SUMMARY
To facilitate investigation of diverse rodent behaviours in rodents’ home cages, we have developed an integrated modular platform, the SmartCage™ system (AfaSci, Inc. Burlingame, CA, USA), which enables automated neurobehavioural phenotypic analysis and in vivo drug screening in a relatively higher-throughput and more objective manner.
The individual platform consists of an infrared array, a vibration floor sensor and a variety of modular devices. One computer can simultaneously operate up to 16 platforms via USB cables.
The SmartCage™ detects drug-induced increases and decreases in activity levels, as well as changes in movement patterns. Wake and sleep states of mice can be detected using the vibration floor sensor. The arousal state classification achieved up to 98% accuracy compared with results obtained by electroencephalography and electromyography. More complex behaviours, including motor coordination, anxiety-related behaviours and social approach behaviour, can be assessed using appropriate modular devices and the results obtained are comparable with results obtained using conventional methods.
In conclusion, the SmartCage™ system provides an automated and accurate tool to quantify various rodent behaviours in a ‘stress-free’ environment. This system, combined with the validated testing protocols, offers powerful a tool kit for transgenic phenotyping and in vivo drug screening.
Keywords: anxiety, behaviour, motor activity, mouse, neurobehaviour, rat, rotarod, sleep, social interaction
INTRODUCTION
Rodents exhibit many behaviours that are similar to those of humans, such as sleep, locomotion, motor coordination, exploration, fear and social approach behaviour. Assessments of behaviours in rodents help us understand disease-altered states and drug treatment responses in humans.1–4 Motor, neurological and mental disturbances are associated with a wide range of central nervous system (CNS) disorders, such as stroke, traumatic brain injury, spinal injury and almost all chronic neurodegenerative disorders (e.g. Alzheimer’s, Parkinson’s and Huntington’s diseases, multiple sclerosis, amyotrophic lateral sclerosis), as well as drug-induced CNS side effects in humans and animals.5–7 Therefore, modelling various components of human disease states and assessment of their behavioural end-points or monitoring treatment responses in preclinical animal models are routinely used in both academia and the pharmaceutical industry.2,3,8–11
Arousal states, wakefulness and sleep are fundamental aspects of global nervous system function and have been examined in rodent models and pharmacological studies in addition to locomotor activity.2,12–14 Typically, sleep/wakefulness is measured using electroencephalography (EEG) and electromyography (EMG).15–17 Analysis of sleep/wake patterns in rodents requires surgery, attachment of wires or invasively implanted wireless transmitters and extensive analysis of the EEG and EMG patterns. Currently, owing to the difficulty associated with the use of EEG and EMG, sleep is not routinely measured in animals subjected to disease modelling, genetic alteration or drug treatment. Thus, a simplified assessment of arousal states is needed, and attempts to meet this need have been reported.18–20
In general, assessments of animal behavioural are conducted in an environment outside of the animal’s home cage for short periods using different apparatuses. Most rodents, particularly mice, are sensitive to environmental conditions (e.g. sound, light and scent). Therefore, there is a need to shift towards assessing rodents’ behaviour in their home cages, especially in cases where the animals are monitored for an extended period. Efforts have been made to conduct rodent behavioural assessment in a home cage-like environment.10,11,14,18–22 However, automation and throughput in behavioural phenotyping and in vivo drug screening still present significant challenges. Here, we describe the development and validation of a rodent behavioural monitoring platform, named SmartCage™ (AfaSci, Inc., Burlingame, CA, USA; see Fig. S1a, available as Supporting information), that integrates a variety of technologies and modular devices to assess a broad range of behaviours, including wake/sleep, locomotor activity, exploration, motor coordination and anxiety-related and social approach behaviours, in a normal home cage. This system can simultaneously monitor up to 16 platforms using one host computer and provides a simple and versatile toolkit for behavioural neuroscience and in vivo drug discovery.
METHODS
Animals
Mice (male and female C57BL/6 or ICR mice weighing 20–30 g) or rats (male Sprague–Dawley rats weighing 250–300 g) were used for the studies. All animals were initially group housed under standard laboratory conditions (lights on 07.00–19.00 hours). For behavioural experiments, individual animals in their acclimated home cage or in a fresh home cage were placed within the SmartCage™ platform (Fig. S1a). For experiments requiring long monitoring periods (i.e. >4 h), animals had access to food and water throughout the monitoring period. All procedures were approved by the Institutional Animal Care and Use Committees of SRI International and AfaSci, Inc., and the studies were conducted in accordance with the US National Research Council’s Guidelines for the Care and Use of Mammals in Neuroscience and Behavioural Research (2003, http://grants.nih.gov/grants/olaw/National_Academies_Guidelines_for_Use_and_Care.pdf).
SmartCage™ system
The SmartCage™ platform provides an inner space of 36.0 × 23.0 × 9.0 cm (length × width × height) for mice or 52.0 × 28.0 × 13.0 cm for rats and acts as a station for ‘parking’ a mouse cage (e.g. PC75JHT (29.8 × 18.0 × 12.8 cm); Allen-town, NJ, USA) or rat cage (e.g. PC10198HT (47.6 × 25.9 × 20.9 cm); Allentown, NJ, USA) with bedding, food and water (Fig. S1a). The infrared (IR) processor, instrument amplifier, motor control and microcontroller units are assembled in the platform. Single USB cables are linked directly or via a hub to the host computer (32- or 64-bit Windows XP (Microsoft, Bellevue, WA, USA)) that provides power and operates upto 16 platforms simultaneously using a uniform graphical user interface and a program termed CageCenter™, which comes with the Smart-Cage™ system.
Data were analysed automatically using the Windows-based program CageScore™ (AfaSci, Inc. Burlingame, CA, USA). Home cage activity variables, including activity counts (counts of breaks in x-, y- and z-axis photo beams), activity time, locomotion (distance travelled and speed) and rearing, are calculated in a user-defined time block. The rodent’s instantaneous position in the cage is determined by the x and y coordinates. A circling movement (or rotation) made by the animal was calculated by its accumulated 360° turning. Counter-clockwise (left) turns are designated (−) circling, whereas clockwise (right) turns are designated (+) circling. The net circling is the summation of the left (−) and right (+) circling, indicating the predominant direction. The rearing activity is detected by the upper row of IR sensors. CageScore™ processes single animal and group data analysis and displays the data as the mean ± SEM in table and graphic formats.
Comparing activity and sleep states captured by SmartCage™ to video image tracking and EEG/EMG
Individual mice were placed into a freshly prepared cage and activity was recorded simultaneously by the SmartCage™ system and a video camera (placed above the cage). The video images were processed using a BSMART Video Tracking System (Harvard Apparatus, Boston, MA, USA). To further evaluate home cage activity monitoring, we quantified changes in activity levels induced by two stimulants, namely cocaine (30.0 mg/kg, i.p., in mice; 20.0 mg/kg, i.p., in rats) and amphetamine (20.0 mg/kg, i.p., in mice), and one sedative compound, the nociceptin/orphanin FQ (NOP) receptor agonist SR14150 (10 mg/kg, i.p.).23
Sleep/wake states were monitored using the vibration floor sensor and the signal was amplified and sampled at 100.0 Hz. A split vibration analogue signal output was sent to the external analogue-to-digital (A/D) converter (USB-2533; Measurement Computing, Norton, MA, USA) to ensure synchronization with the EEG and/or EMG, which were recorded using SleepWave™ software (Biosoft Studio, Hershey, PA, USA), as described previously.15,16 The EEG signals were filtered with low and high cutoffs at 0.3 and 30.0 Hz, respectively, and were digitized at 128.0 Hz, whereas the EMG signals were filtered with low and high cut-offs at 100.0 and 300.0 Hz, respectively, and were digitized at 256.0 Hz. The EEG signals were subjected to fast Fourier transformation (FFT) analysis, yielding power spectra between 0.5 and 40.0 Hz, with a 0.5 Hz frequency resolution every 2 s and then averaged every 10 s. The total sleep, including both rapid eye movement (REM) and slow wave sleep (SWS), was determined by manual scoring of the EEG and/or EMG signals in 10 s segments.
Motor coordination and balance
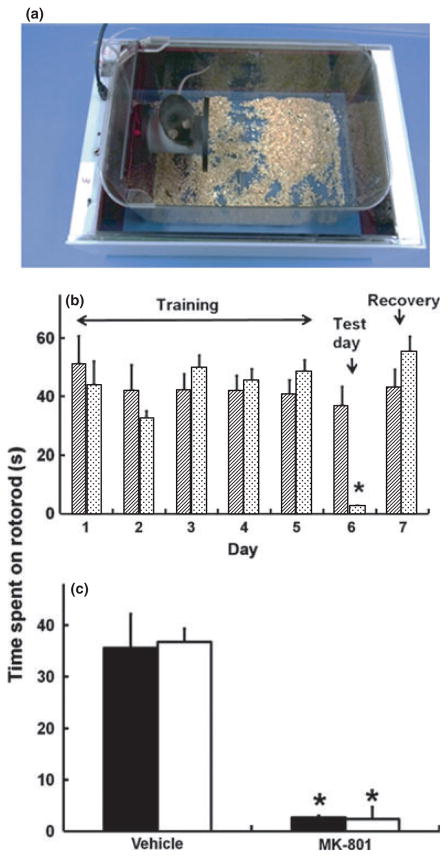
A rotarod module was inserted into a mouse home cage and was operated by the SmartCage™ system (Fig. 5a). The top of the rod (3.5 cm in diameter and 6.0 cm long) is 8.5 cm above the cage floor. A sensor inside the rod automatically detects the animal on and off the rod. There are two speed controls, constant and accelerating (0–60 r.p.m. over a 3 min ramp) modes, using a pulse width modulation circuit. Two groups of mice were trained on rotarod performance daily over 5 days. On Day 6, one group received a treatment with MK-801 (0.1 mg/kg, i.p.), whereas the other group received vehicle. On Day 7, recovery from the drug effect was evaluated. Manual scoring was also conducted to verify the accuracy of SmartCage™ detection.
Fig. 5.
(a) Rotarod insert to measure motor coordination in mice. (b) Duration on the rod measured by SmartCage™ (AfaSci) across 7 days. Days 1–5 were training days and animals did not receive any drug injections. On Day 6, animals received an injection of MK-801 (0.1 mg/kg, i.p.;
 ) or vehicle (▨) and were tested 30 min later. Data are the mean ± SEM of five mice per group. Following a significant overall ANOVA, further Bonferroni analyses revealed a significant effect of MK-801 in decreasing the time spent on the rotarod on Day 6 compared with the vehicle control (*P = 0.0008). (c) Time spent on the rotarod measured by the SmartCage™ (■) compared with that determined manually (□) after vehicle or MK-801 injection. Using both methods, t-tests indicated that animals spent less time on the rotarod following injection of MK-801 compared with the vehicle controls (*P = 0.0006 and 0.0008 for data obtained manually and using the SmartCage™, respectively).
) or vehicle (▨) and were tested 30 min later. Data are the mean ± SEM of five mice per group. Following a significant overall ANOVA, further Bonferroni analyses revealed a significant effect of MK-801 in decreasing the time spent on the rotarod on Day 6 compared with the vehicle control (*P = 0.0008). (c) Time spent on the rotarod measured by the SmartCage™ (■) compared with that determined manually (□) after vehicle or MK-801 injection. Using both methods, t-tests indicated that animals spent less time on the rotarod following injection of MK-801 compared with the vehicle controls (*P = 0.0006 and 0.0008 for data obtained manually and using the SmartCage™, respectively).
Anxiety-related behaviours
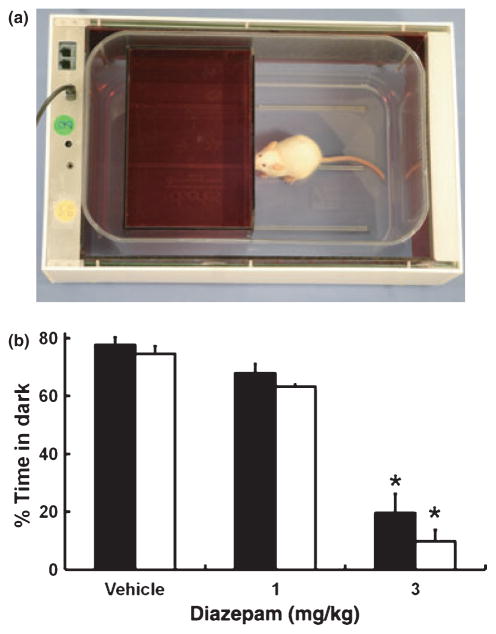
To measure anxiety-related behaviours, a dark red box (made in Plexiglass plastic; 16.5 × 11.0 × 13.5 cm length × width × height) with an opening (3.0 × 3.0 cm) was inserted in a fresh mouse home cage (Fig. 6a). Extra lighting was provided using a 60 W lamp placed 30 cm above the light compartment. The IR array was used to detect the animal’s position and locomotion as described above. CageScore™ automatically calculated and displayed three parameters: (i) latency of first entry into the dark box; (ii) time spent in the light and dark compartments; and (iii) the number of transitions between the light and dark compartments. Mice were treated with diazepam (1.0–3.0 mg/kg, i.p.) or vehicle, then, 30 min after injection, individual animals were placed in the light compartment and tested for 10 min. The results were compared with those obtained using an elevated plus maze using similar parameters as described above.
Fig. 6.
(a) Dark box insert to measure the light/dark preference and anxiety-related behaviour. (b) Effect of diazepam (1.0 a nd 3.0 mg/kg, i.p.) on the percentage of time spent in the dark measured by the SmartCage™ (AfaSci; ■) compared with that determined manually (□). Data are the mean ± SEM of six mice per group. Following a significant ANOVA, Dunnett’s post hoc test revealed that only the 3 mg/kg dose of diazepam produced a significant decrease in time spent in the dark compared with control (*P = 0.05). Findings for time spent in dark box determined manually were identical to those obtained using the SmartCage™.
Automation of social interaction
Two transparent Plexiglass enclosures (8.0 × 6.0 × 12.0 cm length × width × height) with a metal mesh floor were attached at each end of a home cage (Fig. S1c). The enclosures were elevated 6.0 cm above the floor to facilitate the test subject’s exploration of non-social cues (empty enclosures) or social cues (containing a stimulus mouse). Test subjects and stimulus mice were C57BL/6 mice. Stimulus mice were taken from separate housing cages. One day prior to testing, stimulus mice were habituated to the enclosure for 5 min. The social interaction test was conducted in the light phase and consisted of three consecutive 10 min sessions, based on published procedures4,25,26: (i) habituation; (ii) sociability; and (iii) preference for social novelty. During habituation, the test subject explored the cage, which was equipped with two empty enclosures. For the sociability test, a stimulus mouse (Stranger 1) was placed into one enclosure. The location for ‘Stranger 1’ was alternated pseudorandomly for different test mice. Immediately after the sociability session, a new unfamiliar mouse (Stranger 2) was placed into the other empty enclosure. The test subject’s position and movement was monitored by the IR sensors. CageScore™ automatically divided the cage into four virtual even-sized zones and calculated occupancy time (i.e. total time spent), active time and distance travelled by the test mouse in individual zones. The amount of time the test mouse spent sniffing enclosure(s) with or without the stimulus mice was also scored by the experimenter in a separate cohort of animals.
Drugs
SR14150 [1-(1-cyclooctylpiperidin-4-yl)-indolin-2-one], synthesised as a hydrochloride salt,23 and MK-801 (Sigma-Aldrich, St Louis, MO, USA) were dissolved in dimethylsulphoxide (DMSO) and diluted with 0.5% aqueous hydroxypropyl cellulose to final injection solutions containing 2% DMSO. Morphine hydrochloride (Eli Lilly & Co., Indianapolis, IN, USA), diazepam, amphetamine and cocaine hydrochloride (Sigma-Aldrich) were dissolved in water.
Statistical analysis
All data are presented as the mean ± SEM. Correlations were conducted to compare the data (i.e. activity, rearing, sleep time, time spent in a particular zone) obtained using SmartCage™ with those obtained by hand and/or the video tracking system. In experiments investigating drug effects, a repeated-measures ANOVA was used with drug as the between-group variable and time interval as the repeated measure. The first 3–4 h following drug injection were included in the statistical analyses given that results indicate that stimulant and/or depressant effects do not last beyond 3–4 h. Following a significant interaction, Bonferroni tests were used. For the experiment examining motor coordination, the data obtained by SmartCage™ were compared with those obtained manually by the Bonferroni test. The effects of MK-801 across days (baseline, after dose, recovery) were compared with vehicle controls using a repeated-measures ANOVA, followed by Bonferroni comparisons. The effect of diazepam was examined with a one-way ANOVA followed by Dunnett’s post hoc tests. For the social approach data, occupancy time (SmartCage™) and time spent in sniffing behaviour (manual scoring) in Zones 1 and 4 during the different phases were analysed using t-tests (corrected P < 0.05/3 = 0.02). Data were analysed using STATVIEW v.5.0 (SAS Institute, San Francisco, CA, USA) and the significance for the overall two-sided analyses was set at P ≤ 0.05.
RESULTS
Comparing activity measurements captured by SmartCage™ with a video tracking system
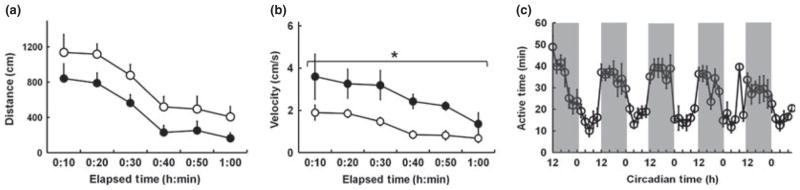
The travelling distance and speed, measured by SmartCage™ and a video tracking system, indicated that the animals were initially more active when placed in fresh home cages, with activity levels declining gradually as the animal acclimated to the cage. Both the SmartCage™ and video tracking systems detected identical patterns in behaviour (r = 0.98; Fig. 1a,b). There was no significant difference in the distance travelled obtained by the SmartCage™ and video tracking systems (F1,30 = 2.0; P = 0.2), whereas there was a significant main effect for measurement of speed between the two systems (F1,30 = 10.8, P = 0.02). The SmartCage™ system allows for continuous recording over a much longer period compared with video recordings. In the example shown in Fig. 1c, the animals’ activity levels were recorded for 5 days. As expected, animals were most active during the dark phase compared with the light phase, showing a clear circadian rhythm.
Fig. 1.
Travel (a) distance and (b) velocity in mice over a 1 h period, as measured by SmartCage™ (AfaSci) and a video tracking system (○). Analysis of the data using ANOVA indicated a significant main effect of tracking system only when looking at velocity data (●), not distance travelled (*P = 0.02; n = 6 per group). (c) Circadian pattern of activity in mice across 5 days of recording with the SmartCage™ system under 12 h light–dark cycle. The shaded area indicates the dark phase of the circadian cycle. Data are the mean ± SEM.
Pharmacological studies using a home cage setting
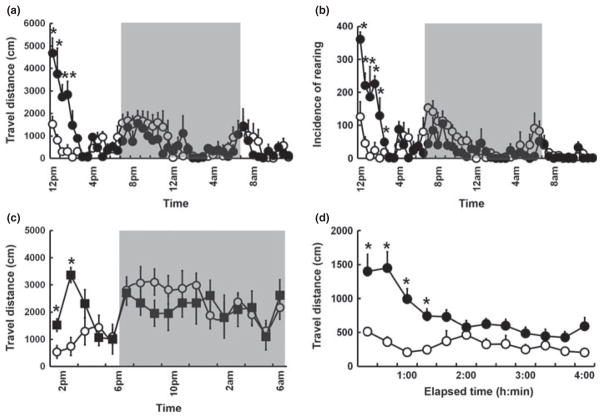
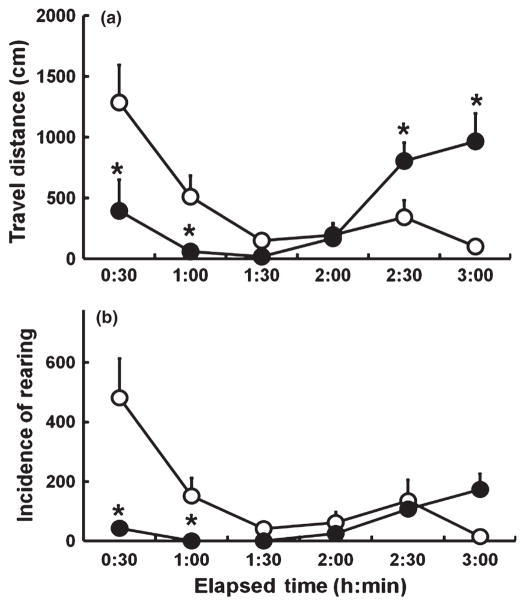
In a series of pharmacological experiments, we quantified changes in activity levels induced by stimulant or depressant compounds using the SmartCage™. Following vehicle injection, the ICR mice exhibited an initial increase in activity and then progressive decreases in activity within an hour, indicative of habituation (repeated ANOVA: F7,28 = 8.5; P = 0.004; Fig. 2a). After 24 h baseline recording, administration of cocaine (30.0 mg/kg, i.p.) to the same cohort of animals at the same circadian time as the vehicle injection resulted in hyperactivity. The data were analysed in 30 min blocks. The overall ANOVA for the 4 h following drug treatment indicated a significant drug–time interaction (F7,70 = 7.3; P < 0.0001) and, compared with the vehicle controls, cocaine hyperactivity lasted for approximately 2 h (Fig. 2a). Moreover, the levels of rearing were almost threefold greater than those observed following vehicle injection, showing that cocaine produced a significant increase in rearing for 3 h (Fig. 2b; F7,70 = 11.0; P < 0.0001). To validate rearing, the values were compared with those obtained by manual scoring. Following vehicle or cocaine injection, rearing obtained by both methods were highly correlated (r = 0.95 and 0.94, respectively), indicating that the SmartCage™ system is reliable in measuring both vertical activity and horizontal movement.
Fig. 2.
Effects of (a,b,d) cocaine (●) and (c) amphetamine (20 mg/kg, i.p.; ■) compared with vehicle injection (●) in mice (a–c) and rats (d) on distance travelled (a,c,d) and the incidence of rearing (b), as measured by SmartCage™ (AfaSci). Data show the mean ± SEM for n = 6 animals in each group. (a) Repeated-measures ANOVA revealed a significant effect of 30.0 mg/kg, i.p., cocaine on the travel distance of mice and Bonferroni tests (calculated P < 0.05/8 = 0.006) indicated drug effects for 2 h after drug injection compared with vehicle (*P values for the first four 30 min intervals are 0.001, 0.001, 0.0007 and 0.003, respectively). (b) Similarly, repeated-measures ANOVA revealed a significant effect of 30.0 mg/kg, i.p., cocaine on the incidence of rearing in mice and Bonferroni tests (calculated P < 0.05/8 = 0.006) indicated drug effects for 3 h after drug injection compared with vehicle (*P values for the first six 30 min intervals are 0.0001, 0.0001, 0.001, 0.0001, 0.003 and 0.005, respectively). (c) Significant effects of amphetamine were detected by ANOVA on distance travelled by mice and Bonferroni tests (calculated P < 0.05/4 = 0.0125) indicated drug effects for 2 h after injection compared with vehicle (*P values for the first two 1 h intervals were 0.01 and 0.0001, respectively). (d) In rats, 20 mg/kg, i.p., cocaine had a significant effect on distance travelled (ANOVA) and Bonferroni tests (calculated P < 0.05/12 = 0.004) indicated drug effects for 3 h after drug injection compared with vehicle (*P values for the first four 20 min intervals were 0.0004, 0.001, 0.0005 and 0.002, respectively).
In a separate experiment using C57BL/6 mice, we examined the effects of amphetamine (20 mg/kg, i.p.). After 3 days baseline recording, as shown in Figs 1c and 2c, administration of amphetamine to the same cohort of animals at the same circadian time as the vehicle injection the previous day resulted in hyperactivity (Fig. 2c). The data were analysed using 1 h blocks. The overall ANOVA for 4 h following drug treatment indicated a significant drug–time interaction (F3,30 = 6.4; P = 0.0017) and, compared with the vehicle controls, amphetamine-induced hyperactivity lasted for 2 h.
We further used the enlarged SmartCage™ to detect changes in activity levels induced by cocaine (20 mg/kg, i.p.) in rats. The overall ANOVA indicated a significant drug–time interaction (F11,110 = 5.5; P < 0.0001), with further analysis revealing that cocaine caused an increase in locomotion relative to vehicle controls during the first 1 h 20 min of testing (Fig. 2d). Cocaine produced not only an increase in activity, but also more circling predominantly in one direction in the movement path (Fig. S2).
To evaluate whether drug-induced hypoactivity could be detected using SmartCage™, we studied the effects of the NOP receptor agonist SR14150, which has been shown previously to inhibit locomotion.23 Injection of SR14150 (10.0 mg/kg, i.p.) produced a decrease in travelling distance and rearing in the first 1 h after injection. The overall ANOVA indicated a significant drug–time interaction for locomotion (F5,65 = 9.0; P < 0.0001) and rearing (F5,65 = 10.5; P < 0.0001). Interestingly, in the last 1 h of testing, animals displayed a significant ‘rebound’ increase in locomotor activity, but not in rearing, compared with vehicle baseline (Fig. 3).
Fig. 3.
Effects of SR14150 (10 mg/kg, i.p.; ●) on (a) distance travelled and (b) rearing in mice, as measured by SmartCage™ (AfaSci). Data are the mean ± SEM (n = 7–8 per group). (●), vehicle (control) group. Following a significant ANOVA, Bonferroni tests (calculated P < 0.05/6 = 0.008) indicated significant SR14150-induced decreases in activity 1 h after drug injection compared with control (*P = 0.004 and 0.005 for the first two 30 min intervals) and increases in activity during the third hour after drug injection (*P = 0.004 and 0.0001 for the last two 30 min intervals).
Noninvasive detection of sleep/awake states
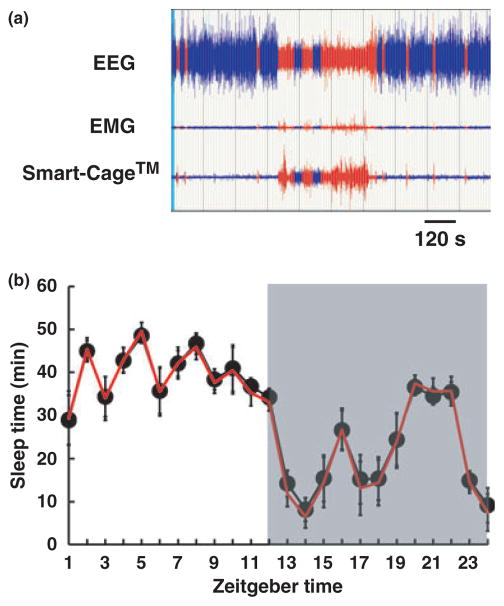
Although the IR array is capable of detecting active/inactive circadian and quantifying locomotion, sleep monitoring requires a more refined measurement. SmartCage™ has a vibration floor sensor for non-invasive sleep monitoring. During sleep, the rodent’s chest presses against the floor and its regular respiration is detected by the vibration sensor. The lower amplitude and regular waveforms (2.5–3.0 Hz for mice; Fig. S1b) have been used as a sleep biomarker in previous studies.18,19 However, the signal size and waveform of such breathing patterns change greatly from hour to hour due to changes in the animal’s position and the bedding location, making consistent automatic scoring difficult. Therefore, we have developed a new method to enhance the contrast between wakefulness and sleep states. The resultant contrast of the vibration signal is two- to threefold higher than the contrast of EMG signals between these two arousal states in mice (Fig. 4a). The automatic scoring algorithm uses such enhanced contrast to differentiate sleep from wakefulness. As shown in Fig. 4b, sleep patterns using SmartCage™ are highly correlated with measures obtained by EEG and/or EMG (total wakefulness: r = 0.862, P = 0.02; hourly variations in wakefulness: r = 0.998, P = 0.0001). Over a 24 h period, the SmartCage™ and EEG/EMG values for wakefulness were 716 ± 23 and 727 ± 22 min, respectively, whereas for sleep they were 724 ± 23 and 713 ± 22 min, respectively. Furthermore, the speed of the new automatic sleep/wake scoring method is rapid (24 h data scored in <5 s), with approximately 90% accuracy compared with manual scoring.
Fig. 4.
Comparison of non-invasive sleep monitoring between the Smart-Cage™ (AfaSci, Burlingame, CA, USA) and electroencephalography (EEG) and electromyography (EMG) recorded simultaneously in the same cohort of mice (n = 7). (a) Screen capture of the automatic scoring of vibration signals and manual scoring of EEG and EMG signals. The segments classified as ‘sleep’ and ‘wake’ are shown in blue and red, respectively. (b) Sleep time quantified by automatically scored vibration signals (●) and manually scored EEG/EMG (—), plotted hourly. The x-axis shows the time of day where Zeitgeber Time 0 = lights on (0700 hours) and Zeitgeber Time 12 = lights off (1900 hours; shaded area).
Evaluation of motor coordination using the rotarod insert
The performance of the rotarod balance by mice improved and reached stable levels following 3–5 days training (Fig. 5b). As seen on Day 5, the baseline performance of two randomly assigned groups was similar. On Day 6, each group received either vehicle or MK-801 (0.1 mg/kg, i.p.). On Day 7 recovery from treatments was assessed. The overall repeated ANOVA for Days 5–7 revealed a significant drug–test day interaction (F2,14 = 11.05; P = 0.001). Further Bonferroni comparison revealed that MK-801 markedly decreased the time mice stayed on the rod compared with control (t8 = 5.6; P = 0.0008), consistent with manually scored data (t8 = 5.8; P = 0.0006; Fig. 5c).
Detection of diazepam-induced anxiolytic effects using the dark box insert
Time spent in the dark compartment for animals receiving vehicle or diazepam (1 or 3 mg/kg) is shown in Fig. 6b. One-way ANOVA revealed a significant main effect of dose (F2,13 = 49.7, P < 0.0001 for SmartCage™ data; F2,13 = 71.45, P < 0.0001 for data collected by hand). Data collected by both methods were similar. Further Dunnett post hoc analysis indicated that mice treated with 3.0 mg/kg diazepam spent less time in the dark box compared with controls (134 vs 483 s, respectively; P < 0.05). To further validate the home cage assessment of drug-induced anxiolytic-like behaviour we compared the results with those of the elevated plus maze. Diazepam (3.0 mg/kg, i.p.) significantly increased the amount of time spent in the open arms compared with the vehicle-injected controls (254 ± 65 vs 65 ± 24 s, respectively; t11 = 4.12, P = 0.001).
Automation of social approach behaviour using enclosure inserts
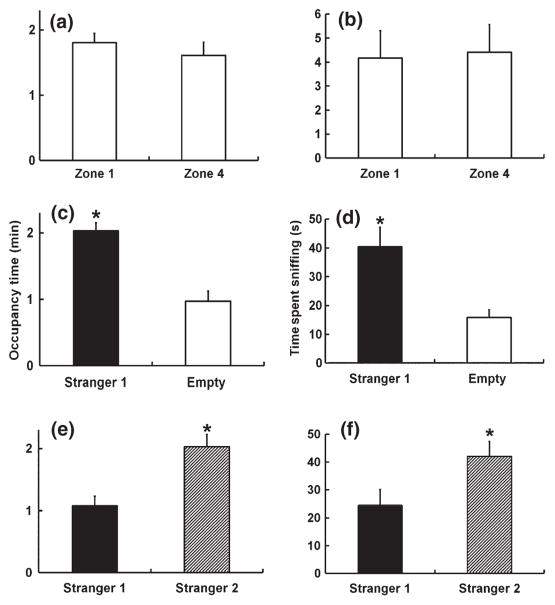
During the habituation session, the test mouse spent a similar amount of time in Zones 1 and 4, where the enclosures were located (Figs 7a,b and S1c). During the sociability session, when a ‘strange’ mouse was placed into one of the enclosures, test mice spent significantly more time investigating the ‘stranger’ compared with the side that had an empty enclosure (t7 = 4.5; P = 0.003; Fig. 7c,d). During the social novelty test, when a novel ‘stranger’ (designated as ‘Stranger 2’) was placed into the other enclosure, the test subject now spent more time with the novel stranger compared with the zone that had the familiar ‘Stranger 1’ (t7 = 4.3; P = 0.003; Fig. 7c).
Fig. 7.
Occupancy time in Zone 1 and 4 were automatically quantified using SmartCage™ (left panels), whereas sniffing behavior was measured by manual scoring (right panels). During the habituation, occupancy time (a) or time spent sniffing (b) in Zones 1 and 4 were similar. During the sociability session, occupancy time (c) and time spent sniffing (d) in the zone with the stranger 1 mouse was significantly greater compared to the zone with the empty cage using t-test (*P = 0.003 and 0.005 for occupancy time and sniffing, respectively). During the social novelty session, occupancy time (e) and time spent sniffing (f) in the zone with ‘Stranger 2’ was significantly greater compared to the zone with the ‘Stranger 1’ (*P = 0.003 and 0.01 for occupancy time and sniffing, respectively).
Time spent sniffing the enclosures (with or without the stimulus mice) by the test subject was also scored manually to validate the automated measure. During habituation, the incidence of sniffing Zones 1 and 4 was similar. During the sociability session, the test mouse spent more time sniffing the enclosure with Stranger 1 compared with the empty enclosure (t11 = 3.6; P = 0.005). During the social novelty session, the test mouse spent more time sniffing Stranger 2 compared with Stranger 1 (t11 = 2.6; P = 0.01). To further verify the social interaction test, we conducted another test using the same procedure but with the enlarged rat version of SmartCage™ to host a bigger cage similar in size to the conventional three-chamber apparatus.4,25,26 We obtained comparable results to those observed using the mouse home cage (data not shown).
DISCUSSION
Robust and subtle changes in behavioural activity can be measured in normal home cages
Behaviour is the functional output of an organism that reflects various biological processes, particularly the nervous and motor systems. Testing rodents in the home cage is desirable because it minimizes an animal’s stress, reduces human labour and increases overall throughput for behavioural phenotypic and pharmacological studies in vivo. Currently, behavioural studies usually use large and specific apparatuses and record for a limited period. Efforts to study rodent activity, circadian and feeding patterns or total sleep in a home cage-like environment have been made.10,11,14,18,19,21 However, most of these systems still limit users to single or a few behavioural assays. Here, we have developed a new integrated modular system that provides automated assessments of a wide range of neurobehaviour, including active/inactive or sleep/wake states, locomotion, motor coordination, anxiety-related behaviours and social interaction. The home cage behavioural assessments can detect drug-induced changes and strain/age/genotype differences (Yang LY and Xie XM et al., unpubl. obs., 2012). Recently, the SmartCage™ system has been shown to detect subtle yet significant differences in active time and travelling distance between interleukin-4-knockout and wild-type mice that were subjected to focal ischaemic insult.27 Using optogenetic stimulation of the locus coeruleus, mice displaying hyperactivity and differential movement pattern from control mice were also revealed by the SmartCage™.28,29
Non-invasive sleep measurement
An important feature of the SmartCage™ system is its integration of activity level detection with non-invasive sleep monitoring. A high correlation between the vibration signals processed by SmartCage™ and EEG and EMG recording demonstrates that the SmartCage™ has the ability to detect total sleep. Individual platforms have a split vibration analogue output, allowing the investigator to simultaneously record EEG ane EMG signals and vibration signals in a synchronized manner. Potentially, the vibration signal could be used as an alternative to EMG and spare that channel for additional EEG recordings.
Rotarod performance
The rotarod test provides an objective evaluation of balance and motor coordination.5,6,25 In our rotarod configuration, the rod is 8.5 cm above the cage floor. Although it is lower than conventional rotarod height (30.0–50.0 cm), the animal is instinctively scared of falling and continually repositions itself to stay on the rotating rod. Injection of MK-801 caused a reversible impairment of performance, consistent with results obtained using conventional methods.8 Because each platform automatically detects the presence and absence of the animal on the rod, the system can conduct rotarod tests on up to 16 animals simultaneously. Each rotarod can be set in different speed modes (constant or accelerating), as appropriate. This is a feature that other conventional rotarod devices do not offer. Moreover, the rod level is low enough for an adult animal to spontaneously climb up and self-initiate a rotarod task. The spontaneous rotarod test could provide a new method to assess not only motor coordination, but also motivation of performance.
Anxiety-related behaviour in home cages
Changes in anxiety levels are characteristic of many neuropsychiatric disorders, including general anxiety disorder, depression, bipolar disorder and post-traumatic stress disorder.24 A dark box inserted in a fresh home cage, with enhanced lighting, has been shown to measure rodent light–dark place preference and is sensitive enough to detect the anxiolytic effect produced by diazepam. The results obtained using SmartCageTM are consistent with reported assessments using conventional light–dark boxes in a home cage setting22 and comparable with those obtained using the elevated plus maze.
Simplification and automation of social interaction
Social dysfunction is a marker for developmental disorders such as autism and schizophrenia.4 Rodents are social animals and social approach behaviour represents an important aspect for evaluation of social interaction. Currently, an apparatus consisting of three separate chambers is often used to evaluate social interaction in a manual or automated manner.4,25,26 We have now simplified the ‘three-chamber apparatus’ with two enclosures placed in an ordinary rodent cage. The pattern of the test mouse social approach behaviour is almost identical to that observed using a larger (rat-size) cage with two enclosures and consistent with results obtained using the three-chamber apparatus.4,26 The SmartCage™ simultaneously quantified the test subject’s active time and travel distance in different zones. Although these two parameters are not as robust as the occupancy time in evaluation of social approach behaviour, they can serve to control for non-specific deficits in arousal status and motor function because they could confound the outcome of the social behaviour test.
CONCLUSION
In the present series of neurobehavioural and pharmacological studies, we have demonstrated that normal behaviour and drug-induced global and subtle behavioural changes can be detected in an ordinary rodent home cage using the SmartCage™ system. Furthermore, with the modular inserts, more specialized behaviours (e.g. sleep, motor coordination, anxiety-related behaviour and social interaction) can be assessed automatically along with basic behaviour. The system is simple, versatile and cost-effective and is suitable for primary transgenic phenotyping and initial in vivo drug screening.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants R44RR017182, R43MH076309, R41HL84990, R43NS073311 and R01MH078194. The authors thank Dr. H. Javitz (SRI International) for statistical input, Rajesh Khanna (SRI International) for help with the graphics and Gregory Frane, Carlos Sanchez, Kefu Li, Patrick Shulman and Garrett Chan (students working as interns at AfaSci Research Laboratories) for assistance with assembling the device and initial testing.
Footnotes
DISCLOSURE
XX is the founder and owner of AfaSci, Inc., and JF is the founder and owner of Biosoft Studio.
Additional Supporting Information may be found in the online version of this article:
Figure S1. (a) An individual SmartCage™(AfaSci) platform. (b) A screenshot of the SmartCage™ #7 shows real-time detection of the animal’s position and movement (upper left portion with a mouse symbol) and sleep/wake waveform (the bottom traces) by the vibration floor sensor. (c) Modular enclosures attached on either end of a mouse home cage to measure sociability.
Figure S2. Panels show representative 20 min movement paths 30 min after injection of (a) vehicle or (b) cocaine in the rat.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Avgustinovich DF, Lipina TV, Bondar NP, Alekseyenko OV, Kudryavtseva NN. Features of the genetically defined anxiety in mice. Behav Genet. 2000;30:101–9. doi: 10.1023/a:1001999020138. [DOI] [PubMed] [Google Scholar]
- 2.Crawley JN, Belknap JK, Collins A, et al. Behavioral phenotypes of inbred mouse strains: Implications and recommendations for molecular studies. Psychopharmacology. 1997;132:107–24. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 3.Crawley JN, Paylor R. A proposed test battery and constellations of specific behavioral paradigms to investigate the behavioral phenotypes of transgenic and knockout mice. Horm Behav. 1997;31:197–211. doi: 10.1006/hbeh.1997.1382. [DOI] [PubMed] [Google Scholar]
- 4.Moy SS, Nadler JJ, Young NB, et al. Mouse behavioral tasks relevant to autism: Phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrante RJ, Andreassen OA, Jenkins BG, et al. Neuroprotective effects of creatine in a transgenic mouse model of Huntington’s disease. J Neurosci. 2000;20:4389–97. doi: 10.1523/JNEUROSCI.20-12-04389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter AJ, Hatcher J, Virley D, et al. Functional assessments in mice and rats after focal stroke. Neuropharmacology. 2000;39:806–16. doi: 10.1016/s0028-3908(99)00262-2. [DOI] [PubMed] [Google Scholar]
- 7.Goulet S, Dore FY, Mirault ME. Neurobehavioral changes in mice chronically exposed to methylmercury during fetal and early postnatal development. Neurotoxicol Teratol. 2003;25:335–47. doi: 10.1016/s0892-0362(03)00007-2. [DOI] [PubMed] [Google Scholar]
- 8.Bradford AM, Savage KM, Jones DN, Kalinichev M. Validation and pharmacological characterisation of MK-801-induced locomotor hyperactivity in BALB/C mice as an assay for detection of novel antipsychotics. Psychopharmacology. 2010;212:155–70. doi: 10.1007/s00213-010-1938-0. [DOI] [PubMed] [Google Scholar]
- 9.Quinn LP, Grundy RI, Campbell CA, et al. A novel behavioural registration system LABORAS and the social interaction paradigm detect long-term functional deficits following middle cerebral artery occlusion in the rat. Brain Res. 2005;1031:118–24. doi: 10.1016/j.brainres.2004.10.036. [DOI] [PubMed] [Google Scholar]
- 10.Quinn LP, Stean TO, Chapman H, et al. Further validation of LABORAS using various dopaminergic manipulations in mice including MPTP-induced nigrostriatal degeneration. J Neurosci Methods. 2006;156:218–27. doi: 10.1016/j.jneumeth.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Quinn LP, Stean TO, Trail B, et al. LABORAS: Initial pharmacological validation of a system allowing continuous monitoring of laboratory rodent behaviour. J Neurosci Methods. 2003;130:83–92. doi: 10.1016/s0165-0270(03)00227-9. [DOI] [PubMed] [Google Scholar]
- 12.Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- 13.Spink AJ, Tegelenbosch RA, Buma MO, Noldus LP. The EthoVision video tracking system: A tool for behavioral phenotyping of transgenic mice. Physiol Behav. 2001;73:731–44. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- 14.Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA. 2008;105(20):575–82. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang J, Wang Y, Krueger JM. Mice lacking the TNF 55 kDa receptor fail to sleep more after TNFalpha treatment. J Neurosci. 1997;17:5949–55. doi: 10.1523/JNEUROSCI.17-15-05949.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan Z, Vgontzas AN, Bixler EO, Fang J. Sleep is increased by weight gain and decreased by weight loss in mice. Sleep. 2008;31:627–33. doi: 10.1093/sleep/31.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grisar T, Lakaye B, Thomas E. Molecular basis of neuronal bio-rhythms and paroxysms. Arch Physiol Biochem. 1996;104:770–4. doi: 10.1076/apab.104.6.770.12915. [DOI] [PubMed] [Google Scholar]
- 18.Flores AE, Flores JE, Deshpande H, et al. Pattern recognition of sleep in rodents using piezoelectric signals generated by gross body movements. IEEE Trans Biomed Eng. 2007;54:225–33. doi: 10.1109/TBME.2006.886938. [DOI] [PubMed] [Google Scholar]
- 19.Donohue KD, Medonza DC, Crane ER, O’Hara BF. Assessment of a non-invasive high-throughput classifier for behaviours associated with sleep and wake in mice. Biomed Eng Online. 2008;7:14. doi: 10.1186/1475-925X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–8. doi: 10.1152/physiolgenomics.00139.2006. [DOI] [PubMed] [Google Scholar]
- 21.Ganea K, Liebl C, Sterlemann V, Muller MB, Schmidt MV. Pharmacological validation of a novel home cage activity counter in mice. J Neurosci Methods. 2007;162:180–6. doi: 10.1016/j.jneumeth.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Tang X, Orchard SM, Sanford LD. Home cage activity and behavioral performance in inbred and hybrid mice. Behav Brain Res. 2002;136:555–69. doi: 10.1016/s0166-4328(02)00228-0. [DOI] [PubMed] [Google Scholar]
- 23.Zaveri NT, Jiang F, Olsen CM, et al. A novel series of piperidin-4-yl-1,3-dihydroindol-2-ones as agonist and antagonist ligands at the nociceptin receptor. J Med Chem. 2004;47:2973–6. doi: 10.1021/jm034249d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould TD. Mood and Anxiety Related Phenotypes in Mice: Characterization Using Behavioral Tests. Humana Press; New York: 2009. [Google Scholar]
- 25.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10:248–58. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 26.Nadler JJ, Moy SS, Dold G, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–14. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 27.Xiong X, Barreto GE, Xu L, Ouyang YB, Xie X, Giffard RG. Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke. 2011;42:2026–32. doi: 10.1161/STROKEAHA.110.593772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter ME, de Lecea L. Optogenetic investigation of neural circuits in vivo. Trends Mol Med. 2011;17:197–206. doi: 10.1016/j.molmed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter ME, Yizhar O, Chikahisa S, et al. Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci. 2010;13:1526–33. doi: 10.1038/nn.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.