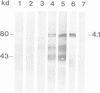
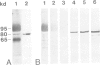
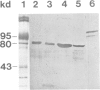
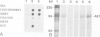Abstract
Linkages of the cytoskeleton to integral membrane proteins of the plasma membrane have been shown to be important for diverse cellular functions. The erythrocyte membrane provides the best studied example of how the spectrin-actin based membrane cytoskeleton is linked via two proteins, ankyrin and protein 4.1, to the anion exchanger (anion exchanger 1, AE1). Although these and other types of cytoskeleton-membrane connections have been well documented by in vitro binding studies it has not been possible to establish any of such interactions by defining the binding interface at the amino acid level. In the present study we have performed binding studies between protein 4.1 and AE1 using peptides and corresponding idiotypic and anti-idiotypic antibodies to show that arginine-rich clusters of the cytoplasmic domain of AE1 (IRRRY/LRRRY) serve as a major binding site for a motif with opposite charge and identical hydrophobicity present on the membrane-binding domain of protein 4.1 (LEEDY). Both motifs appear to be highly conserved during evolution and may also be involved in other types of cytoskeleton-membrane association, i.e. in binding of protein 4.1 to the glycophorins.
Full text
PDF


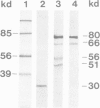
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Lovrien R. E. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984 Feb 16;307(5952):655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. Proteins involved in membrane--cytoskeleton association in human erythrocytes: spectrin, ankyrin, and band 3. Methods Enzymol. 1983;96:313–324. doi: 10.1016/s0076-6879(83)96029-9. [DOI] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. Association between ankyrin and the cytoplasmic domain of band 3 isolated from the human erythrocyte membrane. J Biol Chem. 1980 Jul 10;255(13):6424–6432. [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Blanchard D., Dahr W., Hummel M., Latron F., Beyreuther K., Cartron J. P. Glycophorins B and C from human erythrocyte membranes. Purification and sequence analysis. J Biol Chem. 1987 Apr 25;262(12):5808–5811. [PubMed] [Google Scholar]
- Byers T. J., Branton D. Visualization of the protein associations in the erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6153–6157. doi: 10.1073/pnas.82.18.6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland W. L., Wassermann N. H., Sarangarajan R., Penn A. S., Erlanger B. F. Monoclonal antibodies to the acetylcholine receptor by a normally functioning auto-anti-idiotypic mechanism. Nature. 1983 Sep 1;305(5929):56–57. doi: 10.1038/305056a0. [DOI] [PubMed] [Google Scholar]
- Colin Y., Rahuel C., London J., Roméo P. H., d'Auriol L., Galibert F., Cartron J. P. Isolation of cDNA clones and complete amino acid sequence of human erythrocyte glycophorin C. J Biol Chem. 1986 Jan 5;261(1):229–233. [PubMed] [Google Scholar]
- Conboy J. G., Chan J. Y., Chasis J. A., Kan Y. W., Mohandas N. Tissue- and development-specific alternative RNA splicing regulates expression of multiple isoforms of erythroid membrane protein 4.1. J Biol Chem. 1991 May 5;266(13):8273–8280. [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter A., Harris R. Simplified preparation of rabbit Fab fragments. J Immunol Methods. 1983 Apr 29;59(2):199–203. doi: 10.1016/0022-1759(83)90031-5. [DOI] [PubMed] [Google Scholar]
- Djabali K., Portier M. M., Gros F., Blobel G., Georgatos S. D. Network antibodies identify nuclear lamin B as a physiological attachment site for peripherin intermediate filaments. Cell. 1991 Jan 11;64(1):109–121. doi: 10.1016/0092-8674(91)90213-i. [DOI] [PubMed] [Google Scholar]
- Friedrichs B., Koob R., Kraemer D., Drenckhahn D. Demonstration of immunoreactive forms of erythrocyte protein 4.2 in nonerythroid cells and tissues. Eur J Cell Biol. 1989 Feb;48(1):121–127. [PubMed] [Google Scholar]
- Giebelhaus D. H., Eib D. W., Moon R. T. Antisense RNA inhibits expression of membrane skeleton protein 4.1 during embryonic development of Xenopus. Cell. 1988 May 20;53(4):601–615. doi: 10.1016/0092-8674(88)90576-4. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Appearance of new variants of membrane skeletal protein 4.1 during terminal differentiation of avian erythroid and lenticular cells. Nature. 1985 Jan 17;313(5999):238–241. doi: 10.1038/313238a0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Membrane skeletal protein 4.1 of avian erythrocytes is composed of multiple variants that exhibit tissue-specific expression. Cell. 1984 Jun;37(2):595–607. doi: 10.1016/0092-8674(84)90390-8. [DOI] [PubMed] [Google Scholar]
- Guesdon J. L., Ternynck T., Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979 Aug;27(8):1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Kopito R. R. Molecular biology of the anion exchanger gene family. Int Rev Cytol. 1990;123:177–199. doi: 10.1016/s0074-7696(08)60674-9. [DOI] [PubMed] [Google Scholar]
- Kouklis P. D., Papamarcaki T., Merdes A., Georgatos S. D. A potential role for the COOH-terminal domain in the lateral packing of type III intermediate filaments. J Cell Biol. 1991 Aug;114(4):773–786. doi: 10.1083/jcb.114.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Low P. S. Structure and function of the cytoplasmic domain of band 3: center of erythrocyte membrane-peripheral protein interactions. Biochim Biophys Acta. 1986 Sep 22;864(2):145–167. doi: 10.1016/0304-4157(86)90009-2. [DOI] [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Characterization and partial sequence of di-iodosulphophenyl isothiocyanate-binding peptide from human erythrocyte anion-transport protein. Biochem J. 1982 Sep 1;205(3):465–475. doi: 10.1042/bj2050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack G. R., Anderson R. A., Leto T. L., Marchesi V. T. Interactions between protein 4.1 and band 3. An alternative binding site for an element of the membrane skeleton. J Biol Chem. 1985 Mar 25;260(6):3676–3683. [PubMed] [Google Scholar]
- Rivas C. I., Vera J. C., Maccioni R. B. Anti-idiotypic antibodies that react with microtubule-associated proteins are present in the sera of rabbits immunized with synthetic peptides from tubulin's regulatory domain. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6092–6096. doi: 10.1073/pnas.85.16.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner M. J., Martin P. G., High S. The complete amino acid sequence of the human erythrocyte membrane anion-transport protein deduced from the cDNA sequence. Biochem J. 1988 Dec 15;256(3):703–712. doi: 10.1042/bj2560703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita M., Marchesi V. T. Amino-acid sequence and oligosaccharide attachment sites of human erythrocyte glycophorin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2964–2968. doi: 10.1073/pnas.72.8.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]