Abstract
Cancer chemotherapeutics often fail to reach all diseased cells. To help solve this problem, researchers are investigating novel drug delivery systems. Liposomes are an attractive option due to their low toxicity, high biocompatibility, and potential to carry a large amount of a drug to the tumor site, all while avoiding being eliminated from the body. This study evaluates the penetration of doxorubicin-encased liposomes into three-dimensional cell cultures, or spheroids. Liposomes composed of lipids containing head groups of phosphatidylcholine (PC), phosphatidylethanolamine (PE), and cholesterol were created by extrusion. Doxorubicin is encapsulated within the hydrophilic core of the liposome. The drug is actively released in the spheroid as the lipids bind to cellular lipid bilayers. Spheroids were dosed with liposomal doxorubicin, free doxorubicin, or media control to assess drug distribution over the course of seventy-two hours. Drug penetration was visualized Matrix-Assisted Laser Desorption/Ionization-Imaging Mass Spectrometry (MALDI-IMS) with confirmation by steady state fluorescence microscopy, creating a comprehensive picture of drug distribution. This technique is able to identify both free and liposomal doxorubicin throughout the spheroid after just twelve hours of treatment. Additionally, MALDI-IMS is able to detect three metabolites of doxorubicin, indicating that cells actively metabolize the drug during treatment. Steady state fluorescence microscopy cannot distinguish the drug from its metabolites as they have the same emission spectra. This report summarizes the first study to use MALDI-IMS to analyze drug penetration of a liposomal drug carrier as well as its metabolites.
Keywords: Liposome, Drug Delivery, MALDI-IMS, Fluorescence Microscopy, 3D Cell Culture
TOC image
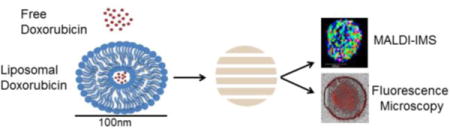
Introduction
One of the common downfalls of current chemotherapy regimens is the failure of active drug to reach all diseased cells. This failure can lead to metastasis, drug resistance, and ultimately death. Findings suggest that drug penetration into the necrotic core of tumors could be lacking and could be responsible for this cancer progression.1–2 To address this issue, researchers in recent years have started to investigate novel drug delivery systems. Liposomes, first discovered in 1965 by Alec Bangham, have become a popular option to enhance drug delivery to tumors.3 Liposomes possess several advantages over other drug delivery vehicles. Liposomes have been shown to have low toxicity and high biocompatibility due to the fact they are composed of naturally occurring phospholipids.4 Additionally, liposomes have the ability for self-assembly and, depending on their size, can deliver a high concentration of drug directly to the tumor while avoiding elimination by the mononuclear phagocyte system (MPS).5–7 Therefore, liposomes have the potential to facilitate greater drug penetration into tissue samples, making treatments more efficient.4–6
Several liposomal-based drugs have reached the market in recent years.7 Doxil and Caelyx are liposomal configurations of the chemotherapeutic doxorubicin and are now used as second-line chemotherapy drugs.8–12 To date there have been eighteen liposomal drugs that are on the market or in Phase III clinical trials.8 However, little research has been performed on overall tissue penetration and efficacy by chemotherapeutic drugs entrapped in liposomes. To date, only two reports have been published that implement Matrix-Assisted Laser Desorption/Ionization-Imaging Mass Spectrometry (MALDI-IMS) to examine the penetration of liposomes.15–16 Fülöp et. al utilized MALDI-IMS to examine the distribution of two liposomal markers and indocyanine green within mice brains looking for the overall integrity of the liposome after administration. Walrant et al. evaluated the ability of cell-penetrating peptides to cross the membrane of liposomes to evaluate direct translocation into cells. This present study is the first to look at a popular chemotherapy drug, doxorubicin, entrapped within a liposome and evaluate drug penetration using MALDI-IMS with confirmation by steady state fluorescence microscopy.
In preclinical trials, it is common for drugs to be tested for efficacy on two-dimensional cell cultures, but this data can be misleading as tumors in vivo grow three-dimensionally. It has been shown that cells that are grown in three-dimensions display different cellular responses than cells grown in two-dimensions. For this reason, it would be beneficial to test drug efficacy using three-dimensional cell cultures.13–14 Three-dimensional cell cultures or spheroids display pathophysiological gradients that are more complex than conventional two-dimensional cell cultures and are higher throughput than animal models.17–18 These cultures have naturally occurring layers of cellular populations that are comparable to in vivo tumors. The physiological layers form in response to decreasing nutrient and oxygen concentrations from the exterior of the spheroid to the core.19–20 These layers consist of an outer region of proliferating cells, a middle layer of quiescent cells, and a necrotic core. Using spheroids, the penetration of a drug into a more complex, tumor-like sample can be investigated.17–23
Eetezadi et. al first described the effects of a doxorubicin delivery system on tissue penetration in a three-dimensional ovarian cell culture system.23 In this study, the penetration of doxorubicin was determined by measuring its fluorescence signal in optical images. Doxorubicin is one of the leading chemotherapy drugs on the market and is commonly used to treat breast, bladder, and several types of blood cancers.24 Doxorubicin is an anthracycline antibiotic that displays antineoplastic activity by acting as an intercalating agent with base pairs in the DNA helix to prevent replication in cells.24–25 The drug is commonly used as a model system drug due to its inherent fluorescence. However, there is a recognized limitation in evaluating the drug’s penetration through tissue masses by fluorescence. The excitation and emission signal have to travel through many layers of cells before visualization.23–24 As a result, signal attenuation and amplification makes it difficult to obtain reliable fluorescence results from a spheroid or tissue. Additionally, doxorubicin’s fluorescence can change as a function of its environment. When the molecule interacts with DNA within cells, the fluorescence signal is significantly quenched, however when doxorubicin interacts with histones the signal is amplified.24–26 To successfully minimize these confounding effects of doxorubicin, previous studies assessed fluorescence at the earliest time point possible.
This is the first study of its kind to visualize liposomal drug distribution into a three-dimensional cell culture system using MALDI-IMS over a period of seventy-two hours. In this proof of concept study, doxorubicin is used due to its ability to be detected using MALDI-IMS with confirmation of drug delivery by steady state fluorescence microscopy. Figure 1 depicts the experimental workflow. Liposomes are created by extrusion, a process by which doxorubicin is entrapped in the core of the liposome. Spheroids are dosed with liposomal doxorubicin, free doxorubicin, or media (control). Spheroids are collected at time points over the course seventy-two hours and embedded in gelatin for cryosectioning. During cryosectioning, alternating 16 μm slices are collected for either MALDI-IMS or fluorescence microscopy analysis. Our approach using MALDI-IMS allows for longer time points since the determination of drug penetration is not based on the fluorescence signal but rather on the doxorubicin analyte itself. In MALDI-IMS analysis, the laser is rastered across a spheroid section in a grid-like pattern to generate spectra of analytes found at each particular spot. In this study, doxorubicin (544.8 m/z) is mapped throughout the entire spheroid section. This method directly allows for the determination of doxorubicin penetration into the spheroids.
Figure 1.

Free doxorubicin or liposomal doxorubicin (90:5:5 POPC:POPE:cholesterol) are administered to spheroids for treatment for 2, 6, 12, 24, 48, or 72 hours. After treatment spheroids are washed in PBS before embedded in gelatin for cryosectioning. During cryosectioning, alternating 16 μm slices were thaw-mounted on slides for either MALDI-IMS or fluorescence microscopy analysis. At least 12 slices from 3 spheroids were collected for analysis.
One substantial advantage of using MALDI-IMS to monitor drug penetration is the ability to map intact doxorubicin and doxorubicin metabolites simultaneously. For many therapeutics, including doxorubicin, the drug and metabolites have the same absorption and emission spectra, making it impossible to distinguish them from each other by steady state fluorescence imaging. MALDI-IMS has much greater specificity, as the drug and metabolites have distinct molecular weights and can be distinguished from each other. As a result, MALDI-IMS, but not steady state fluorescence imaging, can determine if drugs are metabolized and map the spatial localization of the process. In this study, we leverage this substantial advantage to map the metabolism of both free and liposome-encased doxorubicin across a time course.
Methods
Chemicals and Materials
HPLC grade acetonitrile (ACN) and HPLC grade water were purchased from B & J Brand (Honeywell Burdick & Jackson, Muskegon, MI). Chloroform, Trifluoroacetic Acid (TFA), Tris and 2,5 dihydroxybenzoic acid (DHB) were all obtained from Fisher Scientific (Fair Lawn, NJ). Sodium Chloride (NaCl) was obtained from Amreoco Life Science (Solon, OH).
Cell Culture and Spheroid Formation
The colon carcinoma cell line HCT 116 was purchased from ATCC (Manassas, VA) and maintained in McCoy’s 5A cell culture media (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (Thermo Scientific, Gaithersburg, MD) and 1% L-glutamine (Invitrogen, San Diego, CA). Cells were grown in 5% CO2 at 37°C and passed every four days. Cell lines were used within three months after resuscitation of frozen aliquots thawed from liquid nitrogen. The cells were verified by Short Tandem Repeat (STR) sequencing in 2016.
Spheroids were prepared in an agarose-coated 96-well plate as previously described.27–29 Cells were seeded into each well at a density of 7000 cells/well, incubated at 37°C and 50% of cell culture media was changed every 48 hours after four days in culture. After 13 days in culture, spheroids reach their maximum growth and were dosed with liposomal doxorubicin, free doxorubicin, or control media.
Liposome Formation
Lipids, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine (POPE), were purchased from Avanti Polar Lipids (Alabaster, AL) and dissolved in chloroform. A final concentration of 1mM lipid in a 90: 5: 5 POPC: POPE: cholesterol, and 90: 5: 5 POPE: POPC: cholesterol ratio was used. Appropriate amounts of lipids were measured and chloroform was evaporated under the flow of nitrogen. Doxorubicin (EMB Millipore Corp., Billerica, MA) was dissolved in nanoPure water at a final concentration of 50 μM and added to the lipid film. Cholesterol (MP Biomedicals LLC, Solon, OH) was also added to the lipid film at a final concentration of 1 mM. Lipids, doxorubicin, and cholesterol were rehydrated in Tris Buffered Saline (TBS pH 7.8) and briefly vortexed until the solution became cloudy. Solution was extruded 21 times through a 0.1 μm pore polycarbonate membrane using a MiniExtruder system (Avanti Polar Lipids). Liposomes were dialyzed for 2 hours at 4°C in 50:50 TBS:DI water and then overnight in DI water to remove any excess lipid, doxorubicin, or cholesterol that did not form the liposome configuration. Dynamic Light Scattering (DLS) was utilized to ensure liposome size did not change through the dialysis process. Concentration of doxorubicin inside the liposome was determined using a SpectraMax M5 Plate Reader (Molecular Devices, Sunnyvale, CA). The absorbance of doxorubicin was measured at 480 nm. A standard curve of known concentrations of doxorubicin was used to determine amount of drug present within the liposome.
Drug Penetration
To visualize drug penetration, spheroids were treated with 30 μM free doxorubicin or liposomal doxorubicin for various lengths of time (2, 6, 12, 24, 48, and 72 h). For control spheroids, the same volume of media without doxorubicin was added. Doxorubicin was dissolved in nanoPure water and added to McCoy’s 5A media at a final concentration of 30 μM. After specific incubation times, the media was aspirated and the spheroids were washed with 1X phosphate buffered saline (PBS) and embedded in gelatin. The spheroids were then harvested and sectioned into 16 μm-thick slices by using the gelatin-assisted sectioning method previously described.28 Sections were thaw-mounted on ITO coated slides for MALDI-IMS analysis and glass microscopy slides for fluorescence microscopy analysis. A TM sprayer nebulizer (HTX Technologies, Carrboro, NC) was used to apply the matrix for MALDI-IMS analysis. The matrix, DHB was dissolved in 50: 50 acetonitrile: water with 0.1% trifluoroacetic acid (TFA) (EMB, Billerica, MA) to yield a final concentration of 10 mg/mL. Matrix was applied at 75°C for 8 passes over the sample. The flow rate of matrix was 0.1 mL/min at a velocity of 1000 mm/min, track spacing of 2 mm, pressure 10 psi, gas flow rate of 3 L/min, nozzle height 40 mm, and a drying time of 20 seconds between each pass. After matrix was applied, the sample was dried in a desiccator before MALDI-IMS analysis.
MALDI-IMS Analysis
An UltrafleXtreme (Bruker Daltonics, Billerica, MA) was operated in positive ion mode with the reflector on and set to acquire a mass range of 300-800 m/z. The laser raster was set to 35 μm along both the x and y axes. Each MALDI mass spectrum for each pixel is a result of 750 consecutive laser shots. External calibration was performed using a standard peptide mixture on one spheroid. The images were processed using FlexAnalysis 3.0 and FlexImaging 4.1(Bruker Daltonics, Billerica, MA). All spectra were normalized against total ion count, defined as the sum of all intensities in the mass range analyzed to reduce influences by matrix hot spots. All samples were analyzed in biological triplicate and 6 technical replicates.
Steady State Fluorescence Microscopy Analysis
Spheroid slices (16 μm thick) were analyzed using Nikon TE-2000U Epifluorescence Microscope. Brightfield images were taken at an exposure of 100 ms. Fluorescence images were captured at 500 ms to capture doxorubicin fluorescence at 480nm. Doxorubicin fluorescence was determined by subtracting the natural fluorescence detected in control spheroids from the fluorescence signal obtained in treated spheroids. Brightfield and fluorescence images were overlayed and analyzed in ImageJ.
Results and Discussion
Free Doxorubicin Penetration Into Spheroids
Spheroids were treated with free doxorubicin for various amounts of time before being harvested and embedded in gelatin. Six slices from three different spheroids were analyzed with MALDI-IMS. Figure 2 shows free doxorubicin penetration into the spheroid over a period of seventy-two hours in comparison to control (untreated) spheroids. Steady state fluorescence microscopy was used an orthogonal method in order to verify drug delivery due to doxorubicin’s inherent fluorescence. In MALDI-IMS analysis, analytes are ionized when a laser interrogates the sample of interest to generate a spatial map of analyte distributions. Using MALDI-IMS, we detect doxorubicin in the outer proliferative cells of the spheroid as early as 2 hours with full penetration into the core of the spheroid visualized after 12 hours of doxorubicin treatment (Figure 2). The doxorubicin analyte (544.8 m/z) is detected in high intensity in all time points after 12 hours of treatment throughout the entire spheroid. Complementary results to the MALDI-IMS analysis were obtained using steady state fluorescence microscopy (Supplementary Information). Control, untreated spheroids were also analyzed for each time point and no signal corresponding to doxorubicin was detected.
Figure 2.

Doxorubicin (30 μM concentration) penetration visualized by MALDI-IMS over the course of 72 hours. (A) Control spheroids for each time point showed no signal for doxorubicin. (B) Drug is seen in the outer cells of the spheroid as early as 2 hour with full penetration into the core by 12 hours of treatment.
Liposome Formulation and Doxorubicin Concentration Determination
Liposomes (90:5:5 POPC: POPE: cholesterol and 90:5:5 POPE: POPC: cholesterol) were generated through the process of extrusion. POPC and POPE were chosen for this study due to phosphatidylcholine and phosphatidylethanolamine being a common and abundant component in naturally occurring lipid bilayers. Dialysis tubing with a molecular weight cut off of 1 kDa allowed the free doxorubicin, lipid, and cholesterol that was not incorporated into a liposome to diffuse freely leaving the purified product behind in the tubing after an overnight incubation. Dynamic light scattering (DLS) was utilized to determine the integrity and size distribution of the liposomes before and after the dialysis purification to ensure the purification process was not altering the liposome. Supporting Figure 1 shows that dialysis purification of the liposomes does not affect the overall size and integrity and is a reliable method to remove excess doxorubicin, cholesterol, and lipid. A slight shift was seen in the size distribution after dialysis but the shift is not statistically significant.
After DLS confirmed the size distribution and the overall integrity of the liposomes, a standard curve looking at the absorbance of doxorubicin at 480 nm was created in order to determine the concentration of doxorubicin entrapped within the liposomes with a plate reader assay(Figure S-2). A wide range of concentrations of doxorubicin were used to determine the limit of detection (LOD). An LOD of 15 μM was established (Figure S-2). The liposomal doxorubicin configuration entrapped approximately 30 μM of doxorubicin leading to a 60% entrapment yield. Spheroids were treated with liposomal doxorubicin, free doxorubicin, or media control for a pre-determined length of time to assess the penetration of doxorubicin.
Liposomal Doxorubicin Penetration Into Spheroids
The same time points in the free doxorubicin study were used to evaluate the liposomal doxorubicin penetration into the spheroids. Alternating 16 μm slices were collected during cryosectioning for MALDI-IMS and confirmation steady state fluorescence microscopy analysis (i.e. odd slices were used for fluorescence and even slices were for MALDI-IMS analysis). Collecting alternating slices allows for a comprehensive drug penetration picture to be created and minimizes biological differences and experimental bias. Figure 3 shows the time course of drug penetration of 90:5:5 POPC: POPE: cholesterol and 90:5:5 POPE: POPC: cholesterol liposomal doxorubicin analyzed by MALDI-IMS. Control spheroids were also analyzed for the same time course. The data shows that doxorubicin is successfully delivered to the spheroid model system after being encapsulated within the two different liposome configurations.
Figure 3.
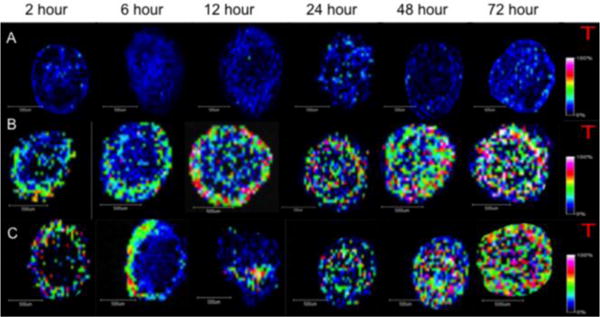
Liposomal doxorubicin (90: 5: 5 POPC :POPE: cholesterol and 90: 5: 5 POPE: POPC: cholesterol) penetration visualized by MALDI-IMS over the course of 72 hours. (A) Control spheroids collected at each time point indicate no drug or interference present. (B) 90: 5: 5 POPC :POPE: cholesterol liposomal doxorubicin is present as early as 2 hours and seen throughout the spheroid by 12 hours, however, an intense ring of drug was detected in the outer cells at this time point. (C) 90: 5: 5 POPE: POPC: cholesterol liposomal doxorubicin shows similar drug penetration to 90: 5: 5 POPC:POPE: cholesterol liposomal doxorubicin and free drug after 24 hours but interesting patterns are visualized at 6 and 12 hours of treatment.
Like the free doxorubicin, 90:5:5 POPC: POPE: cholesterol and 90:5:5 POPE: POPC: cholesterol liposomal doxorubicin can be detected in the outer proliferative cells as early as after two hours of treatment. Looking at the two different liposomal doxorubicin configurations (Figure 3B–C) different drug distribution emerges. The 90:5:5 POPC: POPE: cholesterol liposomal drug continues to penetrate into the spheroid as treatment time increases, with full penetration into the spheroid observed by twelve hours. These results correlate with the free doxorubicin time course, indicating the liposomal encasement does not severely affect the rate of doxorubicin diffusion into the spheroids. One intriguing difference, however, between 90:5:5 POPC: POPE: cholesterol liposomal doxorubicin and free doxorubicin detected by MALDI-IMS is observed in the outer region of cells after twelve hours of treatment. The liposomal doxorubicin shows an intense ring of drug in the outer proliferative cells and low levels of the drug throughout the rest of the spheroid, while in the free drug samples this unique characteristic is seen after 6 hours of treatment. This ring could indicate delayed permeation of the drug into the spheroids and was observed in all three biological and six technical replicates. This intense band of drug near the edge of the spheroid is detected in the free doxorubicin samples at 6 hours in the three replicates. This difference could be due to the fact the doxorubicin must first be released from the liposome through the POPC and POPE binding to cellular bilayers before it has the ability to passively diffuse into cells. The doxorubicin entrapped in the liposome is essentially inactive until it is released. We hypothesize that this distinction could be the cause of the increase in drug concentration in the outer proliferative cells at 12 hours of treatment.
The 90:5:5 POPE: POPC: cholesterol liposomal doxorubicin presents interesting reproducible patterns of drug distribution specifically after 6 (drug visualized intensely on one side of the spheroid in the outer cells) and 12 hours (drug visualized at high intensity in only core of the spheroid) of treatment. These interesting trends of drug distribution will be more thoroughly investigated in future experiments.
Doxorubicin Metabolism and Metabolite Detection
MALDI-IMS has a significant advantage compared to steady state fluorescence microscopy as MALDI-IMS can detect and distinguish metabolites formed by the cells actively metabolizing the drug. If the fluorescent emission spectra are the same, the relative signal obtained using microscopy experiments cannot be distinguished between the parent drug and its metabolites.30 However, if the compounds are ionizable and, as long as the molecular weights of the metabolites are different from the parent drug, they are distinguishable by MALDI-IMS. Three metabolites of doxorubicin: 7-deoxydoxorubicinone (399.1 m/z), 7-deoxydoxorubicinolone (401.1 m/z), and doxorubicinone (397.0 m/z) are detected by MALDI-IMS in this study. Peaks specific for doxorubicin (544.8 m/z) and the three metabolites are distinguishable from one another due to the loss of the sugar ring and hydroxyl groups during metabolism.30 Figure 4 shows the structures of doxorubicin and the three metabolites detected in this study as well as a typical mass spectrum obtained in a standard MALDI-IMS experiment. In the mass spectrum, the parent drug, the metabolites and the lipid mass range are all distinct.
Figure 4.
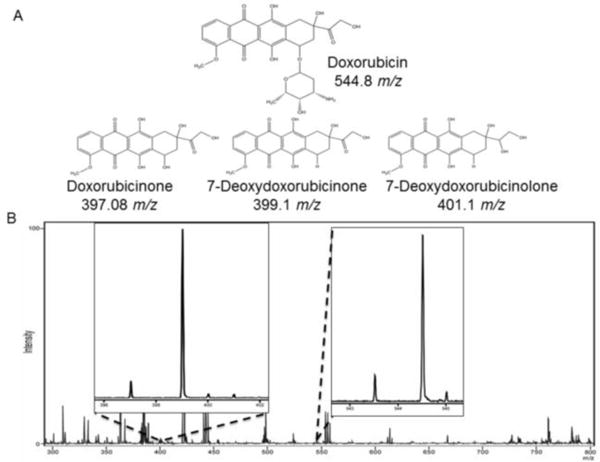
(A) Doxorubicin and its metabolites found within this study. (B) Typical spectrum obtain in MALDI-IMS experiment. The peaks for doxorubicin as well as the three metabolites are distinct from not only one another but all the lipid mass range.
The three metabolites of doxorubicin are detected by MALDI-IMS analysis after just 24 hours of treatment with free and both liposomal doxorubicin configurations. MALDI-IMS data was acquired at 24 and 48 hours but showed a very limited signal from the metabolites. More intense metabolite signals are detected after 72 hours of treatment. Figure 5 shows the distribution of the doxorubicin as well as the three metabolites after 72 hours of treatment with free drug and 90: 5: 5 POPC: POPE: cholesterol liposomal doxorubicin. The proliferative cells within the spheroid appear to metabolize doxorubicin, as a distinct ring of all three metabolites were detected in the outer layer. An additional ring of metabolites is detected in the second layer, also known as the quiescent zone, within the spheroid. Doxorubicin itself is detected throughout the spheroid (outer proliferative, quiescent zone, and necrotic core) at all time points after twelve hours of dosing with either free drug or liposomal doxorubicin. Similar results were obtained for the 90: 5: 5 POPE: POPC: cholesterol liposomal doxorubicin and can be seen in the Supplementary Information.
Figure 5.
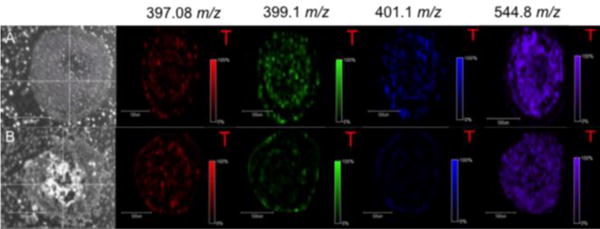
Doxorubicin and three metabolites present after 72 hours of treatment. (A) Free doxorubicin dosed spheroid. (B) 90:5:5 POPC:POPE:cholesterol liposomal doxorubicin spheroid. Outer proliferative cells are processing the drug as seen by the intense ring on the edge of the spheroid.
Conclusions
This study is the first to utilize MALDI-IMS to analyze drug distribution of a liposomal drug carrier in a three-dimensional cell culture system. Steady state fluorescence microscopy was used as an orthogonal method to confirm doxorubicin’s presence with the spheroid sections. Doxorubicin was successfully entrapped in two different liposomal configurations and delivered to the spheroid model system and was visualized throughout the entire spheroid by 12 hours of treatment, which is comparable to free drug distribution. In addition to determining the time course in which doxorubicin enters into the spheroid we also distinguished three metabolites of doxorubicin. These metabolites were produced through active catabolism of the drug by the cells of the spheroid throughout treatment. MALDI-IMS analysis was used to detect these metabolites, a measurement that that is not possible through fluorescence microscopy as their emission spectra are indistinguishable. As many chemotherapeutics are administered as inactive pro-drugs, confident detection of their metabolites is critical to evaluate drug efficacy.
This work is the beginning of a platform that can be utilized to assess the efficacy of numerous liposomal chemotherapeutic drugs in a three-dimensional model system by determining drug distribution. In future studies, we will expand on this approach and quantitatively evaluate the amounts of the drug and metabolites in the different layers of the spheroids.21,31 Now that we have determined that liposomal configurations can be evaluated by imaging mass spectrometry, accurate assessment of the relative amounts of different chemical species is key to understanding the effectiveness and distribution of liposomal drugs.
Supplementary Material
Acknowledgments
We thank Dr. William Boggess in the University of Notre Dame Mass Spectrometry and Proteomics Facility, Carolyn Shirey and Kristen Johnson in the R.V. Stahelin lab for their help with liposome formation and dynamic light scattering, and Dr. Susan Skube for manuscript editing. ABH was supported by the National Institutes of Health (R01GM110406), and the National Science Foundation (CAREER Award, CHE-1351595). EMW was supported by the Walther Cancer Foundation. The UltrafleXtreme instrument (MALDI-TOF-TOF) was acquired through National Science Foundation award #1625944.
References
- 1.Vallabhapurapu SD, Blanco VM, Sulaiman MK, Vallabhapurapu SL, Chu Z, Franco RS, Qi X. Oncotarget. 2015;6:34375–34388. doi: 10.18632/oncotarget.6045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeung T, Terebiznik M, Yu L, Silvius J, Abidi W, Philips M, Levine T, Kapus A, Grinstein S. Science. 2006;313:347–351. doi: 10.1126/science.1129551. [DOI] [PubMed] [Google Scholar]
- 3.Allen TM, Cullis PR. Adv, Drug Delivery Rev. 2013;65:36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 4.Lombardo D, Calandra P, Barreca D, Magazu S, Kiselev MA. Nanomaterials. 2016;6:125. doi: 10.3390/nano6070125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Front Pharmacol. 2015;6:286. doi: 10.3389/fphar.2015.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagayasu A, Uchiyama K, Kiwada H. Adv Drug Delivery Rev. 1999;40:75–87. doi: 10.1016/s0169-409x(99)00041-1. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, Utama RH, Kitiyotsawat U, Babiuch K, Jiang Y, Stenzel MH. Biomater Sci. 2015;3:1085–1095. doi: 10.1039/c4bm00323c. [DOI] [PubMed] [Google Scholar]
- 8.Chang HI, Yeh MK. Int J Nanomed. 2012;7:49–60. doi: 10.2147/IJN.S26766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu Lila AS, Ishida T. Biol Pharm Bull. 2017;40:1–10. doi: 10.1248/bpb.b16-00624. [DOI] [PubMed] [Google Scholar]
- 10.Barenholz Y. J Controlled Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien ME, Wigler N, Inbar M, Rooso R, Grischke E, Santoro A, Catane R, Kieback DG, Tomczak P, Ackland SP, Orlandi F, Mellars L, Alland L, Tendler C, Group, C.B.C.S. Ann Oncol. 2004;3:440–449. doi: 10.1093/annonc/mdh097. [DOI] [PubMed] [Google Scholar]
- 12.Seynhaeve ALB, Dicheva BM, Hoving S, Koning GA, ten Hagen TLM. J Controlled Release. 2013;172:330–340. doi: 10.1016/j.jconrel.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 13.Horning JL, Sahoo SK, Vijayaraghavalu S, Dimitrijev S, Vasir JK, Jain TK, Panda AK, Labhasetwar V. Mol Pharmaceutics. 2013;5:849–862. doi: 10.1021/mp800047v. [DOI] [PubMed] [Google Scholar]
- 14.Yue X, Lukowski JK, Weaver EM, Skube SB, Hummon AB. J Proteome Res. 2016;15:4265–4276. doi: 10.1021/acs.jproteome.6b00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fülöp A, Sammour DA, Erich K, von Gerichten J, van Hoogevest P, Sandhoff R, Hopf C. Sci Rep. 2016;6:33791. doi: 10.1038/srep33791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walrant A, Matheron L, Cribier S, Chaignepain S, Jobin M, Sagan S, Alves ID. Anal Biochem. 2013;438:1–10. doi: 10.1016/j.ab.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Weaver EM, Hummon AB. Anal Chem. 2013;85(13) doi: 10.1021/ac400519c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Hummon AB. Anal Chem. 2015;87:9508–9519. doi: 10.1021/acs.analchem.5b00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirschhaeuser F, Menne H, Dittfeld C, West J, Mueller-Klieser W, Kunz-Schughart LA. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 20.Keithley RB, Weaver EM, Rosado AM, Metzinger MP, Hummon AB, Dovichi NJ. Anal Chem. 2010;85:8910–8918. doi: 10.1021/ac402262e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu X, Hummon AB. J Am Soc Mass Spectrom. 2015;26:577–586. doi: 10.1007/s13361-014-1071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weaver EM, Hummon AB, Keithley RB. Anal Methods. 2015;7:7208–7219. doi: 10.1039/C5AY00293A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eetezadi S, De Souza R, Vythilingam M, Lessa Cataldi R, Allen C. Mol Pharmaceutics. 2015;12:3973–3985. doi: 10.1021/acs.molpharmaceut.5b00426. [DOI] [PubMed] [Google Scholar]
- 24.Tacar O, Sriamornsak P, Dass C. J Pharm Pharmacol. 2012;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 25.Laginha KM, Verwoert S, Charrois GJR, Allen TM. Clin Cancer Res. 2005;11:6944–6949. doi: 10.1158/1078-0432.CCR-05-0343. [DOI] [PubMed] [Google Scholar]
- 26.Mohan P, Rapoport N. Mol Pharmaceutics. 2010;7:1959–1973. doi: 10.1021/mp100269f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedrich J, Seidel C, Ebner R, Kunz-Schughart LA. Nat Protoc. 2009;4:309–324. doi: 10.1038/nprot.2008.226. [DOI] [PubMed] [Google Scholar]
- 28.Wheatcraft Ahlf DR, Liu X, Hummon AB. J Visualized Exp. 2014;94:e52313. doi: 10.3791/52313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H, Hummon AB. Anal Chem. 2011;83:8794–8801. doi: 10.1021/ac202356g. [DOI] [PubMed] [Google Scholar]
- 30.Katzenmeyer JB, Eddy CV, Arriaga EA. Anal Chem. 2010;82:8113–8120. doi: 10.1021/ac1011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feist PE, Sidoli S, Liu X, Schroll MM, Rahmy S, Fujiwara R, Garcia BA, Hummon AB. Anal Chem. 2017;89:2773–2781. doi: 10.1021/acs.analchem.6b03602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


