Abstract
The whole-body positron emission tomography (PET)/magnetic resonance (MR) scan is a cutting edge technology providing comprehensive structural information from MR imaging and functional features from PET in a single session. Recent research findings and clinical experience have shown that 18F-fluorodeoxyglucose (FDG) whole-body PET/MR imaging has a diagnostic performance comparable with or superior to that of PET/CT in the field of oncology, including for breast cancer. In particular, FDG PET/MR mammography in the prone position with the breast hanging in a pendant manner can provide more comprehensive information about the metabolism, anatomy, and functional features of a breast lesion than a whole-body PET/MR scan. This article reports on current state-of-the-art PET/MR mammography in patients with breast cancer and the prospects for potential application in the future.
Key words: PET/MR, FDG, Breast cancer, Staging, Lymphatic metastasis, Attenuation correction
Introduction
Whole-body 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) has been established as an important imaging modality for the diagnosis, staging, restaging, monitoring of response to treatment, and estimation of the long-term prognosis in patients with breast cancer [1–4], but does not accurately show the anatomy of breast lesions because of the weak soft-tissue contrast in the CT component of this imaging technique. However, FDG PET/CT mammography acquired in the prone position with the breast hanging in a pendant manner, which is similar to the patient positioning used in magnetic resonance (MR) breast imaging, has been reported to have better diagnostic performance for local assessment and detection of regional lymph node metastasis in patients with breast cancer [5, 6].
Whole-body FDG PET/MR scanning was developed recently and has been shown to have diagnostic performance comparable with or superior to that of PET/CT for various types of cancer [7–9]. However, for breast cancer, whole-body FDG PET/MR imaging should be supplemented with PET/MR mammography when obtaining PET and MR breast images simultaneously in order to obtain comprehensive information on glucose metabolism, cross-sectional morphology, functional features such as tissue perfusion, enhancement kinetics, and diffusion weighted imaging (DWI) or spectroscopy of breast lesions (Fig. 1, Table 1) [10].
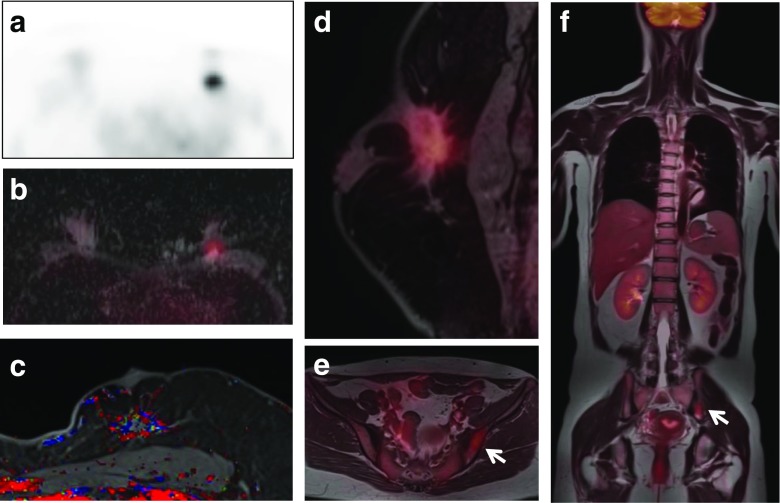
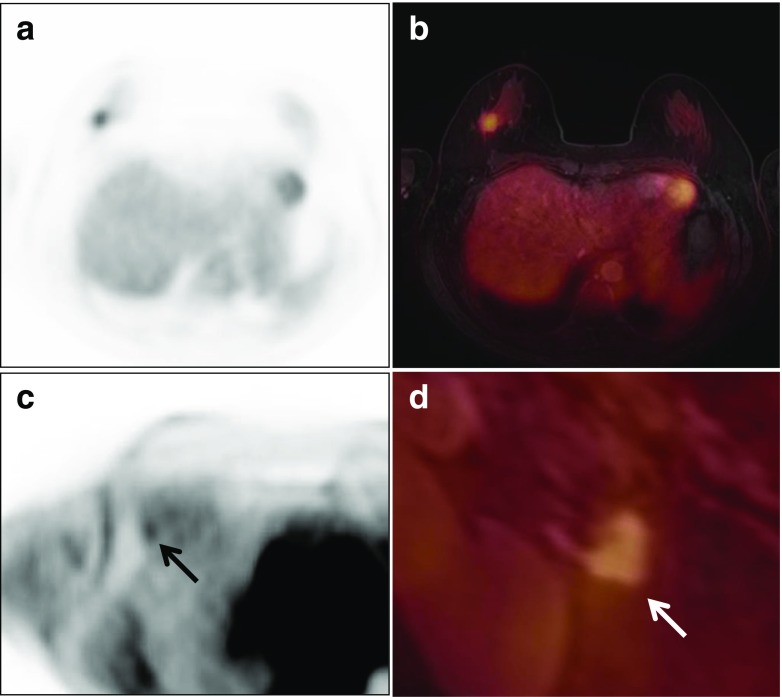
Fig. 1.
Images of whole-body FDG PET/MR scan and PET/MR mammography obtained with a dedicated four-channel PET/MR breast coil in a 41-year-old woman with invasive ductal carcinoma. A spiculated enhancing mass with strong FDG uptake, a low apparent diffusion coefficient (ADC) value, and a washout enhancement pattern is seen at the 12 o’clock position in the left breast on FDG PET (a), FDG/ADC PET/ mammography (b), color-coded map of the maximum slope of enhancement (c), and FDG/T1 fat-saturated gradient-echo (fs GRE) PET/MR mammography (d). Pelvic FDG/T1 and whole body FDG/T2 PET/MR images (e, f) show moderate FDG uptake in the left iliac bone suspicious for bone metastasis (arrows)
Table 1.
Acquisition protocols of clinical torso PET/MR imaging and PET/MR mammography (used at Yeungnam University Hospital)
| TE/TR(ms) | FOV(mm) | Matrix | Resolution | Acquisition time (min) | |
|---|---|---|---|---|---|
| Torso PET/MR | |||||
| PET (5 bed × 3 min/bed) | 594 | 4.2 × 4.2 | |||
| Dixon VIBE for AC | 1.23/3.60 | 400 | 172 × 172 | 1.6 × 1.2 | 00:32 × 5 |
| Axial fs T2 HASTE | 67/1,000 | 400 | 180 × 320 | 1.7 × 1.3 | 00:54 × 5 |
| Coronal T2 HASTE | 76/1,000 | 450 | 232 × 256 | 1.8 × 1.8 | 00:59 × 5 |
| PET/MR mammography | |||||
| PET (1 bed for 25 min) | 594 | 4.2 × 4.2 | |||
| Dixon VIBE for AC | 1.23/306 | 400 | 172 × 172 | 1.6 × 1.2 | 00:32 |
| Axial T1 | 9.6/683 | 340 | 250 × 384 | 1.4 × 0.9 | 01:32 |
| Axial fs T2 | 72/5,200 | 340 | 269 × 384 | 1.3 × 0.9 | 00:18 |
| DWI | 86/10,300 | 340 | 77 × 192 | 2.5 × 2.0 | 03:36 |
| DCE | 1.43/4.02 | 340 | 276*384 | 1.2 × 0.9 | 08:28 |
| Sagittal fs T2 | 3.68/8.29 | 340 | 256 × 320 | 0.8 × 0.6 | 03:16 |
| Axial VIBE | 1.43/4.02 | 340 | 296 × 384 | 1.1 × 0.9 | 02:50 |
AC attenuation correction, DCE dynamic contrast-enhanced imaging, DWI diffusion-weighted imaging, fs fat-saturated, HASTE half-Fourier acquisition single-shot turbo spin echo, VIBE volume-interpolated breath-hold examination
In this review of the early results published in the literature and our preliminary clinical experience, we discuss the potential role of FDG PET/MR mammography in the management of patients with breast cancer along with the relevant technical considerations.
Technical Aspects of Integrated PET/MR Mammography
For MR breast imaging, a multichannel radiofrequency (RF) breast coil is necessary for MR signal detection and to acquire high spatial resolution and high soft-tissue contrast MR images of the breast [11, 12]. However, a conventional radiofrequency (RF) coil for MR signal reception in integrated PET/MR breast imaging causes significant attenuation and scattering of 511-keV positron annihilation photons before they reach the PET detector, resulting in poor PET image quality and image artifacts because the coil lies within the PET field of view during acquisition of PET data [13, 14]. Some studies have reported that use of a conventional RF coil, which contains a substantial amount of plastic and metal material, results in an 11–20 % underestimation of PET activity, causing substantial regional bias in PET quantification [13, 15].
Attenuation correction of PET data obtained by integrated PET/MR breast imaging using a four-channel or 16-channel RF coil was recently developed and evaluated by a CT-based, three-dimensional hardware attenuation map of an RF coil fused with a patient’s MR-based attenuation correction map [16, 17]. When this method is used, small misalignments could create image artifacts and result in incorrect quantification, so the coil needs to be placed as accurately as possible. In addition, the CT scan of the coil, which contains metal components, may produce artifacts that affect the quality of the PET image [16]. In addition, as with the integrated whole-body PET/MR scan [18–21], variable attenuation map artifacts have been observed using the CT-based template attenuation map in PET/MR breast imaging with a four-channel RF coil (Fig. 2).
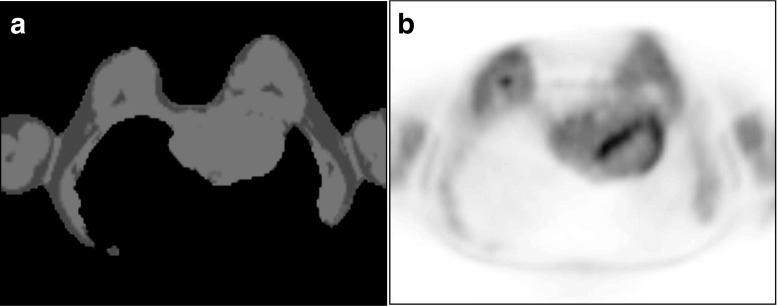
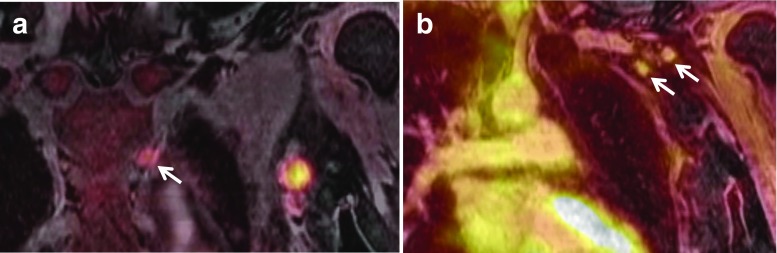
Fig. 2.
A 46-year-old woman with breast cancer. A μ-map of FDG PET/MR mammography (a) show body contour artifact with missing dorsal body contour including both lungs causing failure of attenuation correction on an axial PET image (b)
Further development and validation of a dedicated PET/MR breast coil for MR signal detection without significant attenuation and scattering of photons and including the CT-based template map for PET/MR mammography is required.
The SIGNA PET/MR system (GE Healthcare, Little Chalfont, UK) with a silicon photomultiplier PET detector and time-of-flight PET capability has recently been developed in order to achieve higher sensitivity and spatial resolution of PET imaging, and could be better for detecting malignant breast lesions than the Biograph mMR (Siemens, Munich, Germany), which uses avalanche photodiodes in the PET detector [22]. If the sensitivity of the PET detector used in the PET/MR scanner to or better than that of positron emission mammography (PEM), PET/MR mammography could be used to detect smaller malignant lesions with low FDG avidity and provide more detailed molecular information on small-sized breast cancers.
Local Assessment of Breast Lesions
Dynamic contrast-enhanced MR breast imaging providing information on cross-sectional morphology and the functional features of lesions, including tissue perfusion and enhancement kinetics, plays an important role in characterization of breast lesions and differentiating malignant from benign lesions [11, 12]. These findings can be important for preoperative planning in certain circumstances, such as in young women, women with dense breasts, and where there is a high risk of multifocal/multicentric lesions [23]. However, the specificity of MR imaging for characterization of breast cancer varies widely because of normal enhancing breast tissue and the biological heterogeneity of breast cancer [24–29].
FDG PET has shown high sensitivity and specificity for detection of primary large and palpable breast tumors [30, 31]. However, the sensitivity decreases when the lesions are small and non-palpable, low-grade, or non-invasive neoplasms [31]. In addition, because the CT component of PET/CT is of limited value for characterization of breast lesions, FDG PET/CT is generally not recommended for local assessment of breast cancers [32].
However, there have been some studies of dedicated breast PET devices and use of a breast-positioning device for PET to detect breast cancer and to reduce the risk of false-negative results. Kaida et al. [33] reported that prone breast imaging using a positioning device may help to improve the detection rate of breast cancer in screening. Heusner et al. [6] reported that FDG PET/CT mammography may be more accurate than MR breast imaging in the pretherapeutic differentiation of breast lesions as solitary, multifocal, or multicentric tumors. Further, Koolen et al. [34] reported that response monitoring with FDG PET/CT mammography was possible in 95 % of all tumors in patients scheduled to receive neoadjuvant chemotherapy. Finally, Moon et al. [35] reported that FDG PET/CT mammography provided more correct classification of the focality of lesions than MR breast imaging (95 % vs 90 %).
Positron emission mammography (PEM) has been developed and applied in clinical practice [36]. For detection of breast cancer, PEM uses a dedicated scanner with two parallel photon detectors similar to mammography compressors [37]. PEM has been reported to be more sensitive than whole-body PET/CT in evaluation of breast cancer, with a high sensitivity for tumors smaller than 1 cm and proved to be complementary to MR breast imaging for defining the extent of preoperative mass in the ipsilateral breasts of women with newly diagnosed breast cancer [37, 38]. PEM was more specific than MR breast imaging and less likely to prompt unnecessary biopsies [39].
A study by Moy et al. [40] showed the potential benefit of combining both FDG PET mammography and MR breast imaging acquired separately and later fused with a semiautomatic landmark-based, non-rigid fusion program. In 90 breast lesions, combining both techniques increased the positive predictive value (PPV) and specificity in patients for whom the MR breast-imaging outcome alone would have been nonspecific. The increase in PPV from 77 % in MR breast imaging to 98 % in fused PET/MR breast imaging and the increase in specificity from 53 to 97 % were statistically significant. However, the sensitivity of MR breast imaging was 95 %, that of PET breast imaging was 57 %, and that of fused PET/MR breast imaging was 83 %. The false-negative rate on PET breast imaging was 26.7 %, and after fusion this number was reduced to 9 %.
Taneja et al. [41], who assessed the utility of whole-body FDG PET/MR scanning including PET/MR mammography and MR breast imaging in the initial staging of patients with breast cancer, reported a higher diagnostic confidence for detection of breast cancer using PET/MR mammography than using PET breast imaging or MR breast imaging.
Botsikas et al. [42], who evaluated primary lesions in 58 patients with breast cancer using a sequential whole-body FDG PET/MR scanner (Philips Ingenuity TF PET/MR; Philips Healthcare, Best, The Netherlands), reported that sensitivity for primary cancers with MR breast imaging and fused PET/MR breast imaging was 100 and 77 %, respectively, and specificity for MR breast imaging and fused PET/MR breast imaging was 67 and 100 %.
Grueneisen et al. [43] reported that 47 (96 %) of 49 patients with breast cancer were identified using FDG PET/MR mammography and MR breast imaging, whereas PET/CT detected 46 (94 %) and missed a synchronous carcinoma in the contralateral breast in one patient. PET/MR mammography and MR breast imaging enabled correct identification of multifocal/multicentric disease in three further patients if compared with PET/CT. For the correct T stage, PET/MR mammography and MR breast imaging showed identical results and were correct in significantly more cases than PET/CT. PET/MR mammography could provide more sensitive detection of breast lesions than PET/CT (true positive rate 68.7 % vs 62.7 %, respectively).
Kong et al. [10] compared whole-body FDG PET/MR scan and PET/MR mammography with regard to their ability to detect breast lesions. The overall sensitivity was 79.2 % (38/48) on PET, 87.5 % (42/48) on whole-body PET/MR scan, and 100 % on PET/MR mammography. PET/MR mammography detected small lesions in addition to possible multiple tumors.
FDG PET/MR mammography has been shown to depict the shape, margin, and internal enhancement pattern of breast cancer and show the enhancement pattern over time, and FDG avidity to provide structural and functional information with better soft-tissue contrast and high spatial resolution by MR imaging complementary to the metabolic data obtained from FDG PET (Figs. 3 and 4) [10]. However, more extensive clinical studies are needed to determine the added value of FDG PET/MR mammography when these two modalities are combined.
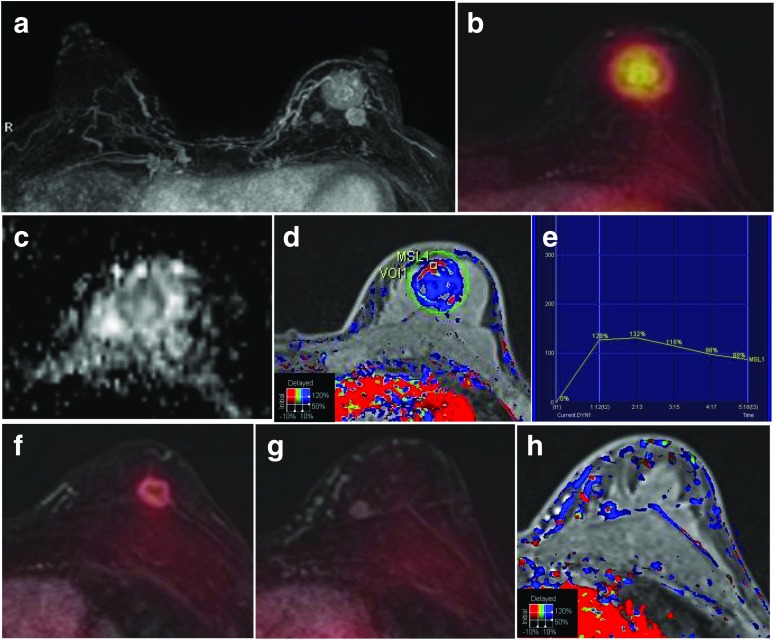
Fig. 3.
A 52-year-old woman with three invasive ductal carcinomas in the left breast. a The MIP of a contrast-enhanced T1 fs GRE subtraction image shows three enhancing masses in the left breast. b FDG/T1 fs GRE PET/MR mammography shows the largest hypermetabolic mass with a low ADC value (0.962 × 10−3 mm2/s) (c) and a washout enhancement (d, e). f, g FDG/T1 fs GRE PET/MR mammography shows two enhancing masses, one with moderate FDG uptake and one with no FDG uptake. h The smallest enhancing mass without FDG uptake is shown as a washout enhancement pattern on the color-coded map
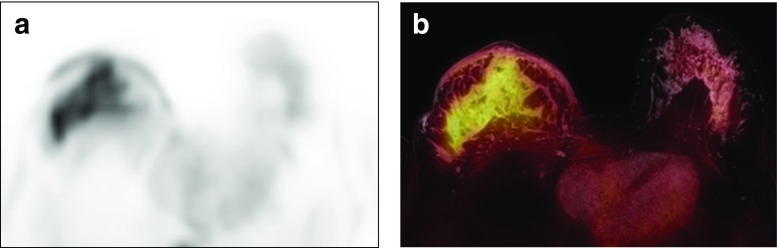
Fig. 4.
A 31-year-old woman with an inflammatory breast cancer in the left breast. The diffuse, infiltrative, non-mass enhancement involving the entire right breast with increased FDG uptake and diffuse FDG uptake overlying thickened skin is shown in the FDG PET image (a) and on FDG/T1 fs GRE PET/MR mammography (b)
Regional Lymph Node Assessment
The status of the axillary lymph nodes is one of the most important prognostic factors in breast cancer. FDG PET/CT could be used for prediction of lymph node metastasis from breast cancer due to its ability to detect malignant pathology by an abnormal increase in glucose metabolism even in non-pathologically enlarged lymph nodes not captured by CT. Several authors have reported that FDG PET and PET/CT have high specificity for evaluation of axillary lymph nodes, but are of limited value because of low sensitivity and high false-negative rates [44, 45].
However, FDG PET/CT could detect previously unsuspected locoregional extra-axillary lymph node metastases and was useful as an additional imaging tool for assessment of extra-axillary lymph node metastases, with a significant impact on patient management [46]. PET/CT has a potential advantage in evaluation of regional lymph nodes in particular locations, such as the internal mammary and infraclavicular lymph nodes [47].
FDG PET/CT mammography reportedly images a wider area of the axillary fossa so that the anatomic structures of the axilla can be more easily differentiated from one another than is possible using whole-body PET/CT, even though there were no significant differences in the number of metastatic axillary lymph nodes that could be detected between these two types of study [5, 35, 48]. Koolen et al. [49] reported that combining prone and standard supine FDG PET/CT detected locoregional lymph node metastases with a sensitivity of 82 % and a specificity of 92 %, which changed the indication for radiotherapy in a substantial proportion of patients based on detection of occult N3 nodes.
MR breast imaging is not considered effective for lymph node staging in the preoperative breast cancer setting, and is not recommended for that purpose. However, careful reading and variable MR imaging parameters, such as morphologic characteristics, and functional parameters, such as patterns of dynamic enhancement and restriction of diffusion, have been proposed for evaluation of metastatic lymph nodes because the axillary and internal mammary lymph nodes are usually included in the field of view when imaging the breast by MR [50].
Botsikas et al. [42], who evaluated 198 lymph node groups (34 malignant, 164 benign) in 58 patients with breast cancer using a sequential whole-body FDG PET/MR scanner (Philips Ingenuity TF PET/MR), reported that sensitivity with MR breast imaging and fused PET/MR breast imaging was 88 and 79 %, respectively, and specificity for MR breast imaging and fused PET/MR breast imaging was 98 and 100 %. However, others reported no significant differences between PET/CT, MR breast imaging, and PET/MR mammography for detection of axillary node-positive patients when a dedicated PET/MR breast-imaging coil was not used. The diagnostic confidence score was higher for PET/MR mammography than for PET or MR breast imaging alone [41, 43].
FDG PET/MR mammography may be useful for axillary, internal mammary, and supraclavicular lymph nodes because the combination of advanced MR imaging sequences for tissue characterization with FDG PET metabolic data could result in more accurate identification of metastatic invasion of lymph nodes (Fig. 5). However, additional FDG PET/MR mammography using an additional volume-interpolated breath-hold examination including the axillary and supraclavicular area might be needed because the field of view of the integrated PET/MR scanner is too small to include both the breast and supraclavicular areas (Fig. 6).
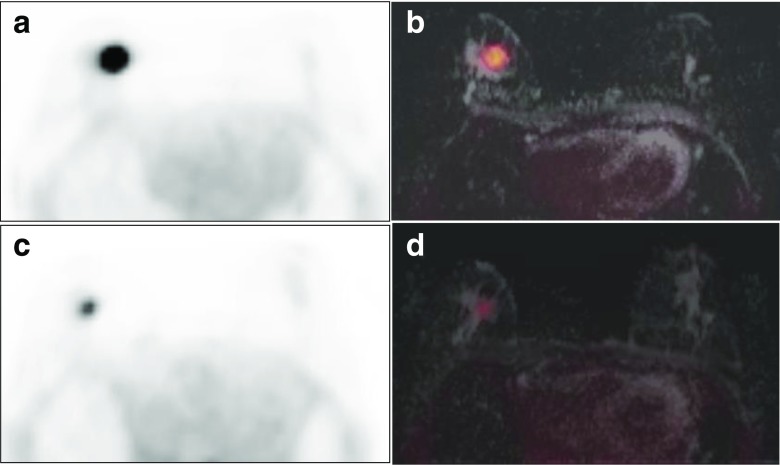
Fig. 5.
A 49-year-old woman with an invasive carcinoma and one axillary lymph node metastasis. Increased FDG uptake is shown in the upper outer quadrant of the right breast on FDG PET (a) and FDG/T1 fs GRE PET/MR mammography (b). Axillary lymph node (arrow) with faint FDG uptake and focal cortical thickening is seen in the right axilla on FDG PET (c) and FDG/T1 fs GRE PET/MR mammography (d)
Fig. 6.
A 29-year-old woman with an invasive carcinoma and axillary and internal mammary lymph node metastasis. FDG/VIBE PET/MR mammography showed multiple hypermetabolic lymph nodes in the left first and second intercostal space (a) and left axilla (level I and II) (b)
Evaluation of Response to Therapy
Neoadjuvant chemotherapy (NAC) has been established as the standard treatment for locally advanced breast cancer. Patients who achieve a pathologic complete response after NAC have longer disease-free and overall survival rates than non-responders [51–53]. For assessment of tumor response after NAC, FDG PET, or PET/CT, DWI, and dynamic contrast-enhanced MR breast imaging is known to provide an accurate assessment [54, 55].
In a recent study by Park et al. [56] comparing DWI with PET/CT for predicting a complete response to NAC in patients with breast cancer, combined use of DWI and PET/CT showed higher diagnostic performance (area under the curve [AUC], 0.94) than PET/CT (AUC, 0.87) or DWI (AUC, 0.91), and could potentially improve the specificity of this prediction. In addition, An et al. [57] showed that combined use of PET/CT with dynamic contrast-enhanced MR breast imaging or DWI could improve the specificity for predicting a pathologic response after NAC.
Therefore, FDG PET/MR mammography could be a useful tool in evaluation of response to NAC (Fig. 7).
Fig. 7.
A 72-year-old woman with an invasive carcinoma. Baseline FDG PET (a) and FDG/ADC PET/MR mammography (b). After the first cycle of neoadjuvant chemotherapy, a significant reduction in tumor FDG uptake from an SUVmax of 13.8 to an SUVmax of 6.4 without a change in the ADC is seen on FDG PET (c) and FDG/ADC PET/MR mammography (d). Histopathology at completion of chemotherapy showed no residual disease in the tumor bed
Additional MR Sequences for FDG PET/MR Mammography
Diffusion-Weighted Imaging
DWI is a quantitative MR imaging technique that provides information on water diffusion in tissues in the form of apparent diffusion coefficient (ADC) values, which in turn provide information about the tissue microstructure. Restricted diffusion is observed in tissues with high cellularity, e.g., tumors, abscess, fibrosis, and cytotoxic edema. Relative free diffusion is observed in tissues with low cellularity or tissues with disrupted cell membranes, e.g., cysts and necrotic tissue [58, 59]. DWI has been reported to reveal information about tumor cellularity and thus to provide prognostic information on breast cancer [60, 61]. However, the clinical value of DWI remains limited, because the frequent pathologic heterogeneity of breast cancers and the necrotic tissues commonly found with aggressive tumors contribute to high ADC values [62–65].
FDG uptake and ADC represent different aspects of the biological features of tumor cells and have not shown a good correlation in breast cancer. However, both types of study may have a role for predicting the prognosis of breast cancer [66–68] (Fig. 8). FDG PET/MR mammography using integrated PET/MR scanning for glucose metabolism and DWI need to be studied more detail.
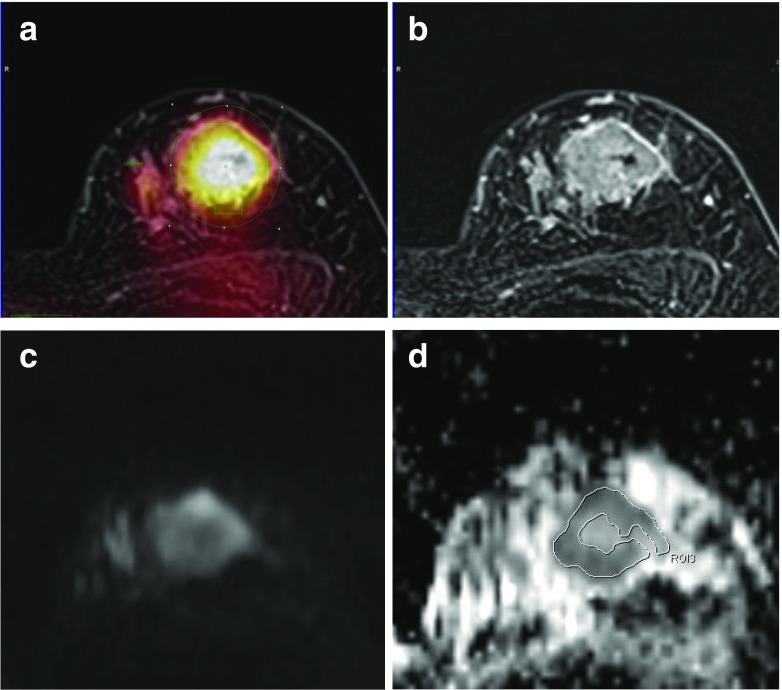
Fig. 8.
Comparison between FDG uptake and the ADC in a 52-year-old woman with a 3.5-cm invasive ductal carcinoma. a Region of interest for measuring SUVmax is drawn on the axial PET/MR mammography (SUVmax, 8.63). b Contrast-enhanced T1 fs GRE subtraction image showed a heterogeneous enhancing mass. c DWI (b value, 800 mm2/s) shows high signal intensity within the tumor. d For measurement of the mean ADC, a region of interest is manually drawn within a mass lesion on an ADC map (mean ADC, 0.825 × 10−3 mm2/s)
MR Spectroscopy
MR spectroscopy noninvasively measures not only metabolites in a selected region, such as choline, reflecting cellular proliferation, but also N-acetyl aspartate, creatine, and lactate, which can be helpful in characterization of some suspicious lesions, although only total choline has been used for breast cancer [69]. Combining this molecular information with glucose metabolism obtained from PET will likely benefit oncologic care. Cho et al. [70] reported good diagnostic performance of MR spectroscopy and FDG PET in the early prediction of the pathologic response to NAC in patients with breast cancer.
Breast MR spectroscopy performed by integrated PET/MR scanner is not acquired with FDG PET data simultaneously yet and takes additional time. However, breast MR spectroscopy during the PET/MR mammography examination using an integrated PET/MR scanner seems promising due to more accurate co-registration between FDG avidity and spectroscopy.
Summary and Conclusion
Unlike FDG PET/CT mammography, FDG PET/MR mammography could be done a breast imaging study additional to a whole-body PET/MR scan in the supine position for patients with breast cancer without adding radiation. It is worthwhile performing FDG PET/MR mammography simultaneously as a preoperative imaging tool to obtain functional and morphologic data as a “one stop shop,” even though it adds a further 30 min to the examination.
FDG PET/MR mammography affords higher diagnostic performance and confidence than whole-body PET/MR imaging and PET/CT mammography for determining the local extent of malignant breast lesions and for planning surgery, and is comparable with MR breast imaging alone [41–43]. The diagnostic performance for detection of axillary lymph node metastasis is similar to that of PET/CT and MR breast imaging [43]. Whole-body FDG PET/MR imaging combined with PET/MR mammography in a single session to stage local breast cancer and detect distant metastasis simultaneously could be a very effective imaging technique in patients with breast cancer. Further, the comprehensive information on glucose metabolism, cross-sectional morphology, functional features such as tissue perfusion, enhancement kinetics, and DWI or spectroscopy of breast lesions obtained by FDG PET/MR mammography would also be useful in the future for individualized treatment strategies in patients with breast cancer according to their tumor biology.
Compliance with Ethical Standards
Conflict of Interest
Ihn-ho Cho and Eung-Jung Kong declare that they have no conflict of interest.
Ethical Statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
References
- 1.Weir L, Worsley D, Bernstein V. The value of FDG positron emission tomography in the management of patients with breast cancer. Breast J. 2005;11:204–9. doi: 10.1111/j.1075-122X.2005.21625.x. [DOI] [PubMed] [Google Scholar]
- 2.Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005;35:84–99. doi: 10.1053/j.semnuclmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Lind P, Igerc I, Beyer T, Reinprecht P, Hausegger K. Advantages and limitations of FDG PET in the follow-up of breast cancer. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S125–34. doi: 10.1007/s00259-004-1535-8. [DOI] [PubMed] [Google Scholar]
- 4.Czernin J. FDG-PET in breast cancer: a different view of its clinical usefulness. Mol Imaging Biol. 2002;4:35–45. doi: 10.1016/S1095-0397(01)00069-3. [DOI] [PubMed] [Google Scholar]
- 5.Heusner TA, Freudenberg LS, Kuehl H, Hauth EA, Veit-Haibach P, Forsting M, et al. Whole-body PET/CT-mammography for staging breast cancer: initial results. Br J Radiol. 2008;81:743–8. doi: 10.1259/bjr/69647413. [DOI] [PubMed] [Google Scholar]
- 6.Heusner TA, Kuemmel S, Umutlu L, Koeninger A, Freudenberg LS, Hauth EA, et al. Breast cancer staging in a single session: whole-body PET/CT mammography. J Nucl Med. 2008;49:1215–22. doi: 10.2967/jnumed.108.052050. [DOI] [PubMed] [Google Scholar]
- 7.Huellner MW, Appenzeller P, Kuhn FP, Husmann L, Pietsch CM, Burger IA, et al. Whole-body nonenhanced PET/MR versus PET/CT in the staging and restaging of cancers: preliminary observations. Radiology. 2014;273:859–69. doi: 10.1148/radiol.14140090. [DOI] [PubMed] [Google Scholar]
- 8.Drzezga A, Souvatzoglou M, Eiber M, Beer AJ, Furst S, Martinez-Moller A, et al. First clinical experience with integrated whole-body PET/MR: comparison to PET/CT in patients with oncologic diagnoses. J Nucl Med. 2012;53:845–55. doi: 10.2967/jnumed.111.098608. [DOI] [PubMed] [Google Scholar]
- 9.Pace L, Nicolai E, Luongo A, Aiello M, Catalano OA, Soricelli A, et al. Comparison of whole-body PET/CT and PET/MRI in breast cancer patients: lesion detection and quantitation of 18F-deoxyglucose uptake in lesions and in normal organ tissues. Eur J Radiol. 2014;83:289–96. doi: 10.1016/j.ejrad.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Kong EJ, Chun KA, Bom HS, Lee J, Lee SJ, Cho IH. Initial experience of integrated PET/MR mammography in patients with invasive ductal carcinoma. Hell J Nucl Med. 2014;17:171–6. doi: 10.1967/s002449910142. [DOI] [PubMed] [Google Scholar]
- 11.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–78. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 12.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672–91. doi: 10.1148/radiol.2443051661. [DOI] [PubMed] [Google Scholar]
- 13.Tellmann L, Quick HH, Bockisch A, Herzog H, Beyer T. The effect of MR surface coils on PET quantification in whole-body PET/MR: results from a pseudo-PET/MR phantom study. Med Phys. 2011;38:2795–805. doi: 10.1118/1.3582699. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald LR, Kohlmyer S, Liu C, Lewellen TK, Kinahan PE. Effects of MR surface coils on PET quantification. Med Phys. 2011;38:2948–56. doi: 10.1118/1.3583697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paulus DH, Braun H, Aklan B, Quick HH. Simultaneous PET/MR imaging: MR-based attenuation correction of local radiofrequency surface coils. Med Phys. 2012;39:4306–15. doi: 10.1118/1.4729716. [DOI] [PubMed] [Google Scholar]
- 16.Aklan B, Paulus DH, Wenkel E, Braun H, Navalpakkam BK, Ziegler S, et al. Toward simultaneous PET/MR breast imaging: systematic evaluation and integration of a radiofrequency breast coil. Med Phys. 2013;40:024301. doi: 10.1118/1.4788642. [DOI] [PubMed] [Google Scholar]
- 17.Dregely I, Lanz T, Metz S, Mueller MF, Kuschan M, Nimbalkar M, et al. A 16-channel MR coil for simultaneous PET/MR imaging in breast cancer. Eur Radiol. 2015;25:1154–61. doi: 10.1007/s00330-014-3445-x. [DOI] [PubMed] [Google Scholar]
- 18.Brendle C, Schmidt H, Oergel A, Bezrukov I, Mueller M, Schraml C, et al. Segmentation-based attenuation correction in positron emission tomography/magnetic resonance: erroneous tissue identification and its impact on positron emission tomography interpretation. Invest Radiol. 2015;50:339–46. doi: 10.1097/RLI.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 19.Keller SH, Holm S, Hansen AE, Sattler B, Andersen F, Klausen TL, et al. Image artifacts from MR-based attenuation correction in clinical, whole-body PET/MRI. MAGMA. 2013;26:173–81. doi: 10.1007/s10334-012-0345-4. [DOI] [PubMed] [Google Scholar]
- 20.Kong E, Cho I. Clinical issues regarding misclassification by Dixon based PET/MR attenuation correction. Hell J Nucl Med. 2015;18:42–7. [PubMed] [Google Scholar]
- 21.Cho I, Kong E, Chun K. Image artifacts from MR-based attenuation correction in dedicated PET/MR breast coil for PET/MR mammography. EJNMMI Phys. 2015;2:A62. doi: 10.1186/2197-7364-2-S1-A62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant AM, Deller TW, Khalighi MM, Maramraju SH, Delso G, Levin CS. NEMA NU 2-2012 performance studies for the SiPM-based ToF-PET component of the GE SIGNA PET/MR system. Med Phys. 2016;43:2334. doi: 10.1118/1.4945416. [DOI] [PubMed] [Google Scholar]
- 23.Biglia N, Bounous VE, Martincich L, Panuccio E, Liberale V, Ottino L, et al. Role of MRI (magnetic resonance imaging) versus conventional imaging for breast cancer presurgical staging in young women or with dense breast. Eur J Surg Oncol. 2011;37:199–204. doi: 10.1016/j.ejso.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 24.DeMartini W, Lehman C, Partridge S. Breast MRI for cancer detection and characterization: a review of evidence-based clinical applications. Acad Radiol. 2008;15:408–16. doi: 10.1016/j.acra.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Lehman CD, Isaacs C, Schnall MD, Pisano ED, Ascher SM, Weatherall PT, et al. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–8. doi: 10.1148/radiol.2442060461. [DOI] [PubMed] [Google Scholar]
- 26.Riedl CC, Ponhold L, Flory D, Weber M, Kroiss R, Wagner T, et al. Magnetic resonance imaging of the breast improves detection of invasive cancer, preinvasive cancer, and premalignant lesions during surveillance of women at high risk for breast cancer. Clin Cancer Res. 2007;13:6144–52. doi: 10.1158/1078-0432.CCR-07-1270. [DOI] [PubMed] [Google Scholar]
- 27.Hylton N. Magnetic resonance imaging of the breast: opportunities to improve breast cancer management. J Clin Oncol. 2005;23:1678–84. doi: 10.1200/JCO.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Lee CH. Problem solving MR imaging of the breast. Radiol Clin N Am. 2004;42:919–34. doi: 10.1016/j.rcl.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Kinkel K, Hylton NM. Challenges to interpretation of breast MRI. J Magn Reson Imaging. 2001;13:821–9. doi: 10.1002/jmri.1117. [DOI] [PubMed] [Google Scholar]
- 30.Scheidhauer K, Walter C, Seemann MD. FDG PET and other imaging modalities in the primary diagnosis of suspicious breast lesions. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S70–9. doi: 10.1007/s00259-004-1528-7. [DOI] [PubMed] [Google Scholar]
- 31.Hodgson NC, Gulenchyn KY. Is there a role for positron emission tomography in breast cancer staging? J Clin Oncol. 2008;26:712–20. doi: 10.1200/JCO.2007.13.8412. [DOI] [PubMed] [Google Scholar]
- 32.Groheux D, Espie M, Giacchetti S, Hindie E. Performance of FDG PET/CT in the clinical management of breast cancer. Radiology. 2013;266:388–405. doi: 10.1148/radiol.12110853. [DOI] [PubMed] [Google Scholar]
- 33.Kaida H, Ishibashi M, Fujii T, Kurata S, Ogo E, Tanaka M, et al. Improved detection of breast cancer on FDG-PET cancer screening using breast positioning device. Ann Nucl Med. 2008;22:95–101. doi: 10.1007/s12149-007-0092-1. [DOI] [PubMed] [Google Scholar]
- 34.Koolen BB, Vrancken Peeters MJ, Wesseling J, Lips EH, Vogel WV, Aukema TS, et al. Association of primary tumour FDG uptake with clinical, histopathological and molecular characteristics in breast cancer patients scheduled for neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2012;39:1830–8. doi: 10.1007/s00259-012-2211-z. [DOI] [PubMed] [Google Scholar]
- 35.Moon EH, Lim ST, Han YH, Jeong YJ, Kang YH, Jeong HJ, et al. The usefulness of F-18 FDG PET/CT-mammography for preoperative staging of breast cancer: comparison with conventional PET/CT and MR-mammography. Radiol Oncol. 2013;47:390–7. doi: 10.2478/raon-2013-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu DF, Freese DL, Levin CS. Breast-dedicated radionuclide imaging systems. J Nucl Med. 2016;57(Suppl 1):40S–5S. doi: 10.2967/jnumed.115.157883. [DOI] [PubMed] [Google Scholar]
- 37.MacDonald L, Edwards J, Lewellen T, Haseley D, Rogers J, Kinahan P. Clinical imaging characteristics of the positron emission mammography camera: PEM Flex Solo II. J Nucl Med. 2009;50:1666–75. doi: 10.2967/jnumed.109.064345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kalinyak JE, Berg WA, Schilling K, Madsen KS, Narayanan D, Tartar M. Breast cancer detection using high-resolution breast PET compared to whole-body PET or PET/CT. Eur J Nucl Med Mol Imaging. 2014;41:260–75. doi: 10.1007/s00259-013-2553-1. [DOI] [PubMed] [Google Scholar]
- 39.Berg WA, Madsen KS, Schilling K, Tartar M, Pisano ED, Larsen LH, et al. Breast cancer: comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radiology. 2011;258:59–72. doi: 10.1148/radiol.10100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moy L, Noz ME, Maguire GQ, Jr, Melsaether A, Deans AE, Murphy-Walcott AD, et al. Role of fusion of prone FDG-PET and magnetic resonance imaging of the breasts in the evaluation of breast cancer. Breast J. 2010;16:369–76. doi: 10.1111/j.1524-4741.2010.00927.x. [DOI] [PubMed] [Google Scholar]
- 41.Taneja S, Jena A, Goel R, Sarin R, Kaul S. Simultaneous whole-body 18F-FDG PET-MRI in primary staging of breast cancer: a pilot study. Eur J Radiol. 2014;83:2231–9. doi: 10.1016/j.ejrad.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Botsikas D, Kalovidouri A, Becker M, Copercini M, Djema DA, Bodmer A, et al. Clinical utility of 18F-FDG-PET/MR for preoperative breast cancer staging. Eur Radiol. 2015;26:2297-307. [DOI] [PubMed]
- 43.Grueneisen J, Nagarajah J, Buchbender C, Hoffmann O, Schaarschmidt BM, Poeppel T, et al. Positron emission tomography/magnetic resonance imaging for local tumor staging in patients with primary breast cancer: a comparison with positron emission tomography/computed tomography and magnetic resonance imaging. Invest Radiol. 2015;50:505–13. doi: 10.1097/RLI.0000000000000197. [DOI] [PubMed] [Google Scholar]
- 44.Crippa F, Gerali A, Alessi A, Agresti R, Bombardieri E. FDG-PET for axillary lymph node staging in primary breast cancer. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S97–102. doi: 10.1007/s00259-004-1531-z. [DOI] [PubMed] [Google Scholar]
- 45.Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, et al. A prospective evaluation of positron emission tomography scanning, sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol. 2004;11:846–53. doi: 10.1245/ASO.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 46.Riegger C, Koeninger A, Hartung V, Otterbach F, Kimmig R, Forsting M, et al. Comparison of the diagnostic value of FDG-PET/CT and axillary ultrasound for the detection of lymph node metastases in breast cancer patients. Acta Radiol. 2012;53:1092–8. doi: 10.1258/ar.2012.110635. [DOI] [PubMed] [Google Scholar]
- 47.Aukema TS, Straver ME, Peeters MJ, Russell NS, Gilhuijs KG, Vogel WV, et al. Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II-III breast cancer. Eur J Cancer. 2010;46:3205–10. doi: 10.1016/j.ejca.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 48.Abramson RG, Lambert KF, Jones-Jackson LB, Arlinghaus LR, Williams J, Abramson VG, et al. Prone versus supine breast FDG-PET/CT for assessing locoregional disease distribution in locally advanced breast cancer. Acad Radiol. 2015;22:853–9. doi: 10.1016/j.acra.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koolen BB, Valdes Olmos RA, Elkhuizen PH, Vogel WV, Vrancken Peeters MJ, Rodenhuis S, et al. Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat. 2012;135:231–40. doi: 10.1007/s10549-012-2179-1. [DOI] [PubMed] [Google Scholar]
- 50.He N, Xie C, Wei W, Pan C, Wang W, Lv N, et al. A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol. 2012;81:2602–12. doi: 10.1016/j.ejrad.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 51.Chia S, Swain SM, Byrd DR, Mankoff DA. Locally advanced and inflammatory breast cancer. J Clin Oncol. 2008;26:786–90. doi: 10.1200/JCO.2008.15.0243. [DOI] [PubMed] [Google Scholar]
- 52.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 53.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002;95:681–95. doi: 10.1002/cncr.10741. [DOI] [PubMed] [Google Scholar]
- 54.Avril S, Muzic RF, Jr, Plecha D, Traughber BJ, Vinayak S, Avril N. 18F-FDG PET/CT for monitoring of treatment response in breast cancer. J Nucl Med. 2016;57(Suppl 1):34S–9S. doi: 10.2967/jnumed.115.157875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lobbes MB, Prevos R, Smidt M, Tjan-Heijnen VC, van Goethem M, Schipper R, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–75. doi: 10.1007/s13244-013-0219-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Park SH, Moon WK, Cho N, Chang JM, Im SA, Park IA, et al. Comparison of diffusion-weighted MR imaging and FDG PET/CT to predict pathological complete response to neoadjuvant chemotherapy in patients with breast cancer. Eur Radiol. 2012;22:18–25. doi: 10.1007/s00330-011-2236-x. [DOI] [PubMed] [Google Scholar]
- 57.An YY, Kim SH, Kang BJ, Lee AW. Treatment response evaluation of breast cancer after neoadjuvant chemotherapy and usefulness of the imaging parameters of MRI and PET/CT. J Korean Med Sci. 2015;30:808–15. doi: 10.3346/jkms.2015.30.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charles-Edwards EM, DeSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–43. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thoeny HC, De Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007;17:1385–93. doi: 10.1007/s00330-006-0547-0. [DOI] [PubMed] [Google Scholar]
- 60.Guo Y, Cai YQ, Cai ZL, Gao YG, An NY, Ma L, et al. Differentiation of clinically benign and malignant breast lesions using diffusion-weighted imaging. J Magn Reson Imaging. 2002;16:172–8. doi: 10.1002/jmri.10140. [DOI] [PubMed] [Google Scholar]
- 61.Kuroki Y, Nasu K, Kuroki S, Murakami K, Hayashi T, Sekiguchi R, et al. Diffusion-weighted imaging of breast cancer with the sensitivity encoding technique: analysis of the apparent diffusion coefficient value. Magn Reson Med Sci. 2004;15:79–85. doi: 10.2463/mrms.3.79. [DOI] [PubMed] [Google Scholar]
- 62.Razek AA, Gaballa G, Denewer A, Nada N. Invasive ductal carcinoma: correlation of apparent diffusion coefficient value with pathological prognostic factors. NMR Biomed. 2010;23:619–23. doi: 10.1002/nbm.1503. [DOI] [PubMed] [Google Scholar]
- 63.Martincich L, Deantoni V, Bertotto I, Redana S, Kubatzki F, Sarotto I, et al. Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol. 2012;22:1519–28. doi: 10.1007/s00330-012-2403-8. [DOI] [PubMed] [Google Scholar]
- 64.Kim SH, Cha ES, Kim HS, Kang BJ, Choi JJ, Jung JH, et al. Diffusion-weighted imaging of breast cancer: correlation of the apparent diffusion coefficient value with prognostic factors. J Magn Reson Imaging. 2009;30:615–20. doi: 10.1002/jmri.21884. [DOI] [PubMed] [Google Scholar]
- 65.Youk JH, Son EJ, Chung J, Kim JA, Kim EK. Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol. 2012;22:1724–34. doi: 10.1007/s00330-012-2425-2. [DOI] [PubMed] [Google Scholar]
- 66.Choi BB, Kim SH, Kang BJ, Lee JH, Song BJ, Jeong SH, et al. Diffusion-weighted imaging and FDG PET/CT: predicting the prognoses with apparent diffusion coefficient values and maximum standardized uptake values in patients with invasive ductal carcinoma. World J Surg Oncol. 2012;10:126. doi: 10.1186/1477-7819-10-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55:736–42. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 68.Kong E, Chun KA, Bae YK, Cho IH. Integrated PET/MR mammography for quantitative analysis and correlation to prognostic factors of invasive ductal carcinoma. Q J Nucl Med Mol Imaging. 2016. In press. [DOI] [PubMed]
- 69.Belkic D, Belkic K. Molecular imaging in the framework of personalized cancer medicine. Isr Med Assoc J. 2013;15:665–72. [PubMed] [Google Scholar]
- 70.Cho N, Im SA, Kang KW, Park IA, Song IC, Lee KH, et al. Early prediction of response to neoadjuvant chemotherapy in breast cancer patients: comparison of single-voxel (1)H-magnetic resonance spectroscopy and (18)F-fluorodeoxyglucose positron emission tomography. Eur Radiol. 2016;26:2279-90. [DOI] [PubMed]