Abstract
The aim of this study was to explore whether the phenomenon of brain tumor-related neurovascular uncoupling (NVU) in resting-state blood oxygen level-dependent functional magnetic resonance imaging (BOLD fMRI) (rsfMRI) may also affect the resting-state fMRI (rsfMRI) frequency domain metrics the amplitude of low-frequency fluctuation (ALFF) and fractional ALFF (fALFF). Twelve de novo brain tumor patients, who underwent clinical fMRI examinations, including task-based fMRI (tbfMRI) and rsfMRI, were included in this Institutional Review Board-approved study. Each patient displayed decreased/absent tbfMRI activation in the primary ipsilesional (IL) sensorimotor cortex in the absence of a corresponding motor deficit or suboptimal task performance, consistent with NVU. Z-score maps for the motor tasks were obtained from general linear model analysis (reflecting motor activation vs. rest). Seed-based correlation analysis (SCA) maps of sensorimotor network, ALFF, and fALFF were calculated from rsfMRI data. Precentral and postcentral gyri in contralesional (CL) and IL hemispheres were parcellated using an automated anatomical labeling template for each patient. Region of interest (ROI) analysis was performed on four maps: tbfMRI, SCA, ALFF, and fALFF. Voxel values in the CL and IL ROIs of each map were divided by the corresponding global mean of ALFF and fALFF in the cortical brain tissue. Group analysis revealed significantly decreased IL ALFF (p = 0.02) and fALFF (p = 0.03) metrics compared with CL ROIs, consistent with similar findings of significantly decreased IL BOLD signal for tbfMRI (p = 0.0005) and SCA maps (p = 0.0004). The frequency domain metrics ALFF and fALFF may be markers of lesion-induced NVU in rsfMRI similar to previously reported alterations in tbfMRI activation and SCA-derived resting-state functional connectivity maps.
Keywords: : frequency domain metrics ALFF (amplitude of low-frequency fluctuation) and fALFF (fractional ALFF), motor activation, neurovascular uncoupling, presurgical mapping, resting-state fMRI
Introduction
In recent years, there has been an increase in interest in the application of resting-state blood oxygenation level-dependent (BOLD) functional magnetic resonance imaging (fMRI) (rsfMRI) for presurgical planning in patients with brain tumors. First proposed by Biswal and associates (1995), rsfMRI focuses on spontaneous low-frequency fluctuations (<0.1 Hz) in the BOLD signal in subjects scanned while at rest (i.e., without performance of any cognitive, language, or motor tasks). Because it does not require patient cooperation for specific task performance, rsfMRI may be particularly useful in patients who are not able to undergo conventional task-based fMRI (tbfMRI) for lesion localization (Fukunaga et al., 2006; Liu et al., 2009; Shimony et al., 2009; Zhang and Raichle, 2010). Also, rsfMRI can identify many networks such as the sensorimotor, visual, and default mode networks (DMN) (Beckmann et al., 2005; Damoiseaux et al., 2006; Kiviniemi et al., 2003; Raichle et al., 2001; Seeley et al., 2007; Smith et al., 2009) simultaneously during a single acquisition.
Although rsfMRI has potential advantages over tbfMRI, rsfMRI (Agarwal et al., 2016a,b; Mallela et al., 2016) also suffers from similar important clinical limitations of false negative BOLD signals as seen in tbfMRI (Holodny et al., 2000; Ulmer et al., 2003; Zacà et al., 2014) due to brain tumor-related neurovascular uncoupling (NVU); thus, practical application of rsfMRI for presurgical planning should take into account the possibility of NVU. NVU is characterized by the presence of abnormally decreased tbfMRI activation in the ipsilesional (IL) hemisphere when the patient neither exhibits substantial neurological deficit that would be indicative of tumor-related destruction of eloquent cortex nor displays inability to perform the required tasks (Zacà et al., 2014).
Studies have demonstrated that NVU in high-grade gliomas is due to tumor angiogenesis, which compromises cerebral autoregulatory capacity in the tumoral and peritumoral regions (Hou et al., 2006; Jiang et al., 2010). This regulation of cerebral blood flow is achieved primarily by arterioles, which either dilate or contract under the influence of complex physiological control systems to deliver sufficient blood containing oxygen and nutrients to the brain tissue for its metabolic need. Previous studies have demonstrated that NVU is also highly prevalent in low-grade gliomas (Pillai and Zacà, 2011, 2012; Zacà et al., 2011, 2014). Glial tumors are infiltrating lesions that can disrupt the neuronal contacts with surrounding microvasculature and astrocytes (Chaitanya et al., 2014; Pelligrino et al., 2011; Watkins et al. 2014), also known as “gliovascular uncoupling”, which subsequently results in reduced cerebrovascular reactivity (CVR). CVR is a measure of change in cerebral blood flow in response to a vasodilatory or vasoconstrictive stimulus.
In a previous study, it was demonstrated that comparison of IL to contralesional (CL) results of both task-based motor activation and breathhold (BH) CVR can be an effective method for assessment of NVU in low-grade perirolandic tumors (Zacà et al., 2014). Recent studies have demonstrated the effects of brain tumor-related NVU on rsfMRI (Agarwal et al., 2016a,b; Mallela et al., 2016). In a recent study, Agarwal and associates (2016b), assessed brain tumor-related NVU quantitatively using two standard rsfMRI analysis methods—independent component analysis (ICA) and seed-based correlation analysis (SCA)—in a group of patients with perirolandic primary glial neoplasms. They demonstrated that tumor-related NVU can adversely affect the resting-state BOLD signal similar to its effect on task-based BOLD activation. In another study, the same group presented a series of cases to demonstrate that the problem of brain tumor-related NVU may also similarly affect rsfMRI at ultrahigh field (7 T), despite known substantial BOLD signal-to-noise ratio advantages for rsfMRI provided by higher field strength, which may not fully mitigate the effects of such NVU (Agarwal et al., 2016b). Mallela and associates (2016), have examined the correlation of rsfMRI data with tumor characteristics and clinical information to characterize functional reorganization of resting-state networks (RSN) and the limitations of this method in high-grade gliomas due to NVU.
Although standard methods for determining various rsfMRI networks include SCA and ICA of voxel time series (Lee et al., 2013; Suril et al., 2015), there are several other rsfMRI metrics such as amplitude of low-frequency fluctuations (ALFF) (Zang et al., 2007) and fractional ALFF (fALFF) (Zou et al., 2008) that have been developed, and their usefulness in characterizing rsfMRI properties in healthy (Di et al., 2013b; Taylor et al., 2012; Yuan et al., 2013) and diseased populations has been studied. However, to our knowledge, no study has been done so far to examine the utility of these frequency domain rsfMRI metrics ALFF and fALFF in the assessment of brain tumor-induced NVU.
In our current study, we aim to explore whether the phenomenon of brain tumor-related NVU in rsfMRI may also affect the rsfMRI frequency domain metrics ALFF and fALFF similar to previously reported effects on BOLD signal on tbfMRI and SCA.
Materials and Methods
Patients
Twelve de novo (i.e., treatment naive) brain tumor patients referred for routine clinical presurgical motor mapping with BOLD fMRI were included in this Institutional Review Board approved study. These patients demonstrated evidence of potential NVU based on results of their clinical tbfMRI scans as described in previous publications (Zacà et al., 2014). In every patient, the decreased/absent tbfMRI activation in the primary IL sensorimotor cortex without associated clinical deficits was highly suggestive of NVU. These patients all presented with primary perirolandic gliomas with NVU affecting the face or hand representation area (RA) of the primary sensorimotor cortex.
Table 1 presents the clinical and demographic data for 12 patients recruited for the study.
Table 1.
Clinical/Demographic Data for 12 Patients Recruited for the Study
| Sex (M; F) | Age in years | Tumor: location/type/grade | Motor tasks demonstrating NVU |
|---|---|---|---|
| M | 24 | Right perirolandic frontoparietal operculum/oligodendroglioma/II | Vertical TM |
| M | 27 | Right parietal lobe/oligodendroglioma/II | Bilateral simultaneous sequential finger tapping |
| M | 34 | Left frontal lobe/oligoastrocytoma/II | Vertical TM |
| F | 36 | Left hemispheric/oligoastrocytoma/II | Vertical TM |
| M | 36 | Right perirolandic/oligoastrocytoma/II | Vertical TM |
| F | 51 | Right frontoparietal operculum/oligodendroglioma/II | Vertical TM |
| F | 61 | Left frontal lobe/oligodendroglioma/II | Bilateral simultaneous sequential finger tapping |
| M | 75 | Right frontal lobe/oligodendroglioma/II | Bilateral simultaneous sequential finger tapping |
| M | 30 | Left frontoparietal mass/anaplastic oligoastrocytoma/III | Bilateral simultaneous sequential finger tapping |
| F | 63 | Right frontal perirolandic/anaplastic astrocytoma/III | Vertical TM |
| F | 44 | Left frontal lobe/glioma with necrosis/IV | Vertical TM |
| M | 58 | Left perirolandic/glioblastoma/IV | Bilateral simultaneous sequential finger tapping |
F, female; M, male; NVU, neurovascular uncoupling; TM, tongue movement.
MRI acquisition
Scanning was performed using our standard clinical sequences for fMRI studies on a 3.0 Tesla (T) Siemens Trio MRI System (Siemens Medical Solutions, Erlangen, Germany) equipped with a 12-channel head matrix coil. The imaging protocol included a three-dimensional T1-weighted imaging sequence (TR = 2300 msec, TI = 900 msec, TE = 3.5 msec, flip angle = 9°, field of view = 24 cm, acquisition matrix = 256 × 256 × 176, slice thickness = 1 mm) as well as an axial two-dimensional (2D) T2 fluid attenuated inversion recovery imaging sequence (TR = 9000 msec, TI = 2500 msec, TE = 116 msec, flip angle = 141°, field of view = 17.2 × 23 cm, acquisition matrix = 240 × 320 × 53, slice thickness = 3 mm with 3 mm gap between slices) for structural imaging and multiple 2D gradient-echo echo planar imaging T2*-weighted BOLD sequences for functional imaging (TR = 2000 msec, TE = 30 msec, flip angle = 90°, field of view = 24 cm, acquisition matrix = 64 × 64 × 33, slice thickness = 4 mm with 1 mm gap between slices, interleaved acquisition) run while patients were performing a motor paradigm as described in more detail in the “Motor paradigms” subsection. For resting-state fMRI, 180 volumes were acquired using the same functional imaging protocol. For the resting-state fMRI acquisition, each patient was instructed to remain still with eyes closed without falling asleep during the scanning period of 6 min.
Motor paradigms
All patients performed one or more motor tasks for sensorimotor activation mapping during scanning. To map the face RA of the primary motor cortex a 3-min long tongue movement (TM) task was used, consisting of three cycles of 30-sec blocks of rest alternating with 30-sec blocks of repetitive vertical TM. The hand RA was mapped using a 3-min duration finger-tapping task, consisting of three cycles of 30-sec blocks of rest alternating with 30-sec blocks of bilateral simultaneous sequential finger tapping.
Instructions for all tasks were visually cued. A comprehensive prescan training session outside the MRI scanner ensured full patient understanding of task instructions and confirmed their ability to adequately perform each of the tasks. Patient motor task performance was monitored during the scan via use of both a LCD monitor in the scan suite and real-time fMRI for patient observation and assessment of activation, respectively.
Task-based motor fMRI analysis
SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK) software was used for preprocessing and postprocessing of tbfMRI data. As preprocessing steps, fMRI raw data were slice-time corrected, spatially realigned to correct for head motion, normalized to Montreal Neurological Institute (MNI) space at 2 mm voxel resolution, and spatially smoothed using a 6 mm isotropic full width at half maximum (FWHM) Gaussian filter. The maximum spatial displacement from the volume taken as reference during motion correction calculation was <1.0 mm, and <1° of rotation was present in all cases (<1 voxel size in each direction), and therefore, no data were discarded (Breiter et al., 2001).
Preprocessed tbfMRI data were then analyzed using the standard statistical parametric mapping (SPM) canonical hemodynamic response function (HRF) (reflecting motor activation vs. rest). A voxel-wise general linear model (GLM) analysis was performed with the expected BOLD response modeled by convolving the stimulus corresponding to each paradigm with a canonical HRF. From the results of the GLM analysis, standard activation maps, expressed as T-value maps for the motor tasks, were created. Z-scores were then calculated from the obtained T-value maps.
Resting-state fMRI analysis
SPM12 software was used for preprocessing of rsfMRI data. As preprocessing steps, rsfMRI raw data were slice-time corrected, spatially realigned to correct for head motion, normalized to MNI space at 2 mm voxel resolution, and spatially smoothed using a 6 mm isotropic FWHM Gaussian filter. The maximum spatial displacement from the volume taken as reference during motion correction calculation was less than 2.0 mm, and the maximum rotation was less than 2° (i.e., <1 voxel size movement in any direction), for each of the patients included in this study; therefore, there was no need to discard any data.
Preprocessed rsfMRI data were analyzed using the REST (version 1.8) (Song et al., 2011) toolkit. After detrending (for removal of systematic linear trend) of preprocessed rsfMRI data, fALFF maps were calculated for each patient. Low-frequency (0.01–0.08 Hz) bandpass filtering was further performed on the detrended preprocessed rsfMRI data, and then ALFF maps were calculated for each case. An automated anatomical labeling template (Smith, 2002; Tzourio-Mazoyer et al., 2002) was used to obtain a seed region (region of interest [ROI]) circumscribing the combination of precentral and postcentral gyri in the CL hemisphere, as well as a homologous IL ROI also comprising the precentral and postcentral gyri for each consecutive imaging slice along the z-axis in MNI space for each patient. The purpose of the ROIs was to limit evaluation to the primary motor and somatosensory cortex. ROIs were then visually inspected to ensure that they encompassed the precentral and postcentral gyri even in the presence of tumor-induced regional mass effects and gyral expansion. Pearson linear correlation coefficients were calculated using the REST toolkit between the seed CL ROI and the corresponding IL ROI comprising the sensorimotor cortex to obtain the seed correlation analysis (SCA)-based functional connectivity map of the sensorimotor network in each patient (Agarwal et al., 2016b). Z-scores were then calculated from the obtained SCA maps. Identical ROIs were used for analysis of the tbfMRI, SCA, ALFF, and fALFF maps.
Results
With respect to the group motor task fMRI data, a paired t-test comparing mean Z-score (1.72 ± 0.42) in IL ROIs to the mean Z-score (2.14 ± 0.46) in the CL ROIs demonstrated significantly decreased motor activation in IL ROIs compared with CL ROIs (p = 0.0005). A similar approach was applied to the resting-state SCA-derived sensorimotor connectivity map, for which the paired t-test comparing mean Z-scores (0.71 ± 0.23) in the IL ROIs to the mean Z-scores (0.89 ± 0.23) in the CL ROIs demonstrated significantly decreased BOLD signals in IL compared with CL ROIs (p = 0.0004).
Voxel values in the CL and IL ROIs of ALFF and fALFF maps were divided by the corresponding global mean of ALFF and fALFF in the cortical brain tissue. Group analysis revealed significantly decreased mean IL voxel ALFF (0.87 ± 0.13, p = 0.02) and fALFF (0.99 ± 0.076, p = 0.03) values (computed as mean of nonzero value voxels in each ROI) compared with the respective normal CL ROIs (1.01 ± 0.14 for ALFF and 1.07 ± 0.082 for fALFF). These ALFF and fALFF findings are similar to the findings of statistically significantly decreased IL BOLD signal for tbfMRI and SCA maps as mentioned in the previous paragraph. All results are tabulated in Table 2.
Table 2.
Mean Z-Scores in Ipsilesional and Contralesional Region of Interests for Task-Based Activation, Resting-State Sensorimotor Network Connectivity Maps, and Frequency Domain Metrics
| Motor task (p = 0.0005) | rsfMRI SCA (p = 0.0004) | rsfMRI ALFF (p = 0.02) | rsfMRI fALFF (p = 0.03) | |
|---|---|---|---|---|
| CL ROI (mean Z scores ± SD) | 2.14 ± 0.46 | 0.89 ± 0.23 | 1.01 ± 0.14 | 1.07 ± 0.082 |
| IL ROI (mean Z scores ± SD) | 1.72 ± 0.42 | 0.71 ± 0.23 | 0.87 ± 0.13 | 0.99 ± 0.076 |
Group level analysis for all four maps (tbfMRI, SCA, ALFF, and fALFF) of all 12 patients, showing statistically significant differences between IL and contralesional ROIs in each map.
ALFF, amplitude of low-frequency fluctuations; CL, contralesional; fALFF, fractional ALFF; IL, ipsilesional; ROI, region of interest; rsfMRI, resting-state functional MRI; SCA, seed correlation analysis; SD, standard deviation; tbfMRI, task-based functional MRI.
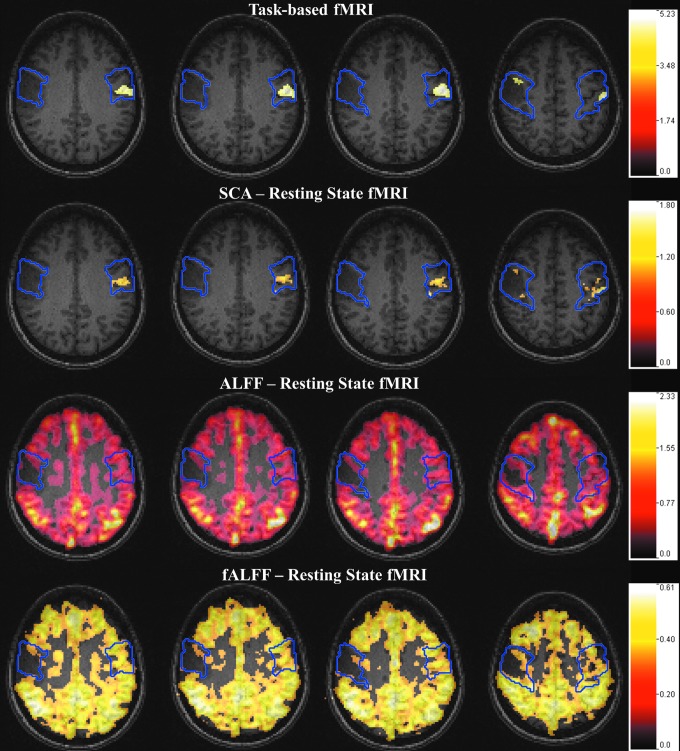
An example of analysis for an individual patient is provided in Figure 1. This figure displays results of motor task-based activation, sensorimotor network resting-state functional connectivity, ALFF, and fALFF all overlaid on T1-weighted anatomic images.
FIG. 1.
Patient with a grade II oligodendroglioma. Top row shows tongue motor fMRI activation map (tbfMRI) (Z-score >4.0) overlaid on T1-weighted structural images. Second, third, and bottom rows show SCA, ALFF, and fALFF maps (SCA >1.0, ALFF >0.4 and fALFF >0.36) from rsfMRI. Blue contours display ROIs from automatically parcellated combined precentral and postcentral gyri. Notice in all maps that there is abnormally decreased signal in the IL perirolandic ROIs compared with the normal CL perirolandic ROIs. In the setting of preserved clinical motor function and excellent task performance, these findings are indicative of tumor-induced NVU. ALFF, amplitude of low-frequency fluctuations; CL, contralesional; fALFF, fractional ALFF; fMRI, functional magnetic resonance imaging; IL, ipsilesional; NVU, neurovascular uncoupling; ROI, region of interest; rsfMRI, resting-state fMRI; SCA, seed correlation analysis; tbfMRI, task-based functional MRI. Color images available online at www.liebertpub.com/brain
Discussion
In this preliminary study, we have shown that regional alterations in the frequency domain rsfMRI metrics ALFF and fALFF correspond to similar IL abnormal activation reductions on tbfMRI and decreased resting-state functional connectivity using SCA in the setting of tumor-induced NVU. The abnormally reduced IL task-based activation in the absence of corresponding neurological deficits or impaired task performance is direct evidence of NVU, whereas the findings on the other maps are resting-state correlates of such NVU.
First proposed by Biswal and associates (1995), rsfMRI focuses on spontaneous low-frequency fluctuations (<0.1 Hz) in the BOLD signal in subjects scanned while resting (i.e., while no cognitive, language or motor tasks are being performed) in a MRI scanner. They found that spontaneous low-frequency (<0.08 Hz) fluctuation is highly synchronous among motor cortices. Standard methods for determining various rsfMRI networks (RSN) include SCA and ICA of voxel time series (Lee et al., 2013). Such analysis methods focus on the similarities of interregional time series, and therefore, investigate temporal synchronization of low-frequency fluctuations, that is, functional connectivity. Studies have found that the relative magnitude of these low-frequency fluctuations can differ between brain regions and between subjects, and thus may act as a marker of individual differences or dysfunction. Biswal and associates (1995) found that ALFF was higher in gray matter than in white matter. In 2007, Zang and associates (2007) proposed ALFF (0.01–0.08 Hz) to investigate the baseline brain function of attention-deficit hyperactivity disorder (ADHD). ALFF was defined as the total power within the frequency range between 0.01 and 0.08 Hz, and thus indexes the strength or intensity of low-frequency fluctuations. Unlike SCA and ICA methods which measure functional connectivity, ALFF measures amplitude of regional activity. In 2008, Zou and associates (2008) proposed fALFF, defined as the ratio of the power spectrum of the low-frequency (0.01–0.08 Hz) range to that of the entire frequency range, as a measure of regional activity. fALFF represents the relative contribution of specific low-frequency fluctuation to the whole frequency range.
ALFF and fALFF have been used to access differences in ALFF both between subjects and between regions. Zang and associates (2007) found that children with ADHD show reduced ALFF amplitude in some brain areas and increased amplitude in others compared with controls. Yan and associates (2009) found increased ALFF amplitude in the DMN during Eyes Open versus Eyes Closed resting periods. Studies (Biswal et al., 2010; Hu et al., 2014) have observed changes in fALFF with aging (Biswal et al., 2010; Hu et al., 2014). Kannurpatti and Biswal (2008) used resting-state fluctuation of amplitude (RSFA) as a measure of vascular-related BOLD variability within regions and between individuals. Studies (Kannurpatti and Biswal, 2008; Wise et al., 2004) have examined the correlation between end-tidal CO2 fluctuations and low-frequency BOLD fluctuations available from rsfMRI and established RSFA as a strong CVR correlate. Biswal and associates (2007) showed that only very low-frequency amplitude (0.01, 0.03, and 0.04 Hz) correlated with BH-related activity. Studies (Di et al., 2013a) have also been performed using ALFF to reduce the distortions in task-based activation due to regional variance in neurovascular coupling.
All these studies were performed to examine the usefulness of ALFF and fALFF in representing resting-state MRI properties in healthy and diseased populations without focal brain lesions; however, no study has been done so far to examine the utility of these frequency domain rsfMRI metrics (ALFF and fALFF) in the assessment of brain tumor-induced NVU. To our knowledge, our current study is the first to explore whether NVU may also affect the rsfMRI frequency domain metrics ALFF and fALFF in perirolandic primary glial neoplasms. Since in this preliminary study we were interested in evaluating the relatively nonlateralized primary sensorimotor network, we only focused on ALFF and fALFF metrics in precentral and postcentral gyri. To avoid manual tracing of such ROIs, we adopted an operator-independent, atlas-based parcellation technique to define identical ROIs in the IL and CL sensorimotor cortex. This ROI approach permitted us to directly evaluate the group performance across the tbfMRI, SCA, ALFF, and fALFF maps consistently.
Some limitations of our study include the following: although our study demonstrated IL regional alterations in ALFF and fALFF, these alterations may be indicative of an inherent property of the tumor itself and may not be necessarily specific to NVU. However, similar IL abnormal activation reductions on tbfMRI of these patients who displayed no motor deficits suggest that the decrease in ALFF and fALFF is sensitive to NVU. Although both ALFF and fALFF are sensitive mostly to signal from gray matter, ALFF is more prone to noise from physiological sources, particularly near the ventricles and large blood vessels (Zou et al., 2008; Zuo et al., 2010). However, reliability of ALFF in gray matter regions tends to be higher than for fALFF (Zou et al., 2008). As it is more reliable, ALFF may be more sensitive to differences between groups and individuals. As such, reporting of both measures is recommended (Zuo et al., 2010). Although we considered the sensorimotor network rather than language or other networks due to the relatively less lateralized nature of this particular network, it is clearly based on prior studies that the sensorimotor network may not be perfectly symmetric and that handedness may play some role in the degree of normal asymmetry seen in this network. However, previous studies have reported that the degree of pathologic BOLD signal asymmetry exceeds the normal degree of lateralization variability seen on activation maps during performance of bilateral simultaneous movement tasks (Dassonville et al., 1997; Jancke et al., 1998; Solodkin et al., 2001; Zaca et al., 2014; Zeng et al., 2007). Also, the relatively small sample size is a limitation of this study. However, such data are challenging to acquire because of stringent clinical inclusion criteria, and we were, nevertheless, able to demonstrate statistically significant results. Finally, as these frequency domain measures require a constant timecourse for power analyses, they cannot be run on scrubbed data (Power et al., 2012), in which volumes with excessive movement have been removed.
The potential advantages of the frequency domain metrics include possible absence of network specificity in the assessment of NVU, but this remains to be determined in the future through studies of RSNs other than the sensorimotor network. Therefore, this preliminary study may serve as a catalyst for future application of such frequency domain metrics in the assessment of brain tumor-related NVU in more lateralize networks such as language.
Conclusion
In conclusion, we have shown in this study that the frequency domain metrics ALFF and fALFF may be markers of lesion-induced NVU involving the sensorimotor network in rsfMRI similar to previously reported alterations in tbfMRI activation and SCA-derived resting-state functional connectivity maps. The possible advantages of the frequency domain metrics include potential future applicability to other RSNs in the assessment of NVU.
Acknowledgment
This work was partially supported by NIH grant R42 CA173976-02 (NCI) (PI: J.J.P.)
Author Disclosure Statement
No competing financial interests exist.
References
- Agarwal S, Sair HI, Airan R, Hua J, Jones CK, Heo HY, et al. 2016a. Demonstration of brain tumor-induced neurovascular uncoupling in resting-state fMRI at ultrahigh field. Brain Connect 6:267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Sair HI, Yahyavi-Firouz-Abadi N, Airan R, Pillai JJ. 2016b. Neurovascular uncoupling in resting state fMRI demonstrated in patients with primary brain gliomas. J Magn Reson Imaging 43:620–626 [DOI] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM. 2005. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Biswal BB, Kannurpatti SS, Rypma B. 2007. Hemodynamic scaling of fMRI-BOLD signal: validation of low frequency spectral amplitude as a scalability factor. Magn Reson Imaging 25:1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. 2010. Toward discovery science of human brain function. Proc Natl Acad Sci U S A 107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. 2001. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 30:619–639 [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Minagar A, Alexander JS. 2014. Neuronal and astrocytic interactions modulate brain endothelial properties during metabolic stresses of in vitro cerebral ischemia. Cell Commun Signal 12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. 2006. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A 103:13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassonville P, Zhu XH, Uurbil K, Kim SG, Ashe J. 1997. Functional activation in motor cortex reflects the direction and the degree of handedness. Proc Natl Acad Sci U S A 94:14015–14018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kim EH, Huang CC, Tsai SJ, Lin CP, Biswal BB. 2013a. The influence of the amplitude of low-frequency fluctuations on resting-state functional connectivity. Front Hum Neurosci 7:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di X, Kannurpatti SS, Rypma B, Biswal BB. 2013b. Calibrating BOLD fMRI activations with neurovascular and anatomical constraints. Cerebral Cortex 23:255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga M, Horovitz SG, van Gelderen P, de Zwart JA, Jansma JM, Ikonomidou VN, et al. 2006. Large-amplitude, spatially correlated fluctuations in BOLD fMRI signals during extended rest and early sleep stages. Magn Reson Imaging 24:979–292 [DOI] [PubMed] [Google Scholar]
- Gohel SR, Biswal BB. 2015. Functional integration between brain regions at rest occurs in multiple-frequency bands. Brain Connect 5:23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holodny AI, Schulder M, Liu WC, Wolko J, Maldjian JA, Kalnin AJ. 2000. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: implications for image-guided neurosurgery. AJNR Am J Neuroradiol 21:1415–1422 [PMC free article] [PubMed] [Google Scholar]
- Hou BL, Bradbury M, Peck KK, Petrovich NM, Gutin PH, Holodny AI. 2006. Effect of brain tumor neovasculature defined by rCBV on BOLD fMRI activation volume in the primary motor cortex. Neuroimage 32:489–497 [DOI] [PubMed] [Google Scholar]
- Hu S, Chao HH, Zhang S, Ide JS, Li CS. 2014. Changes in cerebral morphometry and amplitude of low-frequency fluctuations of BOLD signals during healthy aging: correlation with inhibitory control. Brain Struct Funct 219:983–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Müller-Gärtner H. 1998. Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Brain Res Cogn Brain Res 6:279–284 [DOI] [PubMed] [Google Scholar]
- Jiang Z, Krainik A, David O, Salon C, Troprès I, Hoffmann D, et al. 2010. Impaired fMRI activation in patients with primary brain tumors. NeuroImage 52:538–548 [DOI] [PubMed] [Google Scholar]
- Kannurpatti SS, Biswal BB. 2008. Detection and scaling of task-induced fMRI BOLD response using resting state fluctuations. Neuroimage 40:1567–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Kantola JH, Jauhiainen J, Hyvärinen A, Tervonen O. 2003. Independent component analysis of nondeterministic fMRI signal sources. Neuroimage 19:253–260 [DOI] [PubMed] [Google Scholar]
- Lee MH, Smyser CD, Shimony JS. 2013. Resting-state fMRI: a review of methods and clinical applications. AJNR Am J Neuroradiol 34:1866–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Buckner RL, Talukdar T, Tanaka N, Madsen JR, Stufflebeam SM. 2009. Task-free presurgical mapping using functional magnetic resonance imaging intrinsic activity. J Neurosurg 111:746–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallela AN, Peck KK, Petrovich-Brennan NM, Zhang Z, Lou W, Holodny AI. 2016. Altered resting-state functional connectivity in the hand motor network in glioma patients. Brain Connect 6:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Vetri F, Xu HL. 2011. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol 22:229–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Zacà D. 2011. Clinical utility of cerebrovascular reactivity mapping in patients with low grade gliomas. World J Clin Oncol 2:397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JJ, Zacà D. 2012. Comparison of BOLD cerebrovascular reactivity mapping and DSC MR perfusion imaging for prediction of neurovascular uncoupling potential in brain tumors. Technol Cancer Res Treat 11:361–374 [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59: 2142–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. 2001. A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimony JS, Zhang D, Johnston JM, Fox MD, Roy A, Leuthardt EC. 2009. Resting-state spontaneous fluctuations in brain activity: a new paradigm for presurgical planning using fMRI. Acad Radiol 16:578–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. 2002. Fast robust automated brain extraction. Hum Brain Mapp 17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. 2009. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodkin A, Hlustik P, Noll DC, Small SL. 2001. Lateralization of motor circuits and handedness during finger movements. Eur J Neurol 8:425–434 [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. 2011. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PA, Gohel S, Di X, Walter M, Biswal BB. 2012. Functional covariance networks: obtaining resting-state networks from intersubject variability. Brain Connect 2:203–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. 2002. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289 [DOI] [PubMed] [Google Scholar]
- Ulmer JL, Krouwer HG, Mueller WM, Ugurel MS, Kocak M, Mark LP. 2003. Pseudoreorganization of language cortical function at fMR imaging: a consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol 24:213–217 [PMC free article] [PubMed] [Google Scholar]
- Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. 2014. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun 5:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. 2004. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage 21:1652–1664 [DOI] [PubMed] [Google Scholar]
- Yan C, Liu D, He Y, Zou Q, Zhu C, Zuo X, et al. 2009. Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One 4:e5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Di X, Kim EH, Barik S, Rypma B, Biswal BB. 2013. Regional homogeneity of resting-state fMRI contributes to both neurovascular and task activation variations. Magn Reson Imaging 31:1492–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacà D, Hua J, Pillai JJ. 2011. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol 2:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacà D, Jovicich J, Nadar SR, Voyvodic JT, Pillai JJ. 2014. Cerebrovascular reactivity mapping in patients with low grade gliomas undergoing presurgical sensorimotor mapping with BOLD fMRI. J Magn Reson Imaging 40:383–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, et al. 2007. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29:83–91 [DOI] [PubMed] [Google Scholar]
- Zhang D, Raichle ME. 2010. Disease and the brain's dark energy. Nat Rev Neurol 6:15–28 [DOI] [PubMed] [Google Scholar]
- Zeng L, Chen H, Ouyang L, Yao D, Gao JH. 2007. Quantitative analysis of asymmetrical cortical activity in motor areas during sequential finger movement. Magn Reson Imaging 25:1370–1375 [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, et al. 2008. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172:137–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, et al. 2010. The oscillating brain: complex and reliable. Neuroimage 49:1432–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]