3D tissue printing for soft tissue regeneration. Biomaterials 62, 164, 2015 [DOI] [PubMed] [Google Scholar]
- 93.Pati F., Jang J., Ha D.H., Won Kim S., Rhie J.W., Shim J.H., et al. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun 5, 3935, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Das S., Pati F., Choi Y.J., Rijal G., Shim J.H., Kim S.W., et al. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater 11, 233, 2015 [DOI] [PubMed] [Google Scholar]
- 95.Coleman S.R. Facial recontouring with lipostructure. Clin Plast Surg 24, 347, 1997 [PubMed] [Google Scholar]
- 96.Chajchir A., and Benzaquen I. Fat-grafting injection for soft-tissue augmentation. Plast Reconstr Surg 84, 921; discussion 935, 1989 [PubMed] [Google Scholar]
- 97.Bircoll M. Cosmetic breast augmentation utilizing autologous fat and liposuction techniques. Plast Reconstr Surg 79, 267, 1987 [DOI] [PubMed] [Google Scholar]
- 98.Del Vecchio D.A., and Bucky L.P. Breast augmentation using preexpansion and autologous fat transplantation: a clinical radiographic study. Plast Reconstr Surg 127, 2441, 2011 [DOI] [PubMed] [Google Scholar]
- 99.Khouri R., and Del Vecchio D. Breast reconstruction and augmentation using pre-expansion and autologous fat transplantation. Clin Plast Surg 36, 269, viii, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Coleman S.R., and Saboeiro A.P. Fat grafting to the breast revisited: safety and efficacy. Plast Reconstr Surg 119, 775; discussion 786, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Yoshimura K., Sato K., Aoi N., Kurita M., Hirohi T., and Harii K. Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 32, 48; discussion 56, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolle S.F., Fischer-Nielsen A., Mathiasen A.B., Elberg J.J., Oliveri R.S., Glovinski P.V., et al. Enrichment of autologous fat grafts with ex-vivo expanded adipose tissue-derived stem cells for graft survival: a randomised placebo-controlled trial. Lancet 382, 1113, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Yoshimura K., Shigeura T., Matsumoto D., Sato T., Takaki Y., Aiba-Kojima E., et al. Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208, 64, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Tanikawa D.Y., Aguena M., Bueno D.F., Passos-Bueno M.R., and Alonso N. Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg 132, 141, 2013 [DOI] [PubMed] [Google Scholar]
- 105.Paik K.J., Zielins E.R., Atashroo D.A., Maan Z.N., Duscher D., Luan A., et al. Studies in Fat Grafting: Part V. Cell-assisted lipotransfer to enhance fat graft retention is dose dependent. Plast Reconstr Surg 136, 67, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fu S., Luan J., Xin M., Wang Q., Xiao R., and Gao Y. Fate of adipose-derived stromal vascular fraction cells after co-implantation with fat grafts: evidence of cell survival and differentiation in ischemic adipose tissue. Plast Reconstr Surg 132, 363, 2013 [DOI] [PubMed] [Google Scholar]
- 107.Garza R.M., Rennert R.C., Paik K.J., Atashroo D., Chung M.T., Duscher D., et al. Studies in fat grafting: Part IV. Adipose-derived stromal cell gene expression in cell-assisted lipotransfer. Plast Reconstr Surg 135, 1045, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu F., Li J., Gao J., Ogawa R., Ou C., Yang B., et al. Improvement of the survival of human autologous fat transplantation by using VEGF-transfected adipose-derived stem cells. Plast Reconstr Surg 124, 1437, 2009 [DOI] [PubMed] [Google Scholar]
- 109.Zhu M., Zhou Z., Chen Y., Schreiber R., Ransom J.T., Fraser J.K., et al. Supplementation of fat grafts with adipose-derived regenerative cells improves long-term graft retention. Ann Plast Surg 64, 222, 2010 [DOI] [PubMed] [Google Scholar]
- 110.Johal K.S., Lees V.C., and Reid A.J. Adipose-derived stem cells: selecting for translational success. Regen Med 10, 79, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Levi B., Wan D.C., Glotzbach J.P., Hyun J., Januszyk M., Montoro D., et al. CD105 protein depletion enhances human adipose-derived stromal cell osteogenesis through reduction of transforming growth factor beta1 (TGF-beta1) signaling. J Biol Chem 286, 39497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chung M.T., Liu C., Hyun J.S., Lo D.D., Montoro D.T., Hasegawa M., et al. CD90 (Thy-1)-positive selection enhances osteogenic capacity of human adipose-derived stromal cells. Tissue Eng Part A 19, 989, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McArdle A., Chung M.T., Paik K.J., Duldulao C., Chan C., Rennert R., et al. Positive selection for bone morphogenetic protein receptor type-IB promotes differentiation and specification of human adipose-derived stromal cells toward an osteogenic lineage. Tissue Eng Part A 20, 3031, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gierloff M., Petersen L., Oberg H.H., Quabius E.S., Wiltfang J., and Acil Y. Adipogenic differentiation potential of rat adipose tissue-derived subpopulations of stromal cells. J Plast Reconstr Aesthet Surg 67, 1427, 2014 [DOI] [PubMed] [Google Scholar]
- 115.Rodeheffer M.S., Birsoy K., and Friedman J.M. Identification of white adipocyte progenitor cells in vivo. Cell 135, 240, 2008 [DOI] [PubMed] [Google Scholar]
- 116.Xiao J., Yang X., Jing W., Guo W., Sun Q., Lin Y., et al. Adipogenic and osteogenic differentiation of Lin(−)CD271(+)Sca-1(+) adipose-derived stem cells. Mol Cell Biochem 377, 107, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Rennert R.C., Sorkin M., Januszyk M., Duscher D., Kosaraju R., Chung M.T., et al. Diabetes impairs the angiogenic potential of adipose-derived stem cells by selectively depleting cellular subpopulations. Stem Cell Res Ther 5, 79, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Duscher D., Rennert R.C., Januszyk M., Anghel E., Maan Z.N., Whittam A.J., et al. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 4, 7144, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]