Abstract
Objectives
Previously published studies showed that the standard tumor-to-blood standardized uptake value (SUV) ratio (SUR) was a more accurate prognostic method than tumor maximum standardized uptake value (SUVmax). This study evaluated and compared prognostic value of positron emission tomography (PET) parameters and normalized value of PET parameters by blood pool SUV in non-small-cell lung cancer (NSCLC) patients who received curative surgery.
Methods
Seventy-seven patients who underwent curative resection for NSCLC between January 2010 to December 2013 were enrolled in this study. 18Fluorine-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) was performed before surgery. The mean standardized uptake value (SUVmean), SUVmax, metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of each lesion was measured, on the workstation. SURmean, SURmax, and TLGSUR were calculated by dividing each of them by descending aorta SUVmean. Cox proportional hazards regression was used to analyze the effect of age, sex, pathological parameters, and PET parameters on recurrence and death.
Results
In Cox regression analysis, N stage predicted for both recurrence (p < 0.0001) and death (p < 0.0001). SURmax predicted recurrence (p = 0.0014), not death. Area under the receiver operating characteristic curve of SURmax was 0.759 with cutoff value 4.004. However, SUVmax, SUVmean, MTV, TLG, SURmean, and TLGSUR predicted neither recurrence nor death.
Conclusions
Among PET parameters, SURmax was the independent predictor of recurrence in NSCLC patients who received curative surgery. N stage was the independent prognostic factor for both recurrence and death. Both parameters could be used to stratify the risk of NSCLC patients.
Keywords: Fluorodeoxyglucose F18, Positron-emission tomography, Prognosis, Non-small-cell lung cancer, Recurrence, Survival
Introduction
Lung cancer is the third most commonly diagnosed cancer and the most common cause of cancer death in Korea. The 5-year survival rate for lung cancer is 21.9 % [1]. Non-small-cell lung cancer (NSCLC) comprises about 85 % of all lung cancer [2]. In early NSCLC patients, lobectomy is standard treatment, and patients who are not candidates for surgery have to receive a radiotherapy [3]. In advanced stage, chemotherapy or concomitant chemoradiotherapy are considered as the standard treatment [2, 3]. Therefore, precise staging in patients with NSCLC is important.
18Fluorine-fluorodeoxyglucose (18F-FDG) positron emission tomography/computed tomography (PET/CT) is used in staging, surveillance, radiation therapy planning, and predicting prognosis in lung cancer [4]. Research shows that PET parameters could represent the activity of tumor. Of the PET parameters, the maximum standardized uptake value (SUVmax) is commonly used to report tumor activity. It has a high reproducibility and a low interobserver variability [5]. As SUVmax is defined by the highest voxel value within the region of interest (ROI), it is sensitive to image noise [5]. Therefore, mean standardized uptake value (SUVmean) is important to report tumor activity. As metabolic tumor volume (MTV) represents the amount of high metabolic tumor cells [6] and total lesion glycolysis (TLG) represents tumor size and degree of 18F-FDG [7], MTV and TLG have been proposed for risk stratification of lung cancer. MTV is defined as a volume of the tumor, delineated by a specific threshold of SUV. TLG is derived by multiplying MTV and SUVmean, and represents tumor burden [8]. MTV and TLG are predictive of overall survival (OS) and recurrence in NSCLC [8, 9].
A recent study has proved that tumor-to-blood SUV ratio (SUR), the ratio of tumor SUV and aorta blood SUV, showed a stronger correlation with the metabolic rate of FDG than tumor SUV [10]. In a subsequent study, SUR of esophageal carcinoma was an independent prognostic factor of OS and distant metastasis-free survival [11]. Therefore, the aim of this study was to evaluate and compare prognostic value of positron emission tomography (PET) parameters and normalized value of PET parameters by blood pool SUV in non-small-cell lung cancer (NSCLC) patients who received curative surgery.
Materials and Methods
Patients
Three thousand one hundred forty patients were registered as a lung cancer between January 2010 to December 2013. Inclusion criteria were patients with 1) pathologically confirmed NSCLC (adenocarcinoma and squamous cell carcinoma) and 2) preoperative 18F-FDG PET/CT. Exclusion criteria were patients who 1) received chemotherapy, radiation therapy, or concurrent chemoradiation therapy without surgery, 2) had distant metastasis confirmed by imaging modalities such as chest CT, brain magnetic resonance imaging, bone scintigraphy, and 18F-FDG PET/CT, 3) did not perform 18F-FDG PET/CT at staging, 4) had not enough medical records. Seventy-seven patients met the criteria and they were enrolled in this study. The median follow-up was 33 months and the follow-up ranged from 2 month to 64 month. The endpoint of study was 15 August 2015. Our institutional review board waived informed consent and approved the study.
18F-FDG PET/CT Imaging
According to the standard protocol of our institution, all patients fasted for at least 6 h. Their median blood glucose level was 98 mg/dl (range: 66–143 mg/dl) before 18F-FDG administration. Sixty minutes after injection of 0.14 mCi/kg 18F-FDG, PET/CT scans were performed (Biograph 40, Siemens, Knoxville, TN, USA). Low-dose CT from the base of the skull to the proximal thighs was performed for the purpose of attenuation correction and precise anatomical localization. PET data were obtained using a high-resolution whole-body scanner. The scan time per bed position was two minutes with 500 mm axial field of view. Reconstruction parameters were iterative reconstruction with three iterations, 21 subsets; Gaussian smoothing filter with image size 168; voxel dimension 3.9 × 3.9 mm.
Image Analysis
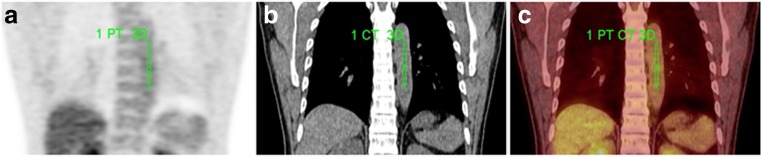
PET/CT images were interpreted directly from the workstation (Siemens TrueD image analysis software, Siemens, Knoxville, TN, USA). A volume of interest (VOI) was drawn over the entire abnormal uptake of lung cancer to include a large amount of radioactivity on axial images semi-automatically. SUVmax of 18F-FDG was measured from VOI. SUVmean and MTV of each lesion were calculated automatically on the workstation and SUV 2.5 was adopted as the threshold. TLG was calculated by multiplying SUVmean by MTV. Blood SUVmean was measured from VOI in the descending aorta (Fig. 1). SUR was derived from the value of SUVmax, SUVmean, and TLG divided by blood SUVmean (Eq. 1). The method of delineation of aorta and calculation of SUR followed published research [11].
| 1 |
Fig. 1.
Delineation of aorta. Delineation was performed on fusion view and was checked by both attenuation CT and PET image
Statistical Analysis
Cox proportional hazards regression was used to analyze the effect of age, sex, pathological parameters such as histopathologic class, pathologic T stage, nodal involvement and stage, and PET parameters on recurrence and death. Recurrence was defined as a tumor recurrence including local recurrence, regional recurrence, and distant metastasis identified by imaging study. The date of death was obtained from patient’s medical records. Two-sided null hypotheses of no difference were rejected if p-values were less than or equal to 0.05 and the 95 % confidence of intervals do not cross 1. Receiver operating characteristic (ROC) curves of PET parameters were drawn and compared to evaluate the capability to predict recurrence and death. The predictive value of the PET parameters was assessed by the area under curve (AUC) of the ROC curve. Recurrence free survival (RFS) was defined as the interval from the operation date to the date of recurrence identified by imaging study or last clinical follow-up and Kaplan–Meier survival analysis with log-rank test was performed according to PET parameters. The cutoffs were determined by median values of each PET parameter. Data analysis was performed by MedCalc v.15.11 software (MedCalc, Mariakerke, Belgium).
Results
Patient Characteristics
Seventy-seven NSCLC patients were included in this study. Patient characteristics are shown in Table 1. The mean age was 62.4 ± 8.7 years. Lymph node metastasis was present in 20 patients (26 %). Eighteen patients (23.4 %) had experienced recurrence and four patients (5.2 %) died during the follow-up (34.0 ± 14.7 months). Median OS and RFS were 34 and 33 months, respectively. The difference of OS and RFS was small. It could be due to short follow-up period or the majority of patients had early stage. The mean value of SUVmean, SUVmax, MTV, TLG, SURmean, SURmax, and TLGSUR were 4.174 ± 1.847, 8.748 ± 5.863, 17.670 ± 28.210 ml, 105.692 ± 190.515 g, 1.937 ± 0.904, 4.024 ± 2.730, and 49.834 ± 96.569, respectively.
Table 1.
Patient characteristics
| Variables | Value (%) |
|---|---|
| Age (years) | |
| Mean ± SD | 62.4 ± 8.7 |
| Sex | |
| Male | 46 (59.7) |
| Female | 31 (40.3) |
| Histology | |
| Squamous cell carcinoma | 21 (27.3) |
| Adenocarcinoma | 56 (72.7) |
| T stage | |
| T1 | 41 (53.2) |
| T2 | 29 (37.7) |
| T3 | 6 (7.8) |
| T4 | 1 (1.3) |
| N stage | |
| N0 | 57 (74.0) |
| N1 | 11 (14.3) |
| N2 | 8 (10.4) |
| N3 | 1 (1.3) |
| TNM stage | |
| I | 48 (62.3) |
| II | 19 (24.7) |
| III | 10 (13.0) |
| IV | 0 (0) |
Recurrence
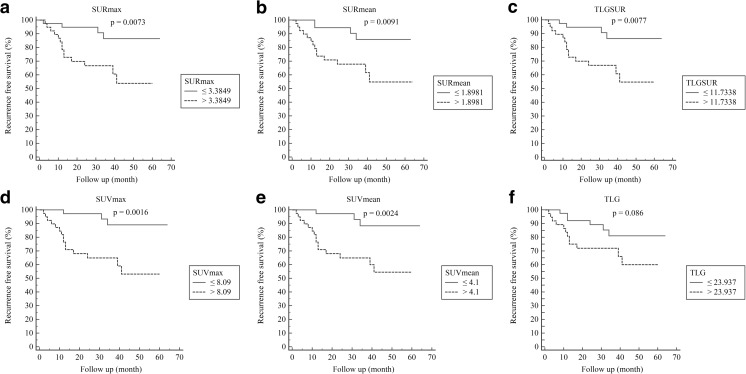
N stage (hazard ratio 2.508, 95 % CI 1.499–4.197, p = 0.0005), TNM stage (2.382, 1.362–4.163, p = 0.0023), SURmax (1.277, 1.099–1.484, p = 0.0014), SURmean (2.299, 1.390–3.802, p = 0.0012), SUVmax (1.113, 1.039–1.192, 0.0022), SUVmean (1.472, 1.163–1.864, p = 0.0013), TLGSUR (1.0051, 1.002–1.009, p = 0.0057), MTV (1.014, 1.001–1.027, p = 0.0377), and TLG (1.003, 1.001–1.004, p = 0.0076) predicted recurrence in univariate analysis (Table 2). Age (1.109, 1.037–1.187, p < 0.0027), N stage (3.381, 1.934–5.910, p < 0.0001), and SURmax (1.381, 1.132–1.685, p = 0.0014) predicted recurrence in multivariate analysis (Table 2). AUC of SURmax, SURmean, TLGSUR, MTV, SUVmax, SUVmean, and TLG were 0.759, 0.735, 0.686, 0.664, 0.744, 0.759, and 0.682, respectively. Although SURmax and SUVmean had higher AUC among the PET parameters, there were no PET parameters which had significantly higher AUC values. AUC of PET parameters for predicting recurrence are in Table 3. Kaplan–Meier survival analysis with log-rank test is in Fig. 2. The cutoff values of PET parameters were derived from median value. SURmax (p = 0.0073), SURmean (p = 0.0091), TLGSUR (p = 0.0077), SUVmax (p = 0.0016), and SUVmean (p = 0.0024) showed significant difference in recurrence.
Table 2.
Univariate and multivariate analysis of recurrence
| Variables | Wald | Univariate | Wald | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |||
| Age | 2.6356 | 1.0487 | 0.9902–1.1106 | 0.1045 | 8.9980 | 1.1091 | 1.0365–1.1867 | 0.0027 |
| Sex | 0.9616 | 0.6115 | 0.2288–1.6342 | 0.3268 | ||||
| T stage | 1.6061 | 1.5433 | 0.7889–3.0194 | 0.2050 | ||||
| N stage | 12.2522 | 2.5080 | 1.4987–4.1970 | 0.0005 | 18.2661 | 3.3807 | 1.9338–5.9104 | <0.0001 |
| TNM stage | 9.2705 | 2.3815 | 1.3622–4.1632 | 0.0023 | ||||
| Histology | 0.06011 | 1.1378 | 0.4054–3.1936 | 0.8063 | ||||
| SURmax | 10.2321 | 1.2772 | 1.0994–1.4838 | 0.0014 | 10.1456 | 1.3814 | 1.1323–1.6853 | 0.0014 |
| SURmean | 10.5215 | 2.2990 | 1.3902–3.8019 | 0.0012 | ||||
| TLGSUR | 7.6536 | 1.0051 | 1.0015–1.0088 | 0.0057 | ||||
| MTV (ml) | 4.3170 | 1.0136 | 1.0008–1.0266 | 0.0377 | ||||
| SUVmax | 9.3900 | 1.1130 | 1.0393–1.1919 | 0.0022 | ||||
| SUVmean | 10.3519 | 1.4724 | 1.1632–1.8637 | 0.0013 | ||||
| TLG (g) | 7.1288 | 1.0025 | 1.0007–1.0044 | 0.0076 | ||||
SUR: standard tumor-to-blood SUV ratio; SUVmax: maximum standardized uptake value; SUVmean: mean standardized uptake value; MTV: metabolic tumor volume; TLG: total lesion glycolysis
Table 3.
Area under the ROC curve (AUC) of PET parameters for predicting recurrence
| Parameters | Cutoff | AUC | 95 % CI | Sen | Sp | LR+ | LR- | p-value |
|---|---|---|---|---|---|---|---|---|
| SURmax | >4.004 | 0.759 | 0.648–0.849 | 83.33 | 66.10 | 2.46 | 0.25 | <0.0001 |
| SURmean | >1.9757 | 0.735 | 0.623–0.829 | 77.78 | 64.41 | 2.19 | 0.35 | 0.0004 |
| TLGSUR | >11.7338 | 0.686 | 0.571–0.787 | 77.78 | 59.32 | 1.91 | 0.37 | 0.0077 |
| MTV (ml) | >4.24 | 0.664 | 0.548–0.768 | 77.78 | 50.85 | 1.58 | 0.44 | 0.0211 |
| SUVmax | >9.46 | 0.744 | 0.632–0.837 | 83.33 | 66.10 | 2.46 | 0.25 | 0.0001 |
| SUVmean | >4.41 | 0.759 | 0.648–0.850 | 83.33 | 69.49 | 2.73 | 0.24 | <0.0001 |
| TLG (g) | >22.6476 | 0.682 | 0.566–0.783 | 77.78 | 55.93 | 1.76 | 0.40 | 0.0110 |
SUR: standard tumor-to-blood SUV ratio; SUVmax: maximum standardized uptake value; SUVmean: mean standardized uptake value; MTV: metabolic tumor volume; TLG: total lesion glycolysis; Sen: sensitivity; Sp: specificity; LR: likelihood ratio
Fig. 2.
Kaplan–Meier curve depicting the recurrence free survival according to SURmax (a), SURmean (b), TLGSUR (c), SUVmax (d), SUVmean (e), and TLG (f)
Death
TNM stage (4.975, 1.249–19.819, p = 0.0229) and SUVmax (1.152, 1.001–1.326, p = 0.0485) predicted death in univariate analysis (Table 4). N stage (3.868, 1.536–9.742, p = 0.0041) was the only parameter which showed significance both in univariate and multivariate analysis (Table 4). AUC of SURmax, SURmean, TLGSUR, MTV, SUVmax, SUVmean, and TLG were 0.781, 0.729, 0.777, 0.764, 0.812, 0.812, and 0.788, respectively. Although SUVmax and SUVmean had higher AUC among the PET parameters, there were no PET parameters which had significantly higher AUC values. AUC of PET parameters for predicting death are shown in Table 5.
Table 4.
Univariate and multivariate analysis of death
| Variables | Wald | Univariate | Wald | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| HR | 95 % CI | P | HR | 95 % CI | P | |||
| Age | 0.3919 | 1.0396 | 0.9206–1.1740 | 0.5313 | ||||
| Sex | NA | NA | NA | NA | ||||
| T stage | 1.4233 | 1.9410 | 0.6529–5.7702 | 0.2329 | ||||
| N stage | 8.2404 | 3.8681 | 1.5359–9.7418 | 0.0041 | 8.2404 | 3.8681 | 1.5359–9.7418 | 0.0041 |
| TNM stage | 0.7052 | 4.9754 | 1.2490–19.8191 | 0.0229 | ||||
| Histology | 1.2138 | 3.0148 | 0.4233–21.4729 | 0.2706 | ||||
| SURmax | 3.0615 | 1.3183 | 0.9673–1.7967 | 0.0802 | ||||
| SURmean | 1.6479 | 1.9075 | 0.7116–5.1128 | 0.1992 | ||||
| TLGSUR | 1.2348 | 1.0044 | 0.9967–1.0121 | 0.2665 | ||||
| MTV (ml) | 2.0618 | 1.0189 | 0.9932–1.0453 | 0.1510 | ||||
| SUVmax | 3.8934 | 1.1522 | 1.0009–1.3262 | 0.0485 | ||||
| SUVmean | 2.7871 | 1.5047 | 0.9314–2.4310 | 0.0950 | ||||
| TLG (g) | 1.5973 | 1.0025 | 0.9986–1.0064 | 0.2063 | ||||
SUR: standard tumor-to-blood SUV ratio; SUVmax: maximum standardized uptake value; SUVmean: mean standardized uptake value; MTV: metabolic tumor volume; TLG: total lesion glycolysis; NA: not applicable
Table 5.
Area under the ROC curve (AUC) of PET parameters for predicting death
| Parameters | Cutoff | AUC | 95 % CI | Sen | Sp | LR+ | LR- | p-value |
|---|---|---|---|---|---|---|---|---|
| SURmax | >4.0359 | 0.781 | 0.672–0.867 | 100.0 | 58.90 | 2.43 | 0 | 0.0007 |
| SURmean | >1.9757 | 0.729 | 0.616–0.824 | 100.0 | 57.53 | 2.35 | 0 | 0.0011 |
| TLGSUR | >15.3114 | 0.777 | 0.668–0.864 | 100.0 | 60.27 | 2.52 | 0 | 0.0005 |
| MTV (ml) | >7.74 | 0.764 | 0.653–0.853 | 100.0 | 57.53 | 2.35 | 0 | 0.0211 |
| SUVmax | >10 | 0.812 | 0.706–0.892 | 100.0 | 61.64 | 2.61 | 0 | 0.0001 |
| SUVmean | >4.88 | 0.812 | 0.706–0.892 | 100.0 | 73.97 | 3.84 | 0 | <0.0001 |
| TLG (g) | >37.2068 | 0.788 | 0.680–0.873 | 100.0 | 60.27 | 2.52 | 0 | 0.0006 |
SUR: standard tumor-to-blood SUV ratio; SUVmax: maximum standardized uptake value; SUVmean: mean standardized uptake value; MTV: metabolic tumor volume; TLG: total lesion glycolysis; Sen: sensitivity; Sp: specificity; LR: likelihood ratio
Discussion
In this study, SURmax, age, and N stage were independent prognostic factors for recurrence in NSCLC patients. N stage was the only independent prognostic parameter for death.
Several studies showed that age was a prognostic factor in lung cancer [12, 13], while other studies did not [14, 15]. In this study, age was an independent prognostic factor for recurrence. The difference of results could be due to different composition of staging, various histology, and different definition of older patient.
TNM staging is the most widely accepted prognostic factor for risk stratification [16]. In this study, N stage was the independent factor for both recurrence and death. However, T stage did not show the significance of prognostic value. It might be due to the fact that the distribution of T stage was biased toward early stage.
Previous studies have presented that SUVmax was a prognostic factor for lung cancer [17]. However, according to Chen et al.’s study, SUVmax did not predict progression free survival independently, which was consistent with our results [18]. As SUVmax is the highest voxel value within the ROI, it is independent of ROI definition [5, 19]. However, it is more susceptible to noise and cannot reflect metabolic activity of the whole tumor volume [5, 19]. As SUVmax is limited in representing tumor metabolic activity, MTV and TLG are proposed for risk stratification of lung cancer [8]. MTV and TLG represent both the degree of 18F-FDG uptake and the size of the tumor [8]. However, MTV is dependent on cutoff values, and it might include inflammation around primary cancer [20]. Therefore, MTV and TLG have weaknesses. Although several studies of volumetric parameters showed association with prognosis in NSCLC patients [8, 9], MTV and TLG were not independent prognostic factors for recurrence in this study. Two thirds of patients who enrolled in this study were stage I and the median follow-up was 33 months. This could influence the results.
Although SUVmax has a high reproducibility, it can vary between different PET scanners [21]. To overcome this shortcoming, tumor SUV is commonly normalized by liver activity. SUV of the normal human liver remains constant if the time delay between tracer injection and PET acquisition is in the range of 50–110 min [22]. Therefore, it can be a reference organ to interpret PET scan [22]. Tumor SUVmax normalized to liver can predict prognosis in various malignancies including NSCLC [23–25]. However, according to Besson et al., SUV of the liver was significantly different between giant cell arteritis patients and control patients [26]. Systemic inflammation affects liver metabolism and can influence the liver uptake ratio [26].
According to the study of Hoff et al., inter-study variability of the arterial input function (AIF) was a crucial factor adversely affecting the correlation between SUV and Km (the metabolic rate of glucose consumption) [10]. As the equation of SUR derived from Patlak formula was less dependent on AIF than the equation of SUV, they expected that SUR had higher linear correlation to Km than SUV and validated their theory by colorectal cancer patients with fully dynamic investigations [10]. As a subsequent study, Butoff et al. showed that SUR was an independent prognostic factor for OS and distant metastasis (DM) in esophageal cancer [11]. In their study, MTV was also an independent prognostic factor for OS and DM. However, the hazard ratio of SUR was higher than MTV [11]. This study demonstrated that SURmax was an independent prognostic factor for recurrence, consistent with previous study.
This study had several limitations. First, it was designed as a retrospective observational study. Second, a small number of patients were included. Third, follow up period was short. Fourth, partial volume effect correction for lung cancer was not performed.
In conclusion, SURmax was the independent prognostic factor for recurrence among the PET parameters in NSCLC patients, while N stage was the independent prognostic factor for both recurrence and death. Both SURmax and N stage could be used to stratify the risk of NSCLC patients. Since this study was retrospective with small sample size, further prospective and larger study should be performed to validate this result.
Acknowledgments
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Compliance with Ethical Standards
Conflict of Interest
Seunghyeon Shin, Seong-Jang Kim, In Joo Kim, Kyoungjune Pak and Bum Soo Kim declare that they have no conflict of interest.
Ethical Statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
Contributor Information
Seunghyeon Shin, Phone: 82-51-240-7389, Email: forladou@naver.com.
Kyoungjune Pak, Phone: 82-51-240-7389, Email: ilikechopin@daum.net.
In Joo Kim, Phone: 82-51-240-7389, Email: injkim@pusan.ac.kr.
Bum Soo Kim, Phone: 82-51-240-7389, Email: bum8112@gmail.com.
Seong Jang Kim, Phone: 82-51-240-7389, Email: growthkim@daum.net, Email: growthkim@pusan.ac.kr.
References
- 1.Jung K-W, Park S, Kong H-J, Won Y-J, Lee JY, Seo HG, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2009. Cancer Res Treat. 2012;44:11–24. doi: 10.4143/crt.2012.44.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bareschino MA, Schettino C, Rossi A, Maione P, Sacco PC, Zeppa R, et al. Treatment of advanced non small cell lung cancer. J Thorac Dis. 2011;3:122–133. doi: 10.3978/j.issn.2072-1439.2010.12.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crino L, Weder W, van Meerbeeck J, Felip E, Group EGW. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol: Off J Eur Soc Med Oncol / ESMO. 2010;21(Suppl 5):v103–v115. doi: 10.1093/annonc/mdq207. [DOI] [PubMed] [Google Scholar]
- 4.Bunyaviroch T, Coleman RE. Pet evaluation of lung cancer. J Nucl Med. 2006;47:451–469. [PubMed] [Google Scholar]
- 5.Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of suv measurements. AJR Am J Roentgenol. 2010;195:310–320. doi: 10.2214/AJR.10.4923. [DOI] [PubMed] [Google Scholar]
- 6.Zhu D, Ma T, Niu Z, Zheng J, Han A, Zhao S, et al. Prognostic significance of metabolic parameters measured by (18)f-fluorodeoxyglucose positron emission tomography/computed tomography in patients with small cell lung cancer. Lung Cancer. 2011;73:332–337. doi: 10.1016/j.lungcan.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Kim K, Kim SJ, Kim IJ, Kim YS, Pak K, Kim H. Prognostic value of volumetric parameters measured by f-18 fdg pet/ct in surgically resected non-small-cell lung cancer. Nucl Med Commun. 2012;33:613–620. doi: 10.1097/MNM.0b013e328351d4f5. [DOI] [PubMed] [Google Scholar]
- 8.Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters of (18)f-fdg pet in non-small-cell lung cancer: a meta-analysis. Eur J Nucl Med Mol Imaging. 2015;42:241–51. [DOI] [PubMed]
- 9.Kim DH, Son SH, Kim CY, Hong CM, Oh JR, Song BI, et al. Prediction for recurrence using f-18 fdg pet/ct in pathologic n0 lung adenocarcinoma after curative surgery. Ann Surg Oncol. 2014;21:589–596. doi: 10.1245/s10434-013-3270-5. [DOI] [PubMed] [Google Scholar]
- 10.van den Hoff J, Oehme L, Schramm G, Maus J, Lougovski A, Petr J, et al. The pet-derived tumor-to-blood standard uptake ratio (sur) is superior to tumor suv as a surrogate parameter of the metabolic rate of fdg. EJNMMI Res. 2013;3:77. doi: 10.1186/2191-219X-3-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butof R, Hofheinz F, Zophel K, Stadelmann T, Schmollack J, Jentsch C, et al. Prognostic value of pretherapeutic tumor-to-blood standardized uptake ratio in patients with esophageal carcinoma. J Nucl Med. 2015;56:1150–1156. doi: 10.2967/jnumed.115.155309. [DOI] [PubMed] [Google Scholar]
- 12.Tas F, Ciftci R, Kilic L, Karabulut S. Age is a prognostic factor affecting survival in lung cancer patients. Oncol Lett. 2013;6:1507–1513. doi: 10.3892/ol.2013.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asmis TR, Ding K, Seymour L, Shepherd FA, Leighl NB, Winton TL, et al. National Cancer Institute of Canada Clinical Trials G: age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of national cancer institute of Canada clinical trials group trials. J Clin Oncol: Off J Am Soc Clin Oncol. 2008;26:54–59. doi: 10.1200/JCO.2007.12.8322. [DOI] [PubMed] [Google Scholar]
- 14.Ye T, Pan Y, Wang R, Hu H, Zhang Y, Li H, et al. Analysis of the molecular and clinicopathologic features of surgically resected lung adenocarcinoma in patients under 40 years old. J Thorac Dis. 2014;6:1396–1402. doi: 10.3978/j.issn.2072-1439.2014.08.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu CL, Chen JH, Chen KY, Shih JY, Yang JC, Yu CJ, et al. Advanced non-small cell lung cancer in the elderly: the impact of age and comorbidities on treatment modalities and patient prognosis. J Geriatr Oncol. 2015;6:38–45. doi: 10.1016/j.jgo.2014.09.178. [DOI] [PubMed] [Google Scholar]
- 16.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The iaslc lung cancer staging project: proposals for the revision of the tnm stage groupings in the forthcoming (seventh) edition of the tnm classification of malignant tumours. J Thorac Oncol: Off Publ Int Assoc Study of Lung Cancer. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 17.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (suvmax) measured on fluorodeoxyglucose positron emission tomography (fdg-pet) is of prognostic value for survival in non-small cell lung cancer (nsclc): a systematic review and meta-analysis (ma) by the European lung cancer working party for the iaslc lung cancer staging project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 18.Chen HH, Chiu N-T, Su W-C, Guo H-R, Lee B-F. Prognostic value of whole-body total lesion glycolysis at pretreatment fdg pet/ct in non–small cell lung cancer. Radiology. 2012;264:559–566. doi: 10.1148/radiol.12111148. [DOI] [PubMed] [Google Scholar]
- 19.Wahl RL, Jacene H, Kasamon Y, Lodge MA. From recist to percist: evolving considerations for pet response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu KP, Murphy JD, La TH, Krakow TE, Iagaru A, Graves EE, et al. Prognostic value of metabolic tumor volume and velocity in predicting head-and-neck cancer outcomes. Int J Radiat Oncol Biol Phys. 2012;83:1521–1527. doi: 10.1016/j.ijrobp.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takahashi Y, Oriuchi N, Otake H, Endo K, Murase K. Variability of lesion detectability and standardized uptake value according to the acquisition procedure and reconstruction among five pet scanners. Ann Nucl Med. 2008;22:543–548. doi: 10.1007/s12149-008-0152-1. [DOI] [PubMed] [Google Scholar]
- 22.Laffon E, Adhoute X, de Clermont H, Marthan R. Is liver suv stable over time in (1)(8)f-fdg pet imaging? J Nucl Med Technol. 2011;39:258–263. doi: 10.2967/jnmt.111.090027. [DOI] [PubMed] [Google Scholar]
- 23.Park J, Chang KJ, Seo YS, Byun BH, Choi JH, Moon H, et al. Tumor suvmax normalized to liver uptake on (18)f-fdg pet/ct predicts the pathologic complete response after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Nucl Med Mol Imaging. 2014;48:295–302. doi: 10.1007/s13139-014-0289-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song MJ, Bae SH, Lee SW, Song do S, Kim HY, Yoo Ie R, et al. 18f-fluorodeoxyglucose pet/ct predicts tumour progression after transarterial chemoembolization in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging. 2013;40:865–873. doi: 10.1007/s00259-013-2366-2. [DOI] [PubMed] [Google Scholar]
- 25.Shiono S, Abiko M, Okazaki T, Chiba M, Yabuki H, Sato T. Positron emission tomography for predicting recurrence in stage i lung adenocarcinoma: Standardized uptake value corrected by mean liver standardized uptake value. Eur J Cardiothorac Surg: Off J Eur Assoc Cardiothorac Surg. 2011;40:1165–1169. doi: 10.1016/j.ejcts.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 26.Besson FL, de Boysson H, Parienti JJ, Bouvard G, Bienvenu B, Agostini D. Towards an optimal semiquantitative approach in giant cell arteritis: An (18)f-fdg pet/ct case–control study. Eur J Nucl Med Mol Imaging. 2014;41:155–166. doi: 10.1007/s00259-013-2545-1. [DOI] [PubMed] [Google Scholar]