Abstract
Background
Serotonergic mechanisms are associated with the development of alcohol dependence (AD), however, studies evaluating serotonergic medications have produced conflicting results. One hypothesis suggests that differential response may be due to a functional polymorphism of the 5-HTTLPR promoter region of the serotonin re-uptake transporter (5-HTT). The L/L genotype is postulated to be associated with early onset alcoholism and the S/S or S/L genotypes associated with late onset alcoholism. The aim of this study was to match and mismatch L/L, S/S, or S/L genotypes with administration of ondansetron and sertraline.
Methods
Fifteen nontreatment seeking alcohol-dependent individuals were randomized to 1 of 2 counterbalanced arms to receive either 200 mg/d of sertraline or ondansetron 0.5 mg/d for 3 weeks followed by an alcohol self-administration experiment (ASAE), then received placebo for 3 weeks followed by a second ASAE. Participants then received the alternate drug for 2 weeks followed by a third ASAE.
Results
At the first ASAE compared to sertraline, ondansetron significantly improved drinking outcomes for the L/L genotype for the ASAE volume consumed (100% reduction based on between-subjects comparison, t = 2.35), and for drinks per drinking day during the 7 days prior to the ASAE (79% reduction and t = 4.34). Compared with ondansetron for S/S or S/L genotypes, outcomes at ASAE 1 for sertraline and S/S or S/L genotypes are opposite than hypothesized. Overall, subjects reduced drinking across their participation in the trial, as there appears to be an order effect.
Conclusion
This study suggests that ondansetron may reduce alcohol consumption in alcohol-dependent individuals who have the L/L genotype as measured naturalistically and during the ASAE. By contrast there was no support that sertraline reduces alcohol use in individuals who have S/S or S/L genotypes. Evidence in the literature suggests that AD in some individuals may be influenced by a gene by socio-environmental interaction making pharmacological treatment with serotonergic drugs complex. Research must consider that typologies may predict successful treatment of AD in future trials.
Keywords: Alcoholism, Ondansetron, Sertraline, Genotypes, 5-HTTLPR, Early onset alcoholism, Late onset alcoholism, L/L, S/S, S/L
Numerous animal and human studies suggest that serotonergic mechanisms are associated with the development and maintenance of alcohol dependence (AD) (Naranjo et al., 2002). However, results evaluating serotonergic medications, such as serotonin specific re-uptake inhibitors (SSRIs) or the 5-HT3 antagonist ondansetron, in heterogeneous populations of alcohol-dependent individuals, have been inconclusive (Johnson et al., 2000; Kranzler et al., 1995; Pettinati et al., 2001). There is evidence that the serotonin transporter gene (5-HTTLPR) is associated with alcoholism (Matsushita et al., 2001) and subtypes of alcoholism (Parsian and Cloninger, 2001). Further, some have proposed to delineate the clinical response based on genetic components linked to serotonin (5-HT) or serotonin transporter (5-HTT) metabolism, in order to differentiate clinical subtypes who may have common genetic and or psychosocial backgrounds with AD (Johnson, 2000).
The 5-HTT is responsible for 5-HT re-uptake into presynaptic neurons and regulates the concentration of 5-HT in the synaptic cleft. There are two 5-HTT alleles designated as long (L) and short (S) (Heils et al., 1996), that result in the biallelic genotypes: L/L, S/S, and S/L. The L allele has a higher transcriptional activity in vitro compared with SS homozygotes (Heils et al., 1996; Lesch et al., 1996). A difference in the distribution of these alleles is proposed to result in the variation of the rate of removal of 5-HT (Heils et al.), and the variation is putatively associated with alcoholic psychopathology (Johnson, 2000). Johnson et al. (2008), for example, demonstrated that 5-HTT expression varies with current and lifetime alcohol consumption in people with the L/L genotype but not S/S or S/L genotypes.
A univariate approach to subtyping suggests that individuals who develop alcoholism after the age of 25 years [late-onset alcoholism (LOA)] may have comparatively normal 5-HT3 functioning due to possessing S/S or S/L, 5-HTT genotypes (Johnson, 2000; Johnson and Ait-Daoud, 2000), though it has been suggested the S allele may actually influence the risk of relapse in abstinent alcohol-dependent subjects (Pinto et al., 2008). By contrast, patients who develop alcoholism before and including the age of 25 years of age [early-onset alcoholism (EOA)], may have reduced 5-HT function resulting in 5-HT3 receptor up-regulation as a compensatory mechanism for low basal levels of intersynaptic 5-HT (Johnson, 2000). When alcohol is consumed, the up-regulation of these receptors is hypothesized to result in an increase in dopamine release in the nucleus accumbens (Hammoumi et al., 1999). Additional evidence also suggests that the L/L genotype of the 5-HTTLPR polymorphism may be more predominant among EOA individuals where family history of alcoholism is thought to be more prevalent (Schuckit et al., 1999). Furthermore, a significant association between the L/L genotype and compulsive alcohol craving has also been reported by alcoholic patients (Bleich et al., 2007). Clinically, there is evidence that a 5-HT3 antagonist such as ondansetron, may be a possible treatment for AD in EOAs but not LOAs (Johnson et al., 2000), and is hypothesized to be able to compensate for 5-HT3 up-regulation associated with the L/L 5-HTT genotype (Johnson, 2000).
A complementary mechanism to the 5-HT3 pathway is proposed for LOAs who putatively have the predominant S/S or S/L genotypes (Johnson, 2000). Theoretically, treatment with an SSRI such as sertraline, facilitates 5-HT transmission and inhibition of dopamine (Pettinati et al., 2000). In the LOA subtype, this is thought to result in an attenuation of reward during acute alcohol consumption. Alternatively however, SSRI administration to the EOA alcohol-dependent individuals with a L/L genotype may be relatively unaffected by the already increased intersynaptic 5-HT levels. This may actually initiate alcohol consumption from a resultant low dopamine level (Johnson et al., 2000).
Results of clinical trials for AD using SSRIs and ondansetron have been disappointing particularly for the main heterogeneous groups of alcoholics (Johnson et al., 2000; Kranzler et al., 1995; Pettinati et al., 2000). On the other hand, further statistical analyses finds that compared with placebo, certain subtypes may respond to treatment based in part on multivariate (Babor et al., 1992) or univariate (Johnson et al., 2000) subtyping, and that some alcohol-dependent subtypes may have an underlying genetic component (Cloninger et al., 1981; Johnson, 2000).
Well studied multivariate typology models include the Cloninger (Cloninger et al., 1981) and Babor (Babor et al., 1992) models that are both characterized by 2 subtypes. The Cloninger subtypes are delineated into one with a socio-environmental-late onset component (Cloninger Type 1), and the other with a genetically based–early onset component (Cloninger Type 2). Recent research suggests there to be 5-HT dysregulation in the hypothalamus and the amydala of Cloninger Type 2 alcoholics (Storvik et al., 2008). Given that epidemiologic studies demonstrate AD to be 50 to 60% heritable (Enoch and Goldman, 1999; Prescott and Kendler, 1999), the prospect that outcomes to drug therapy are at least partly dependent on genetic predisposition in some alcohol-dependent individuals should be considered when discussing alcoholic subtypes. Babor Type B alcoholics have a more severe AD, an earlier onset of alcoholism, more childhood risk factors, greater psychopathology and sociopathy than Babor Type A alcoholics, who by comparison have relatively low vulnerability, low severity-late onset, and are uncomplicated in their alcohol use history.
With respect to differential typological response to SSRIs, Kranzler and colleagues (1995) demonstrated that using 60 mg of fluoxetine was no better than placebo in reducing alcohol consumption. However, further statistical examination of the sample into subtypes as proposed by Babor and colleagues (1992), revealed that for the patients treated with fluoxetine, the less severe Type A patients did not reduce their alcohol consumption, however, compared with the Type A subtype group, the Type B subtype patients actually increased their alcohol consumption.
In a double-blind placebo controlled study conducted by Pettinati and colleagues (2000, 2001), either sertraline or placebo was administered to alcohol-dependent individuals randomized by presence or absence of lifetime depression. Treatment with sertraline was most effective in alcohol-dependent individuals who were never depressed (Pettinati et al., 2001). More relevant, however, analyses demonstrated that Type B alcoholics treated with sertraline reported more drinking days compared with those taking placebo, however, sertra-line treatment in Type A alcoholics was associated with fewer drinking days and a greater likelihood of sustained abstinence.
Another serotonergic medication, ondansetron, has been found to be more effective for a univariate subtype of alcohol-dependent individuals (Johnson et al., 2000; Sellers et al., 1992, 1994). More specifically, ondansetron appears to be more effective for the EOA, but not the LOA subgroup. In a clinical trial that stratified these subtypes, ondansetron increased the number and percentage of days abstinent in EOAs but not LOAs (Johnson et al., 2000).
As noted, one hypothesis for the differential clinical response to these serotonergic medications, suggests that alcoholics may have a genetic predisposition based on a dysregulation of serotonergic function associated with 5-HTT function (Johnson, 2000). Further, the polymorphic repeat polymorphism of the 5-HTT, the 5-HTTLPR, may be at least in part, potentially involved with the differential response of ondansetron and sertraline in subtypes of alcohol-dependent individuals.
One way to test this hypothesis is to assess alcohol consumption when genotyped individuals use both ondansetron and sertraline. Delineating the research to clarify the contribution of the 5-HTTLPR polymorphism to AD is essential to better target alcohol-dependent patients in clinical practice. In particular, understanding the differential effects of SSRIs is clinically relevant as significant numbers of alcohol-dependent persons are prescribed SSRIs by practitioners without regard to subtype, and such treatment may actually be detrimental to some individuals (Croop et al., 1995; Kranzler et al., 1995).
Utilizing genotypes to match and mismatch individuals in the proposed medication cells therefore allowed our group to examine 2 important goals: (1) to evaluate the efficacy of ondansetron for reducing drinking in participants who carry the L/L genotype of the 5-HTTLPR polymorphism by hypothesizing that carriers of the L/L genotype receiving ondansetron compared to either placebo or sertraline, would result in a significant reduction in alcohol consumption as measured by (a) the Timeline Follow-Back (TLFB; Sobell and Sobell, 1992); and (b) alcohol consumed during 3 alcohol self-administration experiments (ASAEs); and (2) to evaluate the efficacy of sertraline for reducing drinking in participants who carry either the S/L or S/S genotypes of the 5-HTTLPR polymorphism, by hypothesizing that alcohol-dependent people with the S/L and S/S genotypes receiving sertraline compared with either placebo or ondansetron, would result in a significant reduction in alcohol consumption as measured by the TLFB and the 3 ASAEs.
METHODS
Subjects
The present sample was recruited with local newspapers in the Providence, RI, area. The study was approved by the Institutional Review Boards of Brown University, Roger Williams Medical Center and the Veterans Administration Medical Center. The study participants were diagnosed as alcohol-dependent as assessed by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders–Text Revision (DSM IV-TR; American Psychiatric Association, 2000), were drinking ≥35 standard drinks/week for men or ≥28 standard drinks/week for women, and were not seeking treatment for AD. Written informed consent was obtained from each individual prior to beginning the study.
Study Design
The experimental study design was a placebo-controlled mixed 2-factor design in 21 consented individuals in which the biallelic 5-HTTLPR alleles (L/L, S/S and S/L) were crossed with the medication conditions (within subjects factor). The researchers and participants were blinded to the medication conditions. All participants received 3 weeks of an active medication, followed by 3 weeks of placebo, followed by 3 weeks of the other active medication. Baseline drinking was statistically controlled for. The order of the active medications was controlled for by randomly assigning half the subjects to receive 200 mg a day of sertraline for the first active medication phase, and the other half to receive 0.5 mg a day of ondansetron for the first medication phase. The proportion of genotypes in this population occurs in roughly 35% for L/L, 49% for S/L, and 16% for S/S in the alcoholic population (Parsian and Cloninger, 2001). Twenty cases had valid allele data with 9 individuals with the L/L genotype and 11 individuals with the S/S or S/L genotypes.
Procedure
An ASAE (see Anton et al., 2004; Drobes et al., 2003; O’Malley et al., 2002) was conducted at the end of each of the 3 medication phases. In brief, the procedure involves administration of a priming drink which must be consumed. The volume of alcohol for all drinks was adjusted for gender, body mass and age (Watson, 1989). Subsequently, individuals were offered 2 trays of 4 drinks, each tray followed by a 45-minute drinking period. As an alternative reinforcement, participants may have received $3.00 for each drink they refused.
Unknown to the participants, the second medication phase was always the placebo condition, however, the bias to treat subjects differently by the study staff was minimized during this period. Although experimenter bias for this condition could not be completely ruled out, our same experienced staff (utilized throughout the study) attempted to minimize such bias by strictly adhering to the ASAE protocol. The single-blind placebo condition not only served as a medication wash out period, but also served as a self-comparison-dependent measure for the active conditions. The main dependent measures for this study was alcohol consumption as measured by the TLFB during the 7-day period leading up to each ASAE, and total milliliters consumed during each ASAE. The TLFB is a common method of assessing naturalistic alcohol consumption that uses a calendar and standardizes drinks, to establish an individual’s consumption over a given period.
The study was conducted in consecutive phases with volunteers in both arms taking the same number of doses a day throughout the study: (1) a 1-week screening period; (2) a 21-day treatment period consisting of a 9-day titration up period for those receiving sertraline and a minimum of 12-days (±3 days) at the target dose; (3) an ASAE on the last day of treatment (±3 days) at the target dose; (4) a 21-day placebo-controlled period; (5) a second ASAE on the last day of placebo (±3 days); (6) a 9-day titration up period for those receiving sertraline last; (7) a minimum of 12 days (±3 days) at the target dose; (8) an ASAE on the last day of treatment at the target dose (±3 days). Sertraline was started at 50 mg for 3 days and increased 50 mg every 3 days to a maximum dose of 200 mg (Pettinati et al., 2000). Whether ondansetron was administered first or last, there were no special dosing considerations required for the dose of 0.25 mg twice a day (total 0.5 mg/d) given for 21 days. Participants remained eligible and included in the analyses as long as they exceed thresholds of 100 mg per day of sertraline and/or 0.25 mg of ondansetron (50% of the maximum target dose). All participants received an interview with the same trained staff who focused on drug side-effects and adherence.
After assessments and labs, participants were administered alcohol beginning at noon (to provide as much time as possible since a last drink). Subjects were presented with a priming drink designed to raise blood alcohol levels (BALs) to 0.03 g/dl and were instructed to consume it within 10 minutes. The volume of the alcohol was adjusted using a formula based on gender, age and body mass (Watson, 1989). The priming dose of alcohol was used to model relapse and assess the influence of abstinence violation on future alcohol consumption. Consistent with previously noted lab studies (Drobes et al., 2003; O’Malley et al., 2002), we waited 50 minutes before presenting more alcohol. Following the priming drink (controlled for in the analyses), each participant received a tab for 2 drinking sessions of 4 drinks, at $3.00 per drink (a maximum of $24), which was provided as an alternative reinforcement for not drinking and intended to approximate the cost of a drink at a bar. The inclusion of the alternate reinforcer in this study with nontreatment seeking alcoholics is considered important as a sensitive test of the value of alcohol (O’Malley et al., 2002). Participants could choose not to drink and take the $3.00 instead for each drink not touched. Each beverage was calculated to raise the BAL by 0.015 g/dl. If the participant’s blood reached 0.1% at any time, then the alcohol consumption period was stopped. After the 90 minute self-administration period, subjects received dinner and waited until their breath alcohol concentration (BrAC) was = 0.00% and they were then brought home by taxi.
Screening assessments such as the physical, the Structured Clinical Instrument for the DSM IV-TR (Ventura et al., 1998), family history of alcohol, genotyping, and age of onset of alcoholism were only done at the start of the study. Clinical and psychological assessments were conducted at baseline, weekly throughout the study, and during a 1-month follow-up visit. Alcohol consumption and medication assessment measures were performed weekly and at follow-up. Pregnancy tests were performed at screening and immediately before alcohol administration in women of child bearing potential.
Inclusion Criteria
Participants were between 21 and 65 years old (inclusive), male or female, and in good health as confirmed by medical history, baseline physical examination, electrocardiogram (ECG), laboratory tests, urinalysis, and vital signs. Female participants were: postmenopausal for at least 1 year, surgically sterile, or practicing an effective method of birth control before entry and throughout the study and had a negative urine pregnancy test at baseline screening and prior to the alcohol challenge sessions. Participants understood that this was not a treatment study; a diagnosis of AD as defined by the DSM-IV-TR (2000). Alcohol dependence as defined by an Alcohol Use Disorders Identification Test (AUDIT) (Bohn et al., 1995) score ≥12 and men must consume ≥35 and women ≥28 standardized alcoholic beverages a week. Participants must have been willing to take oral medication, adhere to the medication regimen, and willing to return for weekly visits and the alcohol challenge sessions. Participants had to be able to read and comprehend written instructions and comprehend and complete all scale and inventories required by the protocol; must have signed an informed consent indicating they understand the purpose of and procedures required for the study and willingness to participate, and had a breath alcohol concentration (BrAC) = 0.000 at the beginning of the alcohol challenge sessions.
Exclusion Criteria
Excluded were individuals with Axis I DSM-IV diagnoses other than alcohol and nicotine dependence; pregnancy or breast-feeding women; positive urine drug screen at baseline for any illegal substance other than caffeine, nicotine, or marijuana; participants were excluded if they had: (a) clinically significant medical abnormalities (i.e. ECG, hematological assessment, bilirubin >150% of the upper limit of normal or ALT or AST elevations >300% the upper limit of normal, biochemistry including urinalysis, electrolytes); medical contraindications for use of sertraline or ondansetron; taking drugs that interfered with the metabolism of either drug that could not be stopped per study physician; allergy to sertraline or ondansetron; individuals with a reasonable expectation of being institutionalized during the course of the trial or pending legal charges; participants who had significant alcohol withdrawal symptoms, Clinical Institute Withdrawal Assessment for Alcohol (CIWA-Ar) revised score >10 (Sullivan et al., 1989); lifetime depression or a history of suicide; history of seizures (e.g. epilepsy) or migraine headaches; or current use of psychotropic medications that could not be discontinued. Persons with medical conditions that were adequately controlled by their primary care physician were not excluded.
Genotyping
Genomic deoxyribonucleic acid (DNA) was isolated from buccal cells using a modification of published methods (Freeman et al., 1997; Lench et al.,1988; Meulenbelt et al., 1995; Sander et al., 1997a,b; Spitz et al., 1996). The cheeks and gums were rubbed for 20 seconds with 3 sterile, cotton-tipped wooden swabs. The analysis was performed at the Veterans Memorial Hospital in Providence, RI. The swabs were placed in a 50-ml capped polypropylene tube containing lysis buffer [500 μl of 1 M Tris–HCl; 200 mM disodium ethylene diaminetetracetic acid (EDTA), pH 8.0; 500 μl of 10% sodium docecyl sulfate; and 100 μl of 5 M sodium chloride]. The subjects then rinsed out their mouth vigorously with 10 ml of bottled water for 20 seconds, and added to the 50-ml tube. The tubes were stored at 4 °C until the DNA was extracted, usually within 48 h. The 5-HTTLPR polymorphism was assayed using a modification of the method of Lesch and colleagues (1996). To each of the tubes, 100 μl of proteinase potassium solution (20 mg/ml) and 100 μl of 5 M sodium chloride will be added. The tubes are incubated at 65 °C for 60 min. Residual lysis buffer is removed from the saturated swabs by centrifugation for 5 min at 1000 rpm (3500 g), and the collected buffer is added back to the original 50-ml collection tube. An equal volume of 100% isopropyl alcohol was then added to each tube to precipitate the DNA, which was collected by centrifugation at 3500 × g for 10 min at room temperature. The liquid was decanted, and the DNA pellet was washed with 1 ml of fresh 50% isopropyl alcohol. After drying at 65 °C, the pellet was resuspended in 1 ml of 20 mM Tris–EDTA, pH 8.0. The yield of DNA was quantified by absorbance at 260 nm [1 optical density unit (O.D.) = 50 μg/ml], and an aliquot was diluted to a concentration of ≤20 ng/ml for a working sample. The primer sequences are as follows: forward, 5′-GGCGTTGCCGCTCTGAATGC-3′ (fluorescently labeled), and reverse, 5′-GAGGGACTGAGCTGGACAACCAC-3′. These primer sequences yield products of 484 or 528 bp. The average yield of DNA in our studies is 40 ± 2 μg. Bands were visualized by ethidium bromide staining under UV illumination.
RESULTS
Twenty-one participants volunteered for the study and provided informed consent. Valid allele datum was collected on 20 participants. Nineteen cases had valid datum on order of medication. Seventeen cases had valid datum for drinks per drinking day (DDD) for the week prior to the first ASAE, and 15 cases had valid data for the third ASAE and DDD for the week prior to the third ASAE. The resulting sample of 15 was used in the statistical analyses. Demographics are shown in Table 1.
Table 1.
Baseline Addiction and Demographic Data of the Final Sample (N = 15)
| Drinks per drinking day (DDD) for the 28-day baseline period | |
| Range | 4.85–25.39 |
| Median | 10.80 |
| Mean (SD) | 12.72 (6.56) |
| Gender n (%) | |
| Males | 12 (80.0) |
| Females | 3 (20.0) |
| Age | |
| Range | 24–57 yrs |
| Median | 44 yrs |
| Mean (SD) | 44.1 (9.5) yrs |
| Education n (%) | |
| High school diploma or GED | 12 (80.0) |
| College degree | 3 (20.0) |
| Employment n (%) | |
| Working full-time | 6 (40.0) |
| Working part-time | 3 (20.0) |
| Retired | 3 (20.0) |
| Unemployed or receiving disability benefits | 3 (20.0) |
| Marital status n (%) | |
| Single (and never married) | 7 (46.7) |
| Divorced | 5 (33.3) |
| Married or cohabiting | 3 (20.0) |
Volume (as measured in milliliters) during the second ASAE had a kurtosis of 4, therefore the 3 volume variables were transformed using a square root transformation. Drinks per drinking day at the 3 time points sufficiently approximated the normal distribution.
There were 4 potential treatment conditions: o(order) g(gene)11 (order of drug administration was first ondansetron, placebo, and then sertraline for those with the L/L genotype), og12 (order of drug administration was ondansetron, placebo, and then sertraline for those with the SS/SL genotype), og21 (order of drug administration was first sertraline, placebo, and then ondansetron for those with the L/L genotype), and og22 (order of drug administration was first sertraline, placebo, and then ondansetron for those with the SS/SL genotype).
In regard to genes and randomization, the 4 cells were well-balanced (n of 3 = og11; 4 = og12; 4 = og21; and 4 = og22). Women were evenly distributed across the 4 cells. There was 1 woman in each of 3 cells [χ2(3,N = 15) = 1.46, p = 0.69]. There was a trend for baseline drinking to be related to gender [t(10.98) = 1.99, p = 0.07]. Overall, the 2 groups were not equivalent on baseline DDD [t(9.89) = 1.91, p = 0.086]. More specifically, for L/L, the 2 groups were equivalent on baseline DDD [t(3.20) = 0.68, p = 0.68], but this was not the case for the groups with SS/SL [t(3.32) = 2.33, p = 0.093].
To test the study hypotheses, 2 separate repeated measure analyses of covariance were conducted for each genetic group. For L/L, with DDD as the outcome variable, medication condition as the factor, and baseline DDD and gender as covariates, there was a significant medication effect F(2,10.70) = 26.5, p < 0.001. When order of medication was entered as a covariate, this medication effect was nonsignificant for DDD, F(2,15.37) = 0.57, p = 0.58. A similar scenario occurred with volume consumed during the ASAE for L/L, F(2,10.80) = 4.96, p = 0.03, but not when order of medication was entered as a covariate for the ASAE, F(2,16.88) = 0.87, p = 0.44. For the other genetic group (S/S and S/L) there was no medication effect whether or not order was entered as a covariate (see Fig. 1).
Fig. 1.
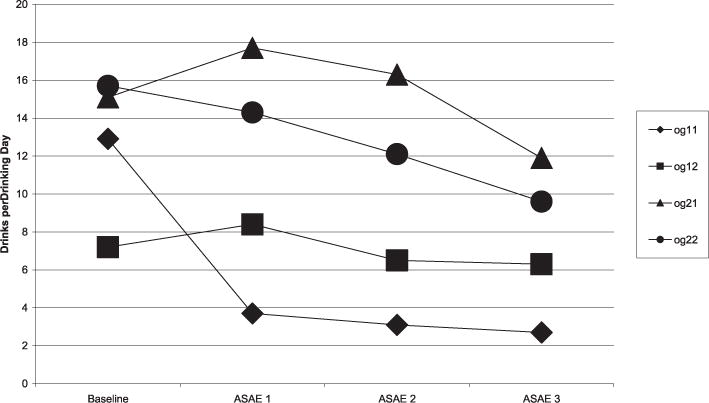
Drinks per drinking day by order and alleles 7-days prior to alcohol self-administration experiments. ◆: og11 (order 1 gene 1)—ondansetron, then placebo, then sertraline; L/L genotype, ■: og12 (order 1 gene 2)—ondansetron, then placebo, then sertraline; SS/SL genotype, ▲: og21 (order 2 gene 1)—sertraline, placebo, then ondansetron; L/L genotype, ●: og22 (order 2 gene 2)—sertraline, placebo, ondansetron; SS/SL genotype.
At ASAE 1 specifically, for those with the L/L alleles there was a medication effect on both dependent measures: Ondansetron (n = 3), DDD mean (SD) = 3.66 (2.40), sertraline (n = 4) 17.69 (5.11), [t(5) = 4.34, p = 0.007]; Ondansetron (n = 3) volume mean in milliliters (SD) = 0 (0), sertraline (n = 4) 81.8 (71.1), [t(5) = 2.35, p = 0.07]. Although not hypothesized, at ASAE 1, subjects with the L/L alleles also demonstrated fewer DDD than subjects with the S/S or S/L, when taking ondansetron L/L (n = 3) DDD mean (SD) 3.66 (2.40), SS/SL (n = 4) 8.40 (1.38), [t(5) = 3.35, p = 0.02] (see Fig. 2).
Fig. 2.
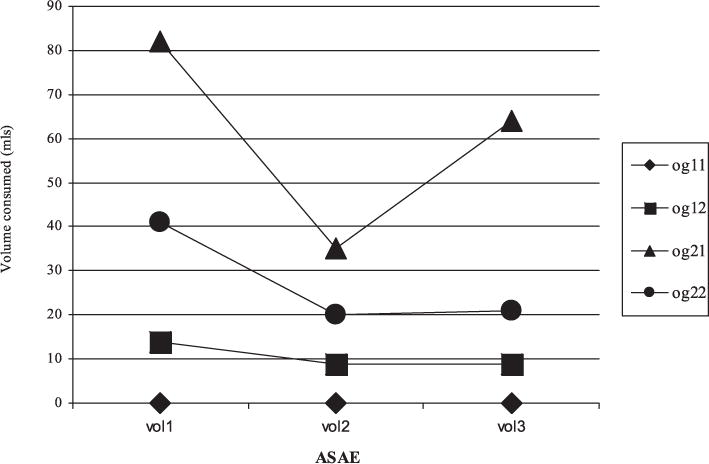
ASAE order by alleles. ◆: og11 (order 1 gene 1)—ondansetron, then placebo, then sertraline; L/L genotype, ■: og12 (order 1 gene 2)—ondansetron, then placebo, then sertraline; SS/SL genotype, ▲: og21 (order 2 gene 1)—sertraline, placebo, then ondansetron; L/L genotype, ●: og22 (order 2 gene 2)—sertraline, placebo, ondansetron; SS/SL genotype.
The careful measurement of the amount of alcohol consumed during controlled environmental conditions (the ASAE) is one of the strengths of this study. Regarding the validity of self-report drinking data outside of the research setting, Gamma glutamyl-transferase (GGT) was assessed at 4 time points and was found to be correlated with DDD the week prior to the first ASAE in particular [r(13) = 0.63, p = 0.02] lending some support to the reliablity of the Time-line Followback data. (The correlations at baseline, ASAE 2, and ASAE 3, between concomitant GGT and DDD were 0.23, 0.22, and 0.04, respectively.)
A chi-square with onset and allele conditions revealed 86% of the L/L group was EOA compared with 63% of the SS/SL group [χ2(1,N = 15) = 1.03, p = 0.31]. Using the age of onset to split the groups rather than the allele, the results were similar, but less pronounced. For late onset, n = 4, and all received the medication in the same order. For those with EOA (n = 11), though not supported by the overall F-test, F(2,23.82) = 0.28, p = 0.76 at ASAE 1 there was partial support for a medication effect on DDD, ondansetron (n = 3), DDD mean (SD) = 5.75 (1.85), sertraline (n = 8) 16.00 (6.50), [t(8.90) = 4.05, p = 0.003]; a parallel, ASAE volume difference became less pronounced, ondansetron (n = 3) volume mean (SD) = 11.8 mls (20.44), sertraline (n = 8) 61.6 mls (60.54), [t(9) = 1.36, p = 0.21].
Instead of reporting the power, we report (d) the effect size (see Table 2). In addition to reporting the results for the medication comparisons at ASAE 1 for L/L participants, we report the complement of results for SS/SL participants. Means and SDs are reported for transformed volume, since these are the values used to calculate t, p, and d values.
Table 2.
Statistical Results for Medication Comparisons at ASAE 1
| Ondansetron
|
Sertraline
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allele | dv | n | M(SD) | n | M(SD) | df | t | p | d |
| L/L | DDD | 3 | 3.66(2.40) | 4 | 17.69(5.11) | 5 | 4.34 | 0.007 | 4.83 |
| sqrt of vol | 3 | 0.00(0.00) | 4 | 7.67(5.53) | 5 | 2.35 | 0.07 | 2.77 | |
| vol | 3 | 0.00(0.00) | 4 | 81.8(71.1) | |||||
| SS/SL | DDD | 4 | 8.40(1.38) | 4 | 14.31(8.05) | 3.2 | 1.45 | 0.239 | 1.02 |
| sqrt of vol | 4 | 2.60(3.06) | 4 | 5.23(4.33) | 6 | 0.99 | 0.360 | 0.70 | |
| Vol | 4 | 13.8(17.1) | 4 | 41.4(49.0) | – | – | – | – | |
dv, dependent variable; df, degrees of freedom; M, mean; SD, standard deviation; d, effect size; n, number; sqrt, squareroot; vol, volume; DDD, drinks per drinking day; L/L, homozygous long 5-HTTLPR alleles; SS, homozygous short 5-HTTLPR alleles; SL, heterozygous short-long 5-HTTLPR alleles.
DISCUSSION
The aim of this pilot study was to evaluate the potential for ondansetron to reduce drinking in nontreatment seeking alcohol-dependent participants who carry the L/L genotype and sertraline for reducing drinking in participants who carry the S/S and S/L genotypes. Our results suggest that at the first ASAE, there is support for ondansetron improving drinking outcomes in nontreatment seeking volunteers who were the L/L genotype, compared with those with the L/L genotype receiving sertraline who actually slightly increased their drinking. The positive effect of ondansetron in our alcoholic subjects with L/L genotype is consistent with results demonstrating the efficacy of ondansetron in treatment seeking AD subjects (Johnson et al., 2000). Furthermore, this is consistent with recent neuroimaging data showing the ability of ondansetron to reduce alcohol cue-induced activation of the ventral striatum (Myrick et al., 2008). This is true for the ASAE volume consumed (100% reduction and t = 2.35), and especially pronounced for DDD naturalistically measured using the TLFB during the 7 days prior to the ASAE (79% reduction and t = 4.34). The similarity of findings for the 2 dependent measures bolsters the validity of the ASAE methodology.
Outcomes at the first ASAE for S/S and S/L genotypes receiving sertraline are in the opposite direction than hypothesized (compared with S/S and S/L genotypes who received ondansetron). As a result the study provides little support for the use of sertraline by individuals who carry the S/S and S/L genotypes who actually reported drinking 14.2 DDD compared to S/S and S/L subjects who received ondansetron and drank only 8.4 DDD.
Overall, subjects reduced drinking across their participation in the trial as there appears to be a very pronounced order effect. Though these participants are not seeking treatment for their AD, perhaps given the opportunity to receive a pharmacological treatment, they may be more motivated to reduce their drinking as the trial progressed. Possibly, adding a measure of motivation to change drinking behavior at baseline and 1 week after each of the ASAEs, may be useful for delineating this hypothesis in the future. Additionally, the order effect may be a function of repeated administrations of the TLFB. Initially, there may be a novelty effect for the first ASAE (the “all you can drink” effect). Participants may not be used to an “open bar,” but subsequently may be embarrassed about consuming so much in a research trial, so the second ASAE volume is lower than the first. We considered performing a baseline ASAE but felt that the study design was valid as performed using each person as their own control. Nonetheless, perhaps assessing the TLFB less frequently (e.g., at baseline and then at the ASAE sessions rather than every week) to maintain a continuous calendar of drinking data, may reduce a hypothesized reactivity to this assessment. Finally, the order effect may also be a function of medication effects from ASAE 1 persisting throughout the whole study, or even a function of social desirability (Crowne and Marlowe, 1960).
Current evidence suggests that having the L/L genotype results in reduced intrasynaptic 5-HT due to higher levels of 5-HTT expression, resulting in more efficient 5-HT transport from the synapse per unit of time (Heils et al., 1996). People with the L/L genotype have lower synaptic 5-HT levels and may demonstrate a greater impulse to consume alcohol compared with people with the S/S or S/L genotype (Johnson, 2000). Consistent with previous findings that reported reduced 5-HT re-uptake in a sample of AD individuals with the L/L genotype (Javors et al., 2005), Johnson et al. (2008) reported that subjects with the L-allele demonstrated a reduced paroxetine binding capacity and reduced functional 5-HT uptake compared with S/S genotype. Furthermore, they found relationships between alcohol use and 5-HT platelet parameters, but only in people with the L/L genotype. Additionally, it was reported that current heavier drinking was associated with increased 5-HT re-uptake but reduced paroxetine binding in the majority of people in the study who had LL alleles. In sum, the researchers suggest that alcohol’s effect on 5-HTT genetic expression differentially affects persons with the L/L, but not S/S genotype (Johnson et al., 2008).
By contrast, it is of interest that there is little effect on alcohol preference in primates in young adulthood reared under optimal conditions compared with alcohol preference and consumption in primates with the 5-HTTLPR S/L genotype reared under stressful conditions (Barr et al., 2004). Studies in humans also demonstrate that 5-HT is an important modulator of the stress response (Goldman et al., 2005) and depression (Caspi et al., 2003). Moreover, strong evidence suggests that AD is associated with dysregulation of 5-HT and that the S-allele of the 5-HTTLPR predicts AD particularly among those with early-onset AD and comorbid psychiatric disorders (Feinn et al., 2005).
There are potentially several explanations why the administration of sertraline in the current study failed to reduce alcohol use in individuals with S/S or S/L genotypes independent of time. Given the effect size has been reported to be notably small (Feinn et al., 2005), it is possible the data from this pilot study did not provide sufficient power to detect group differences. Additionally, while people were excluded from the study who were diagnosed as currently depressed, other socio-environmental interactions such as child abuse (Kaufman et al., 2007) or negative life events were not assessed or controlled for. These may place carriers of the S/S or S/S genotypes at risk for increased alcohol and drug use (Covault et al., 2007). The environmental sources for these interactions may result in different outcomes when combined with genetic predisposition. Therefore, perhaps there may be more than one result of gene by socio-environmental interactions; some responding to pharmacological treatment and some not, but not necessarily based solely on genetic reasons. Certainly larger more powerful studies are required controlling for socio-environmental variables and examining potential gene by environment interactions moderating each possible pair of 5-HTTLPR genotypes and alcohol consumption.
Within a typological framework, the lack of a treatment effect with sertraline may be more closely aligned with the genetic diathesis (Type 2)-social milieu (Type 1) neurochemical model of AD proposed by Cloninger and colleagues (1981), rather than the Babor and colleagues (1992) model. The Cloninger and colleagues (1981) typology identifies a stronger genetic contribution in Type 2 alcoholics (persistent antisocial behavior, strong family history of alcoholism, and early-onset of alcoholism) while socio-environmental factors more strongly influence Type 1 alcoholics (fewer childhood antisocial behaviors). Research on the 5-HTTLPR binding densities reported by Storvik and colleagues (2008) demonstrate a strong positive relationship between 5-HTTLPR binding in the amygdala and paraventricular nucleus (PVN) of the hypothalamus in Type 2 alcoholics and negatively correlate with Type 1 alcoholics. This suggests a dysregulation of serotonergic neurotransmission in the amygdala and PVN in Type 2 alcoholics. While much more research is needed to confirm this hypothesis, the results of such clarification may have significant implications for the pharmacological treatment of AD with serotonergic medications.
Studies demonstrating contradicting effectiveness for sertraline in the treatment of subtypes of AD individuals suggest that treatment with sertraline in a heterogeneous sample of individuals including those with S/L or S/S genotypes may reasonably be associated with a more complex gene by environment interaction as suggested by Cloninger and colleagues (1981). A growing field of research suggests that successful treatment with sertraline may be more related to a neurobiological typology (Leggio and Addolorato, 2008) involving some degree of external childhood physical or psychological insult that interacts with a predisposed genotype facilitating the risk for developing AD as adults, with evidence this excludes depression (Kranzler et al., 2006; Pettinati et al., 2000).
In conclusion, this represents the first study investigating both ondansetron and sertraline in a design considering both a lab and a naturalistic measurement of alcohol. The results support the hypothesis of ondansetron as a medication with efficacy in individuals with a L/L 5-HTT genotype. We also note that this study confirms the validity of the ASAE paradigm. While these results must be considered preliminary, we suggest that future research in larger samples proceed by assessing both genetic and alcoholic subtypes excluding co-morbid depression. Resultant clinical matching of AD patients genetically and by subtype to the proper therapy may therefore be critical to successful treatment outcomes.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders–Text Revision. American Psychiatric Press; Washington, DC: 2000. [Google Scholar]
- Anton RF, Drobes DJ, Voronin K, Durazo-Avizu R, Moak D. Naltrexone effects on alcohol consumption in a clinical laboratory paradigm: temporal effects of drinking. Psychopharm. 2004;173:32–40. doi: 10.1007/s00213-003-1720-7. [DOI] [PubMed] [Google Scholar]
- Babor TF, Hofmann M, DelBoca FK, Hesselbrock V, Meyer RE, Dolinsky ZS, Rounsaville B. Types of alcoholics, I. Evidence for an empirically derived typology based on indicators of vulnerability and severity. Arch Gen Psychiatry. 1992;49:599–608. doi: 10.1001/archpsyc.1992.01820080007002. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Lindell S, Shannon C, Champoux M, Lesch KP, Suomi SJ, Goldman D, Higley JD. Interaction between serotonin transporter gene variation and rearing condition in alcohol preference and consumption in female primates. Arch Gen Psychiatry. 2004;61:1146–1152. doi: 10.1001/archpsyc.61.11.1146. [DOI] [PubMed] [Google Scholar]
- Bleich S, Bönsch D, Rauh J, Bayerlein K, Fiszer R, Frieling H, Hillemacher T. Association of the long allele of the 5-HTTLPR polymorphism with compulsive craving in alcohol dependence. Alcohol. 2007;42:509–512. doi: 10.1093/alcalc/agm068. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Babor TF, Kranzler FR. The Alcohol Use Disorders Test (AUDIT): validation of a screening instrument for use in medical settings. J Std Alcohol. 1995;56:423–432. doi: 10.15288/jsa.1995.56.423. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Bohman M, Sigvardsson S. Inheritance of alcohol abuse. Cross-fostering analysis of adopted men. Arch Gen Psychiatry. 1981;38:861–868. doi: 10.1001/archpsyc.1981.01780330019001. [DOI] [PubMed] [Google Scholar]
- Covault J, Tennen H, Armeli S, Conner TS, Herman AI, Cillessen AH, Kranzler HR. Interactive effects of the serotonin transporter 5-HTTLPR polymorphism and stressful life events on college student drinking and drug use. Biol Psychiatry. 2007;61:609–616. doi: 10.1016/j.biopsych.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Croop RS, Labriola DF, Wrobleski JM, Nibbelink DW. A multicenter safety study of naltrexone as adjunctive pharmacotherapy for individuals with alcoholism. Alcohol Clin Exp Res. 1995;19(Suppl):16A. [Google Scholar]
- Crowne DP, Marlowe D. A new scale of social desirability independent of psychopathology. J Consult Psych. 1960;24:349–354. doi: 10.1037/h0047358. [DOI] [PubMed] [Google Scholar]
- Drobes DJ, Anton RF, Thomas SE, Voronin K. A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: Naltrexone and nalmefene. Neuropsychopharma. 2003;28:755–764. doi: 10.1038/sj.npp.1300101. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Goldman D. Genetics of alcoholism and substance abuse. Psychiatr Clin North Am. 1999;2:289–299. doi: 10.1016/s0193-953x(05)70077-0. [DOI] [PubMed] [Google Scholar]
- Feinn R, Nellissery M, Kranzler HR. Meta-analysis of the association of a functional serotonin transporter promoter polymorphism with alcohol dependence. Am J Med Genet B Neuropsychiatr Genet. 2005;133:79–84. doi: 10.1002/ajmg.b.30132. [DOI] [PubMed] [Google Scholar]
- Freeman B, Powell J, Ball D, Hill L, Craig I, Plowmin R. DNA by mail: An inexpensive and noninvasive method for collecting DNA samples from widely dispersed populations. Behav Genet. 1997;27:251–257. doi: 10.1023/a:1025614231190. [DOI] [PubMed] [Google Scholar]
- Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- Hammoumi S, Payen A, Favre JD, Balmes JL, Benard JY, Husson M, Ferrand JP, Martin JP, Daoust M. Does the short variant of the serotonin transporter linked polymorphic region constitute a marker of alcohol dependence? Alcohol. 1999;17:107–112. doi: 10.1016/s0741-8329(98)00040-8. [DOI] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Javors MA, Seneviratne C, Roache JD, Ait-Daoud N, Bergeson SE, Walss-Bass MC, Akhtar FZ, Johnson BA. Platelet serotonin uptake and paroxetine binding among allelic genotypes of the serotonin transporter in alcoholics. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:7–13. doi: 10.1016/j.pnpbp.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA. Serotonergic agents and alcoholism treatment: rebirth of the subtype concept–an hypothesis. Alcohol Clin Exp Res. 2000;24:1597–1601. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N. Neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Psychopharmacology (Berl) 2000;149:327–344. doi: 10.1007/s002130000371. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Javors MA, Roache JD, Seneviratne C, Bergeson SE, Ait-Daoud N, Dawes MA, Ma JZ. Can serotonin transporter genotype predict serotonergic function, chronicity, and severity of drinking? Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:209–216. doi: 10.1016/j.pnpbp.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: A randomized controlled trial. JAMA. 2000;284:963–971. doi: 10.1001/jama.284.8.963. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Crouse-Artus M, Lipschitz D, Krystal JH, Gelernter J. Genetic and environmental predictors of early alcohol use. Biol Psychiatry. 2007;61:1228–1234. doi: 10.1016/j.biopsych.2006.06.039. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Korner P, Del Boca FK, Bohn MJ, Brown J, Liebowitz N. Placebo-controlled trial of fluoxetine as an adjunct to relapse prevention in alcoholics. Am J Psychiatry. 1995;152:391–397. doi: 10.1176/ajp.152.3.391. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Mueller T, Cornelius J, Pettinati HM, Moak D, Martin PR, Anthenelli R, Brower KJ, O’Malley S, Mason BJ, Hasin D, Keller M. Sertraline treatment of co-occurring alcohol dependence and major depression. J Clin Psychopharmacol. 2006;26:13–20. doi: 10.1097/01.jcp.0000194620.61868.35. [DOI] [PubMed] [Google Scholar]
- Leggio L, Addolorato G. Serotonin transporter (SERT) brain density and neurobiological cloninger subtypes model: a lesson by human autoradiography studies. Alcohol. 2008;43:148–150. doi: 10.1093/alcalc/agm169. [DOI] [PubMed] [Google Scholar]
- Lench N, Stanier P, Williamson R. Simple noninvasive method to obtain DNA for gene analysis. Lancet. 1988;1:1356–1358. doi: 10.1016/s0140-6736(88)92178-2. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Matsushita S, Yoshino A, Murayama M, Kimura M, Muramatsu T, Higuchi S. Association study of serotonin transporter gene regulatory region polymorphism and alcoholism. Am J Med Genet. 2001;105:446–450. doi: 10.1002/ajmg.1405. [DOI] [PubMed] [Google Scholar]
- Meulenbelt I, Droog S, Trommelen GJ, Boomsma DI, Slagboom PE. High-yield noninvasive human genomic DNA isolation method for genetic studies in geographically dispersed families and populations. Am J Hum Genet. 1995;57:1252–1254. [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue-induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo CA, Chu AY, Tremblay LK. Neurodevelopmental liabilities in alcohol dependence: central serotonin and dopamine dysfunction. Neurotox Res. 2002;4:343–361. doi: 10.1080/10298420290034231. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J. Naltrex-one decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacol. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Parsian A, Cloninger CR. Serotonergic pathway genes and subtypes of alcoholism: association studies. Psychiatr Genet. 2001;11:89–94. doi: 10.1097/00041444-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcohol Clin Exp Res. 2000;24:1041–1049. [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A. Double-blind clinical trial of sertraline treatment for alcohol dependence. J Clin Psychopharmacol. 2001;21:143–153. doi: 10.1097/00004714-200104000-00005. [DOI] [PubMed] [Google Scholar]
- Pinto E, Reggers J, Gorwood P, Boni C, Scantamburlo G, Pitchot W, Ansseau M. The Short Allele of the Serotonin Transporter Promoter Polymorphism Influences Relapse in Alcohol Dependence. Alcohol. 2008;43:398–400. doi: 10.1093/alcalc/agn015. [DOI] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Genetic and environmental contributions to alcohol abuse and dependence in a population-based sample of male twins. Am J Psychiatry. 1999;156:34–40. doi: 10.1176/ajp.156.1.34. [DOI] [PubMed] [Google Scholar]
- Sander T, Harms H, Dufeu P, Kuhn S, Rommelspacher H, Schmidt LG. Dopamine D4 receptor exon III alleles and variation of novelty seeking in alcoholics. Am J Med Genet. 1997a;74:483–487. doi: 10.1002/(sici)1096-8628(19970919)74:5<483::aid-ajmg5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Sander T, Harms H, Lesch KP, Dufeu P, Kuhn S, Hoehe M, Rommelspacher H, Schmidt LG. Association analysis of a regulatory variation of the serotonin transporter gene with severe alcohol dependence. Alcohol Clin Exp Res. 1997b;21:1356–1359. [PubMed] [Google Scholar]
- Schuckit MA, Mazzanti C, Smith TL, Ahmed U, Radel M, Iwata N, Goldman D. Selective genotyping for the role of 5-HT2A, 5-HT2C, and GABA alpha 6 receptors and the serotonin transporter in the level of response to alcohol: a pilot study. Biol Psychiatry. 1999;45:647–651. doi: 10.1016/s0006-3223(98)00248-0. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Higgins GA, Sobell MB. 5-HT and alcohol abuse. Trends Pharmacol Sci. 1992;13:69–75. doi: 10.1016/0165-6147(92)90026-3. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18:879–885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-Back: a technique for assessing self reported alcohol consumption. In: Litten RZ, Allen J, editors. Measuring Alcohol Consumption: Psychological and Biological Methods. Humana Press; Towota, NJ: 1992. pp. 41–72. [Google Scholar]
- Spitz E, Moutier R, Reed T, Busnel MC, Marchaland C, Roubertoux PL, Carlier M. Comparative diagnoses of twin zygosity by SSLP variant analysis, questionnaire, and dermatoglyphic analysis. Behav Genet. 1996;26:55–64. doi: 10.1007/BF02361159. [DOI] [PubMed] [Google Scholar]
- Storvik M, Haukija¨rvi T, Tupala E, Tiihonen J. Correlation between the SERT binding densities in hypothalamus and amygdala in Cloninger type 1 and 2 alcoholics. Alcohol. 2008;43:25–30. doi: 10.1093/alcalc/agm157. [DOI] [PubMed] [Google Scholar]
- Sullivan JT, Sykora K, Schneidermann J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiat Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Watson PE. Total body water and blood alcohol levels: Updating the fundamentals. In: Crow K, Batt R, editors. Human Metabolism of Alcohol (Vol. 1): Pharmacokinetics, medicolegal aspects, and general interest. CRC Press; Boca Raton, FL: 1989. pp. 41–58. [Google Scholar]


