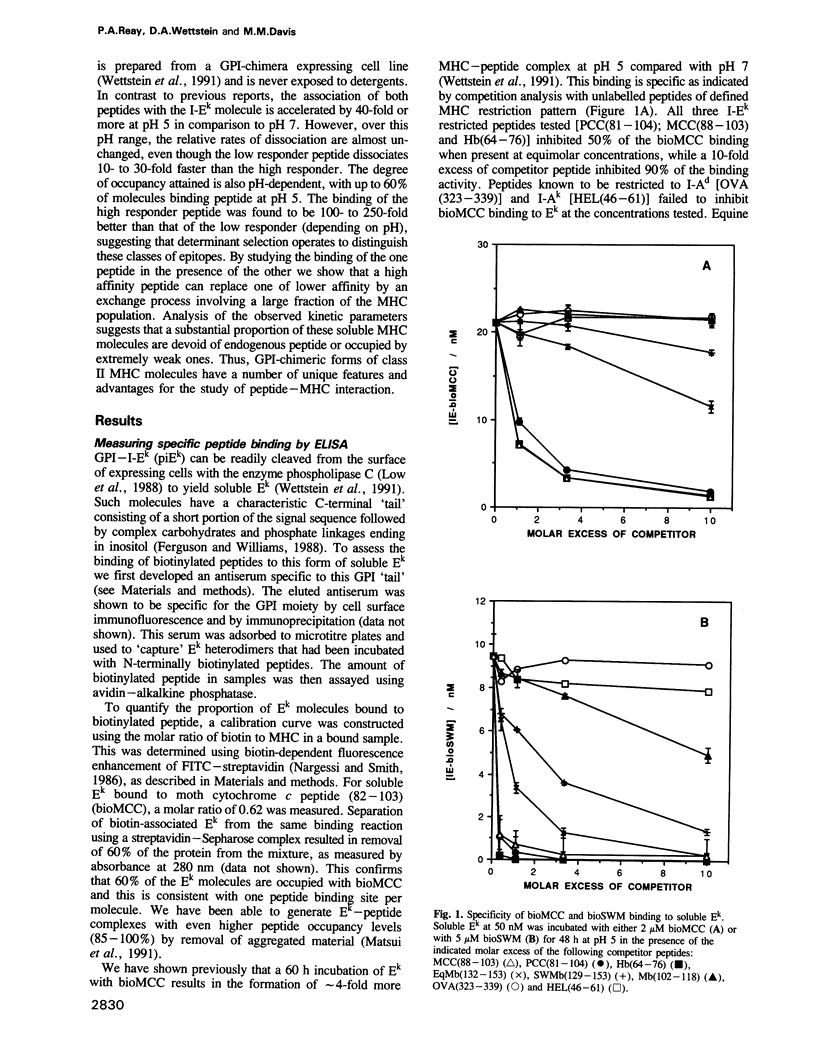
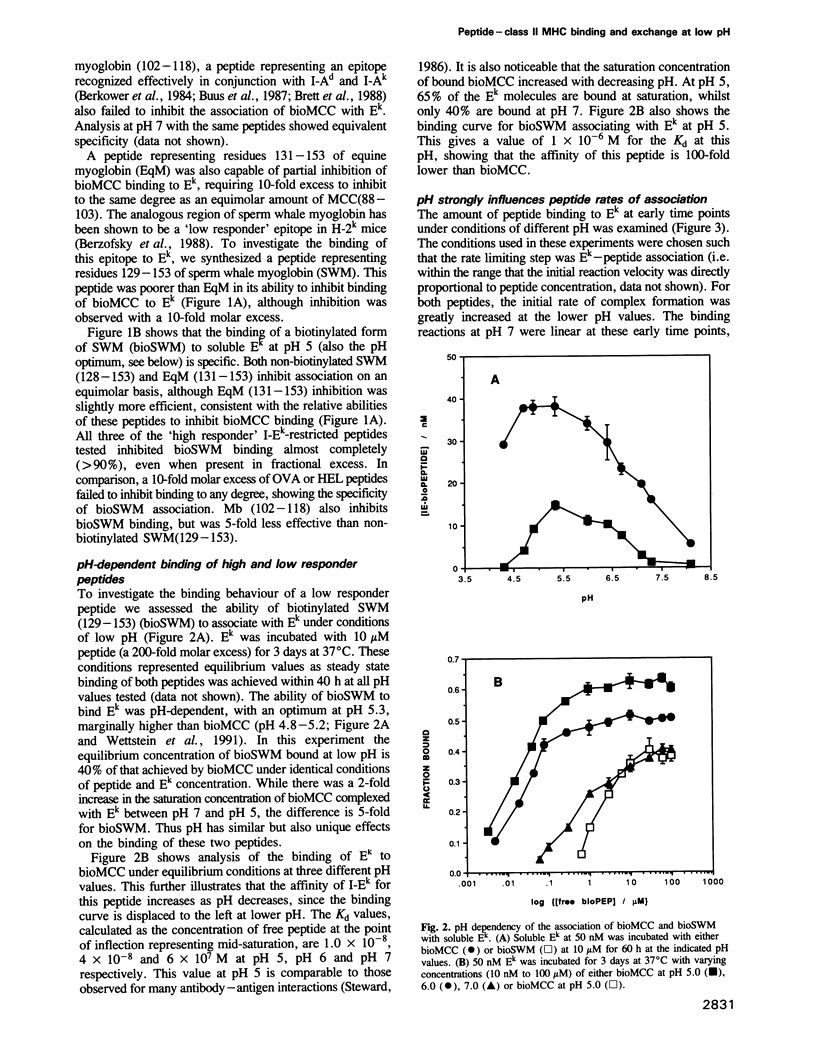
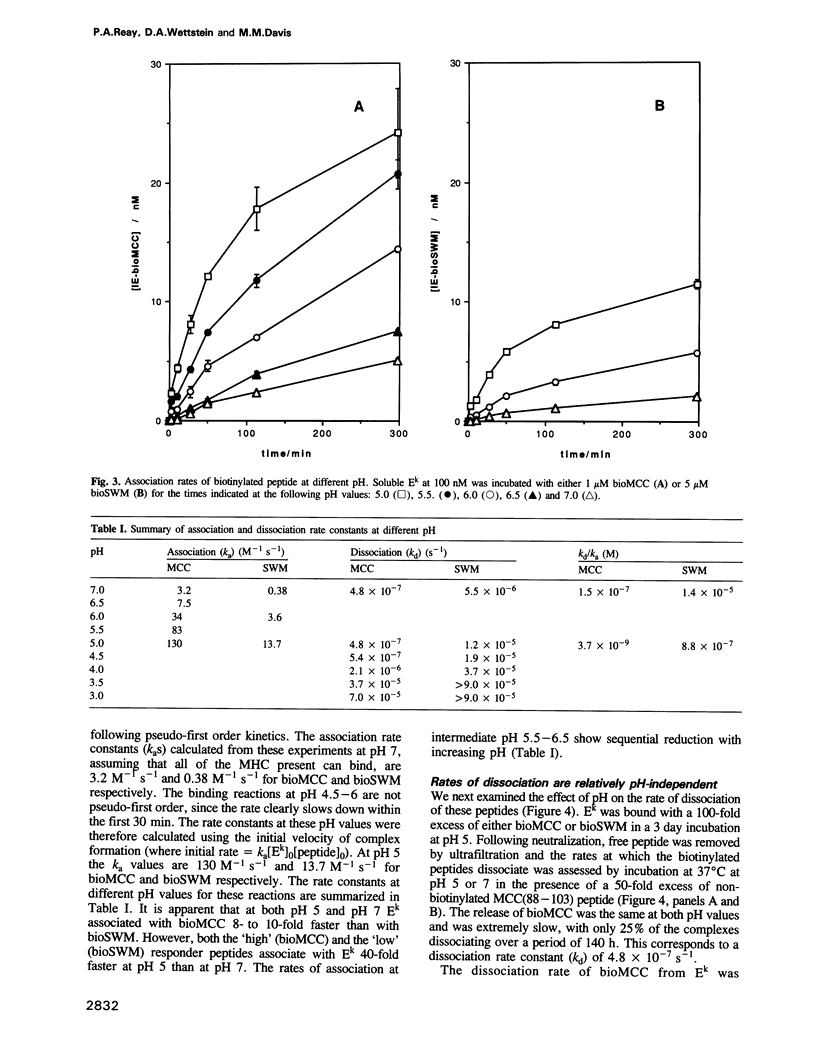
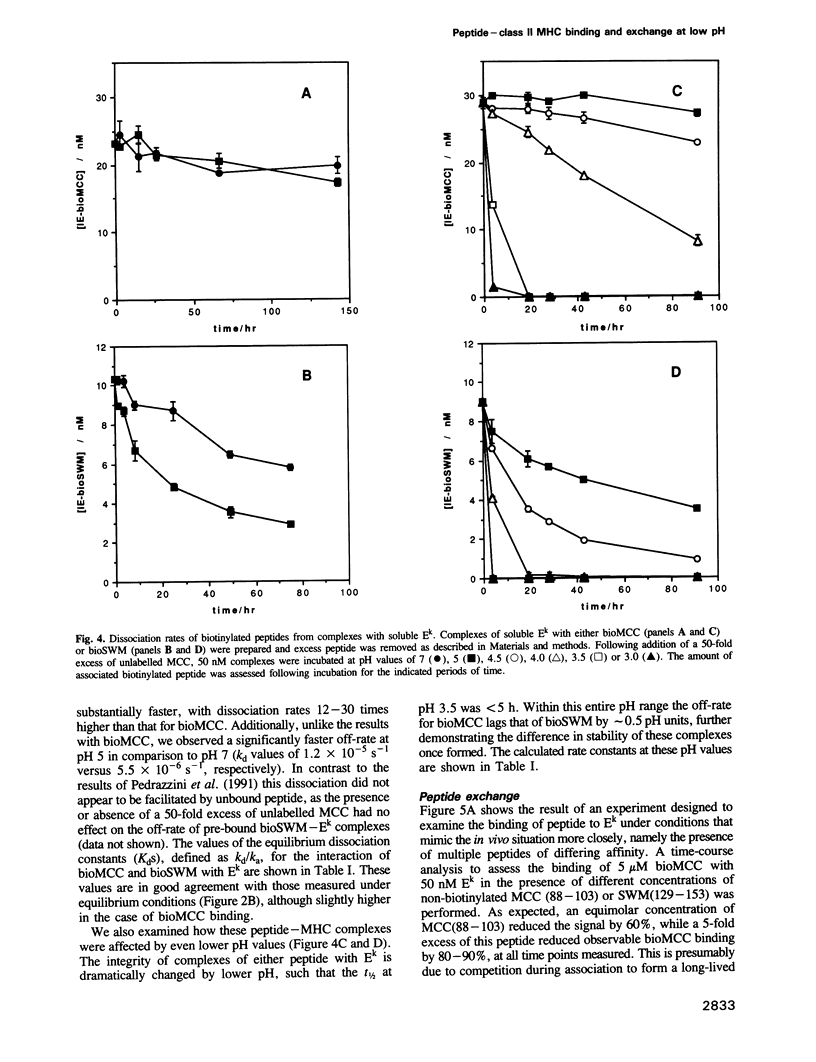
Abstract
We have compared the binding kinetics of two antigenic peptides to a soluble class II MHC molecule. One of the peptides provokes a strong T cell response and the other a much weaker one. Both show greatly increased (approximately 40-fold) association rates at pH 5 in comparison to neutral pH, consistent with the low pH environment of late endosomes being most conducive to class II MHC--peptide binding. Interestingly, the weak peptide has a much faster off-rate that is significantly increased at pH 5 and it can be entirely replaced in an exchange reaction by the stronger one. This suggests that one characteristic of immunodominant peptides is that of nearly irreversible binding, such that they will be strongly selected for in the course of class II MHC transit and recycling through endosomal compartments. Modelling the parameters of this peptide exchange also suggests that a large fraction of the GPI-chimeric MHC molecules used in this study are 'empty' with respect to endogenous peptides, or else occupied with extremely weak ones, consistent with their inability to load processed peptides intracellularly.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adorini L., Appella E., Doria G., Cardinaux F., Nagy Z. A. Competition for antigen presentation in living cells involves exchange of peptides bound by class II MHC molecules. Nature. 1989 Dec 14;342(6251):800–803. doi: 10.1038/342800a0. [DOI] [PubMed] [Google Scholar]
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Benacerraf B. A hypothesis to relate the specificity of T lymphocytes and the activity of I region-specific Ir genes in macrophages and B lymphocytes. J Immunol. 1978 Jun;120(6):1809–1812. [PubMed] [Google Scholar]
- Berkower I., Matis L. A., Buckenmeyer G. K., Gurd F. R., Longo D. L., Berzofsky J. A. Identification of distinct predominant epitopes recognized by myoglobin-specific T cells under the control of different Ir genes and characterization of representative T cell clones. J Immunol. 1984 Mar;132(3):1370–1378. [PubMed] [Google Scholar]
- Berzofsky J. A., Brett S. J., Streicher H. Z., Takahashi H. Antigen processing for presentation to T lymphocytes: function, mechanisms, and implications for the T-cell repertoire. Immunol Rev. 1988 Dec;106:5–31. doi: 10.1111/j.1600-065x.1988.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Bevan M. J. Antigen recognition. Class discrimination in the world of immunology. Nature. 1987 Jan 15;325(6101):192–194. doi: 10.1038/325192b0. [DOI] [PubMed] [Google Scholar]
- Brett S. J., Cease K. B., Berzofsky J. A. Influences of antigen processing on the expression of the T cell repertoire. Evidence for MHC-specific hindering structures on the products of processing. J Exp Med. 1988 Jul 1;168(1):357–373. doi: 10.1084/jem.168.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Colon S., Smith C., Freed J. H., Miles C., Grey H. M. Interaction between a "processed" ovalbumin peptide and Ia molecules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Jenis D. M., Grey H. M. Isolation and characterization of antigen-Ia complexes involved in T cell recognition. Cell. 1986 Dec 26;47(6):1071–1077. doi: 10.1016/0092-8674(86)90822-6. [DOI] [PubMed] [Google Scholar]
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Ceppellini R., Frumento G., Ferrara G. B., Tosi R., Chersi A., Pernis B. Binding of labelled influenza matrix peptide to HLA DR in living B lymphoid cells. Nature. 1989 Jun 1;339(6223):392–394. doi: 10.1038/339392a0. [DOI] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presentation by B cells and its significance in T-B interactions. Adv Immunol. 1986;39:51–94. doi: 10.1016/s0065-2776(08)60348-x. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Reid P. A., Lanzavecchia A., Watts C. Processed antigen binds to newly synthesized MHC class II molecules in antigen-specific B lymphocytes. Cell. 1991 Oct 4;67(1):105–116. doi: 10.1016/0092-8674(91)90575-j. [DOI] [PubMed] [Google Scholar]
- Davis M. M. T cell receptor gene diversity and selection. Annu Rev Biochem. 1990;59:475–496. doi: 10.1146/annurev.bi.59.070190.002355. [DOI] [PubMed] [Google Scholar]
- Demotz S., Grey H. M., Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990 Aug 31;249(4972):1028–1030. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- Falo L. D., Jr, Haber S. I., Herrmann S., Benacerraf B., Rock K. L. Characterization of antigen association with accessory cells: specific removal of processed antigens from the cell surface by phospholipases. Proc Natl Acad Sci U S A. 1987 Jan;84(2):522–526. doi: 10.1073/pnas.84.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson M. A., Williams A. F. Cell-surface anchoring of proteins via glycosyl-phosphatidylinositol structures. Annu Rev Biochem. 1988;57:285–320. doi: 10.1146/annurev.bi.57.070188.001441. [DOI] [PubMed] [Google Scholar]
- Guagliardi L. E., Koppelman B., Blum J. S., Marks M. S., Cresswell P., Brodsky F. M. Co-localization of molecules involved in antigen processing and presentation in an early endocytic compartment. Nature. 1990 Jan 11;343(6254):133–139. doi: 10.1038/343133a0. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Allen P. M., Unanue E. R. Effects of pH and polysaccharides on peptide binding to class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2740–2744. doi: 10.1073/pnas.88.7.2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Unanue E. R. Turnover of Ia-peptide complexes is facilitated in viable antigen-presenting cells: biosynthetic turnover of Ia vs. peptide exchange. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4230–4234. doi: 10.1073/pnas.86.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990 Aug 9;346(6284):574–576. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Unanue E. R., Slot J. W., Schwartz A. L., Geuze H. J. Functional and ultrastructural evidence for intracellular formation of major histocompatibility complex class II-peptide complexes during antigen processing. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5553–5557. doi: 10.1073/pnas.87.14.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Enhanced binding of peptide antigen to purified class II major histocompatibility glycoproteins at acidic pH. J Exp Med. 1991 Nov 1;174(5):1111–1120. doi: 10.1084/jem.174.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen P. E. Regulation of antigen presentation by acidic pH. J Exp Med. 1990 May 1;171(5):1779–1784. doi: 10.1084/jem.171.5.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M., Cease K. B., Buckenmeyer G. K., Berzofsky J. A. Limiting dilution comparison of the repertoires of high and low responder MHC-restricted T cells. J Exp Med. 1988 Mar 1;167(3):1100–1113. doi: 10.1084/jem.167.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Watts T. H. On the dissociation and reassociation of MHC class II-foreign peptide complexes. Evidence that brief transit through an acidic compartment is not sufficient for binding site regeneration. J Immunol. 1990 Mar 1;144(5):1829–1834. [PubMed] [Google Scholar]
- Lin A. Y., Devaux B., Green A., Sagerström C., Elliott J. F., Davis M. M. Expression of T cell antigen receptor heterodimers in a lipid-linked form. Science. 1990 Aug 10;249(4969):677–679. doi: 10.1126/science.1696397. [DOI] [PubMed] [Google Scholar]
- Ljunggren H. G., Stam N. J., Ohlén C., Neefjes J. J., Höglund P., Heemels M. T., Bastin J., Schumacher T. N., Townsend A., Kärre K. Empty MHC class I molecules come out in the cold. Nature. 1990 Aug 2;346(6283):476–480. doi: 10.1038/346476a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Stiernberg J., Waneck G. L., Flavell R. A., Kincade P. W. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988 Oct 4;113(1):101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Matsui K., Boniface J. J., Reay P. A., Schild H., Fazekas de St Groth B., Davis M. M. Low affinity interaction of peptide-MHC complexes with T cell receptors. Science. 1991 Dec 20;254(5039):1788–1791. doi: 10.1126/science.1763329. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R., Yamashiro D. J. Endosome acidification and the pathways of receptor-mediated endocytosis. Adv Exp Med Biol. 1987;225:189–198. doi: 10.1007/978-1-4684-5442-0_16. [DOI] [PubMed] [Google Scholar]
- Nargessi R. D., Smith D. S. Fluorometric assays for avidin and biotin. Methods Enzymol. 1986;122:67–72. doi: 10.1016/0076-6879(86)22150-3. [DOI] [PubMed] [Google Scholar]
- Neefjes J. J., Stollorz V., Peters P. J., Geuze H. J., Ploegh H. L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990 Apr 6;61(1):171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Pedrazzini T., Sette A., Albertson M., Grey H. M. Free ligand-induced dissociation of MHC-antigen complexes. J Immunol. 1991 May 15;146(10):3496–3501. [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Powis S. J., Townsend A. R., Deverson E. V., Bastin J., Butcher G. W., Howard J. C. Restoration of antigen presentation to the mutant cell line RMA-S by an MHC-linked transporter. Nature. 1991 Dec 19;354(6354):528–531. doi: 10.1038/354528a0. [DOI] [PubMed] [Google Scholar]
- Reid P. A., Watts C. Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature. 1990 Aug 16;346(6285):655–657. doi: 10.1038/346655a0. [DOI] [PubMed] [Google Scholar]
- Roche P. A., Cresswell P. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J Immunol. 1990 Mar 1;144(5):1849–1856. [PubMed] [Google Scholar]
- Roof R. W., Luescher I. F., Unanue E. R. Phospholipids enhance the binding of peptides to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1735–1739. doi: 10.1073/pnas.87.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosnek E., Demotz S., Corradin G., Lanzavecchia A. Kinetics of MHC-antigen complex formation on antigen-presenting cells. J Immunol. 1988 Jun 15;140(12):4079–4082. [PubMed] [Google Scholar]
- Rosenthal A. S. Determinant selection and macrophage function in genetic control of the immune response. Immunol Rev. 1978;40:136–152. doi: 10.1111/j.1600-065x.1978.tb00404.x. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B., Gefter M. L. Interactions between immunogenic peptides and MHC proteins. Annu Rev Immunol. 1991;9:527–565. doi: 10.1146/annurev.iy.09.040191.002523. [DOI] [PubMed] [Google Scholar]
- Rudensky AYu, Preston-Hurlburt P., Hong S. C., Barlow A., Janeway C. A., Jr Sequence analysis of peptides bound to MHC class II molecules. Nature. 1991 Oct 17;353(6345):622–627. doi: 10.1038/353622a0. [DOI] [PubMed] [Google Scholar]
- Schumacher T. N., Heemels M. T., Neefjes J. J., Kast W. M., Melief C. J., Ploegh H. L. Direct binding of peptide to empty MHC class I molecules on intact cells and in vitro. Cell. 1990 Aug 10;62(3):563–567. doi: 10.1016/0092-8674(90)90020-f. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Sette A., Southwood S., O'Sullivan D., Gaeta F. C., Sidney J., Grey H. M. Effect of pH on MHC class II-peptide interactions. J Immunol. 1992 Feb 1;148(3):844–851. [PubMed] [Google Scholar]
- Tampé R., McConnell H. M. Kinetics of antigenic peptide binding to the class II major histocompatibility molecule I-Ad. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4661–4665. doi: 10.1073/pnas.88.11.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Townsend A., Ohlén C., Bastin J., Ljunggren H. G., Foster L., Kärre K. Association of class I major histocompatibility heavy and light chains induced by viral peptides. Nature. 1989 Aug 10;340(6233):443–448. doi: 10.1038/340443a0. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Watts T. H. T cell activation by preformed, long-lived Ia-peptide complexes. Quantitative aspects. J Immunol. 1988 Dec 1;141(11):3708–3714. [PubMed] [Google Scholar]
- Wettstein D. A., Boniface J. J., Reay P. A., Schild H., Davis M. M. Expression of a class II major histocompatibility complex (MHC) heterodimer in a lipid-linked form with enhanced peptide/soluble MHC complex formation at low pH. J Exp Med. 1991 Jul 1;174(1):219–228. doi: 10.1084/jem.174.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt S. N., McConnell H. M. A first-order reaction controls the binding of antigenic peptides to major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8164–8168. doi: 10.1073/pnas.88.18.8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R. The binary logic of antigen processing and presentation to T cells. Cell. 1990 Jul 27;62(2):203–206. doi: 10.1016/0092-8674(90)90356-j. [DOI] [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]


