Abstract
Background
Reorganization of the corticospinal tract (CST) can occur in unilateral spastic cerebral palsy (USCP). The affected hand can be controlled via (1)typical contralateral projections from the lesioned hemisphere, (2)ipsilateral projections from the non-lesioned hemisphere, (3)a combination of contralateral and ipsilateral projections (i.e. bilateral). Intensive bimanual therapy and constraint-induced movement therapy (CIMT) improve hand function of children with USCP. Earlier it was suggested that the CST connectivity pattern may influence the efficacy of CIMT
Objective
To examine whether CST projection pattern influences the efficacy of intensive bimanual therapy in children with USCP.
Participants
Thirty-three children with USCP (age 8.9±2.6 years, 16 females). Methods: Bimanual therapy was provided in a day-camp setting (90 hours). Participants were involved in different bimanual play and functional activities actively engaging both hands. Hand function was tested before and after the intervention with the Jebsen-Taylor Test of Hand Function (JTTHF), Assisting Hand Assessment (AHA), ABILHAND-Kids, and the Canadian Occupational Performance Measure (COPM). Single-pulse TMS was used to determine each child’s CST projection pattern (i.e. ipsilateral, contralateral, or bilateral).
Results
Children whose affected hand was controlled only by ipsilateral CST projections had worse JTTHF and AHA scores than children in the contralateral group at baseline. Bimanual hand use and functional hand use was independent of CST projection pattern. After bimanual therapy, improvements on all outcome measures were observed, and these improvements were independent of the CST connectivity pattern.
Conclusion
The efficacy of bimanual therapy on hand function in children with USCP appears to be independent of CST connectivity pattern.
Keywords: Cerebral Palsy, Hemiplegia, Exercise therapy, Rehabilitation, Corticospinal tract, Reorganization
Introduction
Intensive bimanual therapy1, such as Hand-Arm Bimanual Intensive Training (HABIT)2, encourages skillful hand use in structured bimanual activities. Both bimanual therapy and constraint-induced movement therapy (CIMT), a unimanual training approach, have been found to improve hand function in children with Unilateral Spastic Cerebral Palsy (USCP).3–5 Intensive, skilled, goal-directed training appears essential for obtaining long-term effects of these training approaches, both on the functional and cortical level.5,6 Neverthelesss, considerable variability in treatment outcomes exists. In our HABIT trials, 57% of participants showed clinically important improvements in bimanual performance4,5. Although the key factors influencing efficacy of intensive therapy are still unclear, several factors have been implicated, such as the type, location and extent of the lesion and subsequent neuroplastic changes, age, and impairment level.7–9
The corticospinal tract (CST) is the main pathway for control of voluntary, skilled hand movements.10 In healthy individuals, the CST shows a strongly lateralized contralateral corticospinal projection, i.e. the left hemisphere controls the right upper extremity and vice versa.10 However, after perinatal unilateral brain lesions, some children develop an ipsilateral CST in which the contralesional hemisphere controls both hands or have bilateral CST projections so that the affected hand is controlled by both the lesioned and the contralesional hemisphere.11,12
Three studies have examined the influence of CST connectivity on the efficacy of CIMT in individuals with USCP. Kuhnke and colleagues13 showed that children with a contralateral CST increased dexterity after CIMT, whereas children with an ipsilateral CST decreased dexterity (i.e. became slower). These findings were reinforced by a follow up study9 that showed that the two groups also showed differences in exercise-induced neuroplasticity. In contrast, Islam et al.7 concluded that improvements in hand use after CIMT were present in all participants, irrespective of their CST connectivity pattern. Based on these studies, each with a small sample size, it is difficult to draw conclusions about the effect of CST connectivity pattern on the efficacy of CIMT. More importantly, no study has examined the effect of CST connectivity pattern on efficacy of bimanual therapy.
Since evidence of efficacy and clinical use of bimanual therapy is mounting, it is important to understand the factors that influence efficacy. Therefore, the aim of this study was to examine the effect of CST laterality on the efficacy of bimanual therapy in children with USCP. Individuals with a contralateral CST are believed to benefit from CIMT because it might target the dis-balanced interhemispheric inhibition (IHI).13 In healthy individuals each hemisphere inhibits the other hemisphere. However, individuals with hemiparesis can have an unbalanced IHI in which the more active contralesional hemisphere inhibits the less active lesioned hemisphere.14 CIMT lowers activity in the contralesional hemisphere (by constraining the less-affected arm) and increases the activity in the lesioned hemisphere, which in turn can ‘rebalance’ the (unbalanced) IHI.13 For individuals with an ipsilateral CST however, IHI cannot be targeted since both motor representations are located in the contralesional hemisphere.13 Intensive bimanual therapy increases activity in both hemispheres.15 For individuals with a contralateral CST this is hypothesized to maximize activity in both hemispheres and better interaction between the two hemispheres. For children with an ipsilateral CST the use of both hands will increase activity of the contralesional hemisphere, which controls both hands. Therefore, it was hypothesized that improvements after intensive bimanual therapy are not dependent on the CST connectivity pattern.
Methods
Participants
Thirty-three children with USCP and a unilateral brain lesion (confirmed with a Magnetic Resonance Image, MRI) participated in the study (Table 1). Six children (7.9±2.0 years, 4 females) participated in a day-camp (see below) at Teachers College, Columbia University, New York, USA (n=4) or at the Université Catholique de Louvain in Brussels, Belgium (n=2) between 2009 and 2012 as part of previous studies. They returned to the laboratory subsequently (between 2012 and 2014; maximum time between therapy and TMS was 3 years) for this study to determine their CST connectivity pattern with Transcranial Magnetic Stimulation (TMS) and are therefore a sample of convenience of previous participants. The other 27 children (9.1±2.7 years, 12 females) participated in studies on the efficacy of bimanual therapy between 2010 and 2012 at Teachers College (n=19) and 2013 and 2014 at Université Catholique de Louvain (n=8). For this group, CST connectivity pattern was determined before they received intensive bimanual therapy. The behavioral data of some children included in this paper have been published elsewhere.4,5,16–18
Table 1.
Participant characteristics.
| Contralesional hemisphere | Lesioned hemisphere | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Less-affected hand | Affected hand | Affected hand | |||||||
| Participant | CST projection pattern | Lesion side/type (1=brain malformation; 2=PVL; 3=cortical/subcortical) | MACS | Motor threshold, % | Mean latency, ms | Motor threshold, % | Mean latency, ms | Motor threshold, % | Mean latency, ms |
| 1 | Ipsilateral | R/1 | 1 | 52 | 20.4 | 52 | 20.4 | ||
| 2 | Ipsilateral | L/2 | 3 | 56 | 21.4 | 56 | 20.3 | ||
| 3 | Ipsilateral | R/3 | 2 | 86 | 20.1 | 86 | 20.5 | ||
| 4 | Ipsilateral | L/2 | 2 | 76 | 22.8 | 76 | 23.1 | ||
| 5 | Ipsilateral | L/2 | 3 | 88 | 21.8 | 88 | 25.6 | ||
| 6 | Ipsilateral | L/2 | 2 | 72 | 20.9 | 72 | 20.5 | ||
| 7 | Ipsilateral | R/2 | 2 | 46 | 19.9 | 50 | 20.0 | ||
| 8 | Ipsilateral | L/2 | 2 | 68 | 20.1 | 68 | 22.5 | ||
| 9 | Ipsilateral | R/3 | 2 | 72 | 36.5 | 70 | 30.9 | ||
| 10 | Ipsilateral | R/3 | 2 | 52 | 23.0 | 54 | 24.3 | ||
| 11 | Ipsilateral | L/2,3 | 2 | 75 | 16.7 | 75 | 13.8 | ||
| 12 | Ipsilateral | L/2 | 2 | 60 | 21.9 | 60 | 27.1 | ||
| 13 | Ipsilateral | R/2,3 | 2 | 70 | 22.1 | 70 | 21.8 | ||
| 14 | Ipsilateral | R/2 | 2 | 80 | 15.4 | 80 | 16.5 | ||
| 15 | Contralateral | L/2 | 2 | 46 | 20.9 | 56 | 20.4 | ||
| 16 | Contralateral | L/2 | 1 | 63 | 21.2 | 60 | 20.5 | ||
| 17 | Contralateral | L/2 | 3 | 68 | 17.0 | 68 | 15.1 | ||
| 18 | Contralateral | R/2 | 2 | 57 | 19.3 | 82 | 24.6 | ||
| 19 | Contralateral | L/3 | 3 | 74 | 21.0 | 70 | 18.5 | ||
| 20 | Contralateral | L/2 | 2 | 90 | 21.9 | 99 | 40.3 | ||
| 21 | Contralateral | L/3 | 1 | 70 | 17.1 | 75 | 18.3 | ||
| 22 | Contralateral | R/2 | 2 | 80 | 20.2 | 80 | 21.1 | ||
| 23 | Contralateral | L/2 | 2 | 72 | 19.7 | 60 | 19.6 | ||
| 24 | Bilateral | L/2 | 2 | 62 | 20.9 | 62 | 44.3 (wr) | 74 | 15.8 (wr) |
| 25 | Bilateral | R/3 | 2 | 48 | 21.8 | 48 | 32.8 | 84 | 23.4 |
| 26 | Bilateral | L/3 | 1 | 64 | 19.1 | 64 | 22.1 | 66 | 20.9 |
| 27 | Bilateral | L/2 | 3 | 66 | 24.5 | 66 | 15.2 | 66 | 19.9 |
| 28 | Bilateral | L/2 | 1 | 60 | 81a | 60 | 19.3 | 55 | 47.6 (wr) |
| 29 | Bilateral | R/2 | 2 | 70 | 23.0 | 70 | 38.7 | 66 | 22.4 |
| 30 | Bilateral | L/3 | 2 | 39 | 24.5 | 45 | 25.1 | 82 (wr) | 23.8 (wr) |
| 31 | Bilateral | L/3 | 2 | 55 | 18.4 | 55 | 45.7 | 55 | 43.9 |
| 32 | Bilateral | R/3 | 1 | 45 | 20.2 | 47 | 20.3 | 70 | 25.2 |
| 33 | Bilateral | L/2 | 2 | 53 | 19.7 | 65 | 19.4 | 55 | 19.7 |
this participant did not have enough responses in less-affected hand to calculate mean latency.
Motor threshold and latency were calculated based on measures in the FDI muscle unless otherwise indicated (wr = wrist).
Participants were recruited through our website (http://www.tc.columbia.edu/centers/cit), our Facebook page (Center for Cerebral Palsy Research) and online support groups. Potential participants were first screened via telephone. If they appeared eligible for the study they were invited for an on-site physical examination or, in case they were unable to come to the university, for an examination videotaped by their physical/occupational therapist (PT/OT). Inclusion criteria for the intervention performed in this study were: age between 6 and 17 years, diagnosis of USCP, being able to lift the affected arm >15 cm above the table surface and grasp light objects. Participants were excluded from the study if they had any other medical illness unrelated to USCP, visual problems interfering with intervention/testing, severe muscle tone (Modified Ashworth Scale>3.5), surgery in the affected upper limb within one year prior to the intervention, Botulinum Toxin in the upper limb within 6 months prior to the intervention.3–5,19 For the TMS study, children were excluded if they had seizures after the age of 2 years, current medication use to lower the seizure threshold, claustrophobia, pregnancy, or presence of metallic object(s) in the body other than dental hardware. Informed consent was obtained from all children and their caregivers. The study was approved by the Institutional Review Boards of Teachers College, Columbia University Medical Center, Université Catholique de Louvain, Burke-Cornell Medical Research Institute, and Weill-Cornell Medical College.
Intervention
The intervention consisted of HABIT2,3,16,20 provided in a day-camp setting at Teachers College, Columbia University, New York, USA and at Université Catholique de Louvain, Brussels, Belgium.
Procedures in New York: participants were engaged in age-appropriate bimanual activities for 6 hours per day for 15 days (90 hours). During HABIT, participants were in a room with 4 to 5 other children of similar ages. An experienced PT or OT supervised the room. Each child was paired with an interventionist so that there was at least a 1:1 interventionist:participant ratio. The interventionists were trained PTs, OTs, graduate, undergraduate and graduate students in Kinesiology and related fields and undergraduates.4,5 Activities included child-friendly fine and gross motor bimanual activities using motor learning approaches. Examples of activities include playing board games, playing ball, making lunch, and arts and crafts. Skill demands of the activities were increased over the course of therapy and tailored to the abilities of each child. Children also practiced 3 to 5 different functional goals (e.g. tying shoe laces, carrying a tray) as determined by participants and their parents. Further details of the therapy are described elsewhere.3,4
Procedures in Brussels: this camp was conducted in a single room near the university. Therapy was provided for 9 hours per day for 10 consecutive days (90 hours). In addition to bimanual therapy, which involved activities and goals similar to the camp in New York, participants received simultaneous lower extremity training (HABIT-ILE16), such as ball sitting, standing, standing on balance board, running and jumping. Staff from the New York camp was present to ensure the intensity and approach to upper extremity treatment were identical at each site.
Determination of CST connectivity pattern
A detailed explanation of the determination of the CST connectivity pattern can be found in the online-only material.
Single-pulse TMS was performed7,9,13 using a Magstim 200 stimulator and a figure-of-eight coil (Magstim, UK). Motor-evoked potentials (MEPs) were recorded bilaterally from the first dorsal interosseus muscle (FDI) and the wrist flexor using surface electromyography (EMG) electrodes connected to a Neuroprax amplifier (Neuroconn GmbH, Germany). When a hotspot for the FDI of the affected hand was found (in the lesioned hemisphere, contralesional hemisphere, or both hemispheres), the coil was held over that spot to determine the resting motor threshold (MT). MT was defined as the stimulator output at which an MEP equal or bigger than 50 microvolt could be elicited in 6 out of 10 consecutive stimulations delivered at a frequency <0.1Hz. Subsequently, a 1 cm circular grid of 10cm in diameter, centered around the affected FDI hotspot, was superimposed over the hemisphere. Single TMS pulses were delivered to each grid point at a stimulus intensity of 110% MT at a frequency of 0.1Hz. During the analyses, the total number of responses in the affected FDI or wrist was calculated for each hemisphere. To determine the CST connectivity pattern, we calculated the ratio between the number of responses in the affected FDI and wrist flexor obtained from the lesioned and contralesional hemispheres. This ratio was termed the Laterality Index (LI)21, 22:
A participant was classified as having a contralateral CST connectivity pattern if the LI was between 0.9 and 1, meaning that 90–100% of the responses in the affected hand come from the lesioned hemisphere. A participant was classified as having an ipsilateral CST connectivity pattern if the LI was between 0 and 0.1, meaning that 0 to 10% of the responses in the affected hand come from the lesioned hemisphere. If the LI was between 0.1 and 0.9, the participant was classified as having a bilateral CST connectivity pattern.
Outcome measures
Participants were evaluated prior to the intervention (pretest) and within 4 days after the intervention (posttest) by a physical therapist. Unimanual capacity of the affected upper limb was measured with the Jebsen-Taylor Test of Hand Function (JTTHF), a standardized test measuring manual dexterity as the time to complete a set of simulated functional unimanual activities.23 Six of the seven subtests were used (writing task excluded) and the maximum time for each activity was 180s.24,25
Bimanual performance was tested with the Assisting Hand Assessment (AHA, version 4.326), which measures the spontaneous use of the affected hand as an assisting hand in bimanual activities. The test was videotaped and scored by an experienced blinded evaluator. It has good validity27 and reliability (interrater 0.97 and intrarater 0.9928). Transformed logit data (AHA units) were used for analysis.29
To measure the participant’s ability to perform daily manual activities, the ABILHAND-Kids was used.30 This is a reliable and valid measure that consists of a list of 21 manual activities that caregivers score on the amount of difficulty their child experiences while performing the activities. The score is reported in logit-based units. Lastly, the Canadian Occupational Performance Measure (COPM) was conducted with the parents to establish and evaluate the participant’s functional goals in terms of performance and caregiver satisfaction.31 Functional goals were identified in the areas of self-care, school, or leisure activities. Each goal was scored before and after the intervention on a 10-point scale. The COPM is an individualized measure intended to capture change meaningful to the children and their parents32 and is valid and reliable.33
Statistical analyses
Using SPSS (IBM, version 21) an independent samples t-test was used to examine whether the two study populations (New York and Brussels) were comparable in age, baseline measures, and amount of improvement after therapy (Table 2). The two populations from each two centers were combined for further analysis.
Table 2.
Comparison of the baseline and improvement values of the JTTHF and AHA between the New York camp site and the Brussels camp site.
| Measure | New York (n = 23) Mean (SE) |
Brussels (n = 10) Mean (SE) |
p-value, difference between sites |
|---|---|---|---|
| Age (years) | 9.2 (0.6) | 8.2 (0.4) | 0.29 |
| Baseline JTTHF (sec) | 246.6 (39.8) | 317.7 (102.8) | 0.44 |
| % improvement JTTHF | 25.9% (5.3%) | 5.6% (15.2%) | 0.12 |
| Baseline AHA (units) | 60.9 (2.0) | 64.2 (4.3) | 0.44 |
| Improvement AHA (units) | 2.74 (0.94) | 1.40 (1.45) | 0.44 |
A 2×2 repeated measures ANOVA with within factor test (pretest vs. posttest) and between factor CST group (ipsilateral vs. contralateral) was used to evaluate changes in scores of each measure before and after treatment. In addition, for the JTTHF posttest score an ANCOVA was performed with the pretest JTTHF score as covariate. A Fisher’s exact test was used to compare the number of participants with and without improvements bigger than the minimal clinically important differences (MCID) for the AHA and the COPM. Participants with a bilateral CST were analyzed separately, comparing pre- and posttest results with a paired-samples t-test (see below). The significance level was set at 0.05.
Results
Hand function outcomes in children with ipsilateral versus contralateral CST
Unimanual capacity
Thirty-three children (Table 1) participated in the study. We compared improvements on the JTTHF between the ipsilateral (n=14) and the contralateral (n=9) groups. While the ipsilateral group was slower overall (main effect of group F(1,21)=5.41, p=0.030, ηp2 =0.21) and both groups improved (main effect of test F(1,21)=16.68, p=0.001, ηp2 =0.44), there was a greater improvement in movement speed for the ipsilateral than the contralateral group (test × group interaction F(1,21)=5.08, p=0.035, ηp2 =0.20). Given that the baseline JTTHF score for the ipsilateral group was significantly higher compared to the contralateral group (Table 3; p=0.02), we performed an ANCOVA with the pretest JTTHF score as covariate. When correcting for the baseline difference between the groups, the JTTHF score on the posttest was not significantly different between the ipsilateral and contralateral groups (F(1,23)=0.86, p=0.37, ηp2 =0.041). Thus, children in both groups had similar improvements in unimanual dexterity after HABIT. Furthermore, there was no significant difference in percentage improvement in JTTHF between the groups (t(17)=−1.41, p=0.18; ipsilateral: 29.9±4.44%, contralateral: 16.8±9.95%; Figure 1).
Table 3.
Mean (SD) values for the outcome measures for the ipsilateral, contralateral and bilateral group and the p-value for the between- and within-group comparisons and the interaction effects between ipsilateral and the contralateral group. JTTHF = Jebsen Taylor Test of Hand Function; AHA = Assisting Hand Assessment; COPM = Canadian Occupational Performance Measure; sat = satisfaction; perf = performance; LHL = left hemispheric lesion; RHL = right hemispheric lesion.
| Measure | Ipsilateral (n = 14) |
Contralateral (n = 9) |
Bilateral (n = 10) |
p-value, difference between groups (ipsi vs. contra; independent sample t-test*) | p-value, difference within groups (pre- vs. posttest; ANOVA*) | p-value, interaction effect (ANOVA *) |
|---|---|---|---|---|---|---|
| Age (SE) | 9.1 (0.78) | 8.2 (0.53) | 9.3 (0.97) | 0.45 | ||
| Gender | 7F; 7M | 3F; 6M | 6F; 4M | n/a | ||
| Side of Lesion | 7 LHL; 7 RHL | 7 LHL; 2 RHL | 7 LHL; 3 RHL | n/a | ||
| Baseline JTTHF (sec) | 310.87 (45.46) | 145.14 (37.21) | 319.08 (111.66) | 0.02 | ||
| % improvement JTTHFa | 24.84% (6.58%) | 15.03% (6.79%) | 17.04% (17.01 %) | 0.33 | ||
| Baseline AHA units | 56.36 (1.07) | 71.67 (4.40) | 60.90 (3.20) | 0.008 | ||
| Improvemen t AHA units | 1.36 (1.36) | 4.0 (1.08) | 2.20 (1.44) | 0.18 | 0.01 | 0.18 |
| Baseline Abilhand-Kids | 1.98 (0.43) | 2.15 (0.51) | 1.81 (0.25) | 0.81 | ||
| Improvemen t Abilhand-Kids | 0.83 (0.31) | 0.67 (0.37) | 1.43 (0.37) | 0.74 | 0.006 | 0.18 |
| Baseline COPM perf | 4.0 (0.35) | 3.13 (0.42) | 3.80 (0.48) | 0.14 | ||
| Improvemen t COPM perf | 3.05 (0.42) | 3.11 (0.5) | 3.55 (0.59) | 0.93 | <0.001 | 0.93 |
| Baseline COPM sat | 4.69 (0.54) | 3.78 (0.65) | 4.78 (0.79) | 0.30 | ||
| Improvemen t COPM sat | 3.04 (0.59) | 3.23 (0.69) | 3.63 (0.70) | 0.83 | <0.001 | 0.83 |
| Minimal clinically important difference AHA(#yes/#no) | 3/14 | 3/9 | 0.64b | |||
| Minimal clinically important difference COPM perf(#yes/#no) | 9/13 | 7/9 | 1.0b | |||
| Minimal clinically important difference COPM sat(#yes/#no) | 8/13 | 7/9 | 0.65b |
note that the between group, within group and interaction effects are only for the ipsilateral vs. the contralateral group. The bilateral group was analyzed separately.
note that because of the baseline difference on the JTTHF we here indicate the percentage (%) improvement of the JTTHF in order to correct for the baseline differences.
For the comparison of the minimal clinically important differences the p-value of the Fisher’s exact tests are reported.
Figure 1.
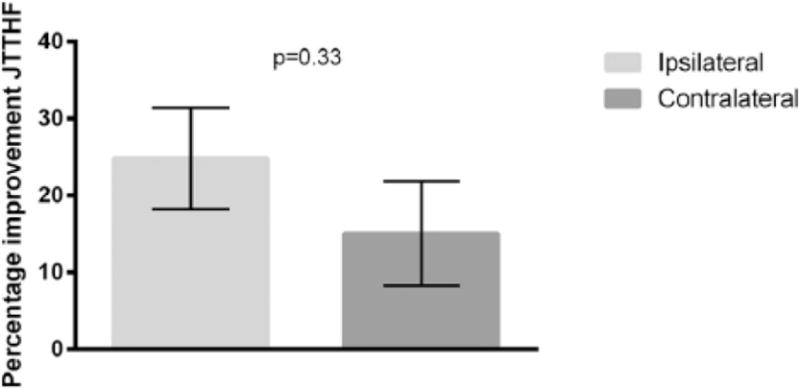
Percentage improvement on the Jebsen-Taylor Test of Hand Function (JTTHF) for the ipsilateral and the contralateral groups.
Bimanual performance
For the AHA, there was an overall increase from the pretest (64.0±1.87) to the posttest (66.7±2.09; F(1,21)=7.88, p=0.01, ηp2 =0.27). In addition, children with a contralateral CST on average had a higher score than children with an ipsilateral CST (73.67±3.00 vs. 57.04±2.41 AHA units; main effect of group F(1,21)=18.67, p<0.001, ηp2 =0.47). There was no interaction between the two variables (F(1,21)=1.92, p=0.18, ηp2 =0.08), indicating that both groups improved similarly (Table 3; Figure 2). The lack of difference between the two groups was also visible for the MCID. An MCID (i.e. ≥534) was achieved in 21% of the participants in the ipsilateral group and 33% in the contralateral group (p=0.64; Table 3).
Figure 2.
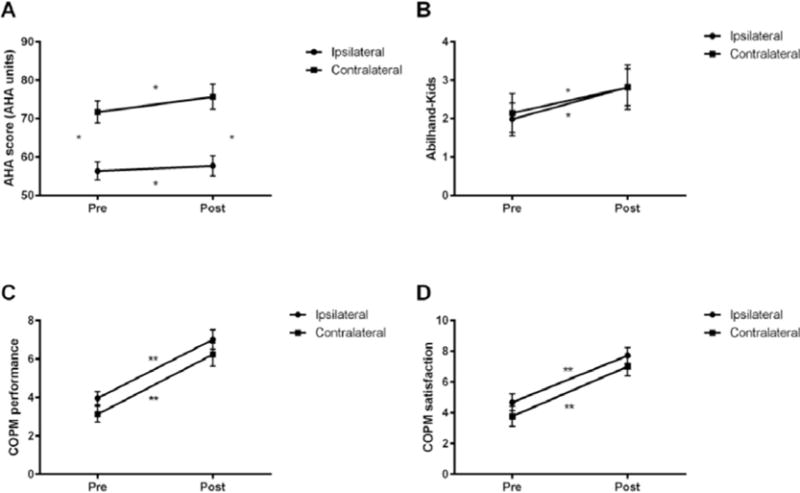
Intensive bimanual therapy improves scores on the (a) AHA, (b) Abilhand-Kids, (c) COPM performance, (d) COPM satisfaction measure for the ipsilateral and the contralateral groups. *p<0.05; **p<0.001.
Functional outcomes
ABILHAND-Kids and COPM scores were available for 22 children (9 contralateral and 13 ipsilateral).
On the ABILHAND-Kids there was an average improvement from 2.06±0.33 to 2.82±0.38 (F(1,20)=9.52, p=0.006, ηp2 =0.32). However, no significant test × group interaction was revealed (F(1,20)=0.11, p=0.74, ηp2 =0.006), suggesting that the improvements were irrespective of CST connectivity pattern (Table 3). For the COPM Performance measure, overall, a significant improvement was observed after HABIT from 3.55±0.27 to 6.63±0.40 points (F(1,20)=90.07, p<0.001, ηp2 =0.82). However, there was no significant interaction between test and CST group (F(1,20)=0.008, p=0.93, ηp2 =0.00). Similarly, for the COPM Satisfaction measure, there was a significant improvement after HABIT from 4.23±0.43 to 7.37±0.41 points (F(1,20)=47.63, p<0.001, ηp2 =0.70) but no test × CST group interaction (F(1,20)=0.47, p=0.83, ηp2 =0.002), suggesting that both groups increase their COPM score to a similar degree after HABIT (Table 3). Likewise, the number of participants with an improvement greater than the MCID (i.e.>2)33 was not different between groups. An MCID on the COPM performance measure was achieved in 69% of the participants in the ipsilateral group and 78% in the contralateral group (p=1.0; Table 3). For the COPM satisfaction measure these values were 62% and 78% for the ipsilateral and contralateral groups respectively (p=0.65; Table 3).
Hand Function Outcomes in Children with a Bilateral CST
This study focused on the differential efficacy of bimanual therapy in children with ipsilateral versus contralateral CST patterns. However, one third of the present study sample (and 15% to 40% of participants in previous studies7,11,12,35–37) had bilateral patterns. Since their CST pattern is more complex, we analyzed their recovery separately.
Unimanual capacity
Figure 3 shows the percentage improvement on the JTTHF for all participants. The participants with a bilateral CST (n=10; triangles) showed a large variety in LI, varying from 0.29 to 0.79 indicating that some children have stronger contralateral control, while other children have stronger ipsilateral control of the affected hand. The average improvement of 69 seconds in this group showed a trend towards significance (t(9)=2.23, p=0.053). Our sample size (n=10) does not allow statistical correlations between LI and improvement in hand function, but in this group it seems to be unrelated. Importantly 60% of the participants improved more than 20% on the JTTHF, which is comparable to the contralateral (55%) and ipsilateral (64%) groups. This suggests that bimanual therapy can improve unimanual dexterity in children with a bilateral CST, irrespective of whether they have stronger ipsilateral or contralateral connections.
Figure 3.
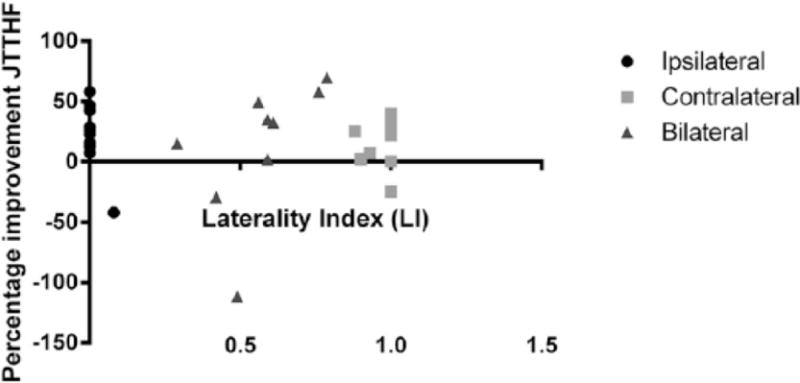
Percentage improvement in JTTHF score plotted against the Laterality index (LI) for the ipsi-, contra-, and bilateral groups.
Bimanual performance
Children with bilateral CST patterns showed an improvement from 60.9±3.20 to 63.1±4.02 AHA units, which is comparable to the ipsilateral and contralateral groups, but was not significant (t(9)=−1.52, p=0.162). Improvements larger than the MCID were achieved by 40% of the participants in the bilateral group, which is also comparable to the other groups.
Functional outcomes
Children with a bilateral CST significantly improved 1.43 points on the ABILHAND-Kids (t(9)=−2.26, p=0.004), which is slightly higher than improvements in the ipsilateral (0.84) and contralateral (0.67) groups.
Children with a bilateral CST significantly improved on both the COPM Performance (t(9)=−6.03, p<0.001; mean difference 3.5) and Satisfaction measures (t(9)=5.22, p =0.001; mean difference 3.6). This improvement is similar to what was found in children with a contralateral or ipsilateral CST. Similar to the other groups, 80% and 70% of the participants in the bilateral group showed an improvement larger than the MCID for the Performance and Satisfaction measure, respectively.
Discussion
This study examined the influence of CST organization on the efficacy of HABIT in children with unilateral cerebral palsy. The main finding was that children with an ipsilateral CST versus a contralateral CST improve similarly on hand function and functional measures. This suggests that the pattern of CST connectivity is not a key factor impacting the efficacy of bimanual therapy. Previously, the ipsilateral CST was suggested to be maladaptive and associated with poorer hand function.13,35 Indeed, we also found that children with an ipsilateral CST have worse baseline hand function than children with a contralateral CST. Two possible mechanisms have been suggested for the relationship between baseline hand function and CST pattern.35 First, if the ipsilateral CST is regarded as a compensatory mechanism following brain injury, the worse baseline hand function in children with an ipsilateral CST suggests that the compensation mechanism is not sufficient to fully compensate for the damage and restore function. Second, impaired sensory function is suggested to play a role. In children with an ipsilateral CST there is a dissociation of sensory input and motor output. The sensory cortex is located in the lesioned hemisphere, whereas the motor cortex is relocated to the contralesional hemisphere.38,39 This disrupts the sensorimotor loop and integration, which is important for skillful hand movements.
Nevertheless, despite their greater impairment, hand function in children with an ipsilateral CST improved after bimanual therapy to a similar degree as children with a contralateral CST. Moreover, a similar percentage of participants in each group achieved an MCID on the AHA and the COPM. Interestingly, about 30% of the children achieved an improvement on the AHA that was equal or greater than the MCID, whereas this was 50–75% of the children for the COPM. As suggested by Brandao et al.5, this difference between the outcome measures might be related to the older age of the children in this study. It is possible that dexterity and functional use may improve whereas older children may have well-established strategies for using the affected hand in bimanual activities. Nevertheless, the present findings are important since they imply that responsiveness to bimanual therapy, such as HABIT, is not limited by CST connectivity pattern. Friel et al.17 also suggested that a large range of children could benefit from bimanual therapy. In their study they showed that peduncle asymmetry (a measure of CST dysgenesis) was not related to improvements in hand function after HABIT. Although peduncle asymmetry has not been shown to be related to CST connectivity pattern18, this study provides further evidence that bimanual therapy can be beneficial for a large range of children with CP.
Another important finding of the present study is that children with a bilateral CST, who are often left out of analyses because of their complicated connectivity pattern, also improve (to the same extent) after bimanual therapy. This supports the suggestion that bimanual therapy is suitable for children with USCP regardless of their CST connectivity pattern. Moreover, it shows the importance of including children with bilateral connectivity patterns, who comprise between 15 and 40%7,11,12,35–37 of the USCP population, in rehabilitation studies.
Our findings add to the existing literature on the influence of CST connectivity pattern on the efficacy of hand therapy. Although this literature is equivocal and had small samples, there seems to be some evidence that CIMT is more effective for children with a contralateral than with an ipsilateral CST9,13 (in a homogeneous subpopulation of children with USCP40). Our study suggests that this is not the case for bimanual therapy. This raises the question why the efficacy of this approach does not seem to be influenced by CST connectivity pattern whereas CIMT may be. Neurophysiological assessments may help to delineate this matter18. However, based on the suggestions by Kuhnke and colleagues13 the question seems not so much why HABIT could work for both groups but rather why CIMT may not be ideal for children with an ipsilateral CST. Juenger et al.9, using TMS, functional MRI and Magnetoencephalography to study exercise-induced neuroplasticity after CIMT in individuals with an ipsilateral vs. a contralateral CST, showed for both groups increased synaptic activity in S1. However, only the contralateral group showed increased excitability of the M1 controlling the affected hand and increased synaptic activity during active movement of the affected hand. The ipsilateral group showed a decrease in both parameters. This difference might be related to the dissociation of S1 and M1 in the ipsilateral group and is possibly caused by an inhibitory interhemispheric interaction from the exercise-induced increase of activity in S1 in the lesioned hemisphere to M1 in the contralesional hemisphere. Still, Juenger et al.9 did not study bimanual therapy and studies that focus on the underlying mechanisms of bimanual therapy and CIMT (i.e. rebalancing IHI and/or involvement of the sensorimotor loop13) are needed.
Our study is, to our knowledge, the first to look at the influence of CST connectivity pattern on the efficacy of bimanual therapy. However, there are several limitations to this study. This study does not include follow-up data. It is possible that CST patterns influence retention of gains. In addition, there was heterogeneity in our population in terms of the timing of the determination of the CST connectivity pattern and the sites the children received the therapy. The timing of the determination of the CST connectivity pattern was either done before or some years after therapy. Although we cannot rule out changes in CST connectivity pattern over time, to the best of our knowledge, there is currently no evidence that the CST connectivity pattern changes during training or development (after the age of 2 years41,42). Similarly, we cannot rule out that including children from two different sites who received slightly different therapies (HABIT vs. HABIT-ILE) influenced the outcomes. However, for the upper extremity there was 100% overlap of focus and activities between the two sites. In addition, we showed (Table 2) that the baseline values and the size of the improvements were similar between the groups. Bleyenheuft et al.20 showed effect sizes with HABIT-ILE that were congruent with previous bimanual therapy studies, which suggests that adding simultaneous lower extremity training does not decrease the possibility to improve upper extremity function. Moreover, we repeated our analyses, excluding the children from the Brussels site and the children with retrospective CST determination. All results were the same for each of the outcome variables when these children were excluded. Thus these factors did not appear to greatly influence our findings. Although we included more participants than previous studies, we still have a limited number of participants, which makes it difficult to draw firm conclusions. Moreover, similar to the study population from Islam et al.7, our study population is rather heterogeneous, which makes it difficult to correct for other issues such as lesion size, lesion location and timing of the injury, which may also have an influence.
In addition, bimanual therapy and CIMT are both commonly used in the rehabilitation of children with USCP. Existing studies (including the present study) have not directly compared the impact of CST connectivity between the two approaches. This does not allow us to determine which type of therapy is optimal for each individual. Therefore, a randomized controlled trial with sufficient number of participants that compares the efficacy of bimanual therapy and CIMT between children with an ipsilateral and children with a contralateral CST could provide insight into this complex but important question. This could potentially allow therapies to be tailored to individuals.
In conclusion, this study showed that bimanual therapy can improve hand function of children with USCP irrespective of CST pattern and can therefore be provided to the general USCP population. Moreover, this study provided a new, objective way to determine CST pattern by using the LI, which may allow us to examine the relation between laterality and outcomes in a more comprehensive manner in children with USCP.
Acknowledgments
We thank Dr. Jason Fuller, Daniela Ebner and Dr. Inmaculada Riquelme Agulló for assistance with TMS procedures. We thank the volunteer interventionists for their help during the camp and we thank the participants and their families for their participation.
Footnotes
Conflicts of interest statement
This study was funded by the NIH grants R03HD073515, R01HD076436, K01NS062116. The study in Brussels was also funded by the Fondation van Goethem-Brichant Prize for Rehabilitation 2012 and Fonds de la recherche clinique fellowship, clinique universitaires St. Luc.
References
- 1.Eliasson AC. Bimanual training for children with unilateral CP - is this something new? Dev Med Child Neurol. 2007;49:806. doi: 10.1111/j.1469-8749.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 2.Charles J, Gordon AM. Development of hand-arm bimanual intensive training (HABIT) for improving bimanual coordination in children with hemiplegic cerebral palsy. Dev Med Child Neurol. 2006;48:931–6. doi: 10.1017/S0012162206002039. [DOI] [PubMed] [Google Scholar]
- 3.Gordon AM, Schneider JA, Chinnan A, Charles JR. Efficacy of a hand-arm bimanual intensive therapy (HABIT) in children with hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2007;49:830–8. doi: 10.1111/j.1469-8749.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- 4.Gordon AM, Hung YC, Brandao M, et al. Bimanual training and constraint-induced movement therapy in children with hemiplegic cerebral palsy: a randomized trial. Neurorehabil Neural Repair. 2011;25:692–702. doi: 10.1177/1545968311402508. [DOI] [PubMed] [Google Scholar]
- 5.Brandao MB, Ferre C, Kuo HC, et al. Comparison of Structured Skill and Unstructured Practice During Intensive Bimanual Training in Children With Unilateral Spastic Cerebral Palsy. Neurorehabil Neural Repair. 2013;28:452–61. doi: 10.1177/1545968313516871. [DOI] [PubMed] [Google Scholar]
- 6.Friel KM, Kuo HC, Fuller J, et al. Skilled Bimanual Training Drives Motor Cortex Plasticity in Children With Unilateral Cerebral Palsy. Neurorehabil Neural Repair. 2016 doi: 10.1177/1545968315625838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam M, Nordstrand L, Holmstrom L, Kits A, Forssberg H, Eliasson AC. Is outcome of constraint-induced movement therapy in unilateral cerebral palsy dependent on corticomotor projection pattern and brain lesion characteristics? Dev Med Child Neurol. 2014;56:252–8. doi: 10.1111/dmcn.12353. [DOI] [PubMed] [Google Scholar]
- 8.Sakzewski L, Gordon A, Eliasson AC. The state of the evidence for intensive upper limb therapy approaches for children with unilateral cerebral palsy. J Child Neurol. 2014;29:1077–90. doi: 10.1177/0883073814533150. [DOI] [PubMed] [Google Scholar]
- 9.Juenger H, Kuhnke N, Braun C, et al. Two types of exercise-induced neuroplasticity in congenital hemiparesis: a transcranial magnetic stimulation, functional MRI, and magnetoencephalography study. Dev Med Child Neurol. 2013;55:941–51. doi: 10.1111/dmcn.12209. [DOI] [PubMed] [Google Scholar]
- 10.Martin JH. The corticospinal system: from development to motor control. Neuroscientist. 2005;11:161–73. doi: 10.1177/1073858404270843. [DOI] [PubMed] [Google Scholar]
- 11.Carr LJ, Harrison LM, Evans AL, Stephens JA. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–47. doi: 10.1093/brain/116.5.1223. doi: [DOI] [PubMed] [Google Scholar]
- 12.Staudt M, Grodd W, Gerloff C, Erb M, Stitz J, Krageloh-Mann I. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–37. doi: 10.1093/brain/awf227. doi: [DOI] [PubMed] [Google Scholar]
- 13.Kuhnke N, Juenger H, Walther M, Berweck S, Mall V, Staudt M. Do patients with congenital hemiparesis and ipsilateral corticospinal projections respond differently to constraint-induced movement therapy? Dev Med Child Neurol. 2008;50:898–903. doi: 10.1111/j.1469-8749.2008.03119.x. [DOI] [PubMed] [Google Scholar]
- 14.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol. 2006;5:708–12. doi: 10.1016/S1474-4422(06)70525-7. [DOI] [PubMed] [Google Scholar]
- 15.Neva JL, Legon W, Staines WR. Primary motor cortex excitability is modulated with bimanual training. Neurosci Lett. 2012;514:147–51. doi: 10.1016/j.neulet.2012.02.075. [DOI] [PubMed] [Google Scholar]
- 16.Bleyenheuft Y, Gordon AM. Hand-arm bimanual intensive therapy including lower extremities (HABIT-ILE) for children with cerebral palsy. Phys Occup Ther Pediatr. 2014;34:390–403. doi: 10.3109/01942638.2014.932884. [DOI] [PubMed] [Google Scholar]
- 17.Friel KM, Kuo HC, Carmel JB, Rowny SB, Gordon AM. Improvements in hand function after intensive bimanual training are not associated with corticospinal tract dysgenesis in children with unilateral cerebral palsy. Exp Brain Res. 2014;232:2001–9. doi: 10.1007/s00221-014-3889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bleyenheuft Y, Dricot L, Gilis N, et al. Capturing neuroplastic changes after bimanual intensive rehabilitation in children with unilateral spastic cerebral palsy: A combined DTI, TMS and fMRI pilot study. Res Dev Disabil. 2015:43–44. 136–49. doi: 10.1016/j.ridd.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gordon AM, Chinnan A, Gill S, Petra E, Hung YC, Charles J. Both constraint-induced movement therapy and bimanual training lead to improved performance of upper extremity function in children with hemiplegia. Dev Med Child Neurol. 2008;50:957–8. doi: 10.1111/j.1469-8749.2008.03166.x. doi: [DOI] [PubMed] [Google Scholar]
- 20.Bleyenheuft Y, Arnould C, Brandao MB, Bleyenheuft C, Gordon AM. Hand and Arm Bimanual Intensive Therapy Including Lower Extremity (HABIT-ILE) in Children With Unilateral Spastic Cerebral Palsy: A Randomized Trial. Neurorehabil Neural Repair. 2015;29:6450657. doi: 10.1177/1545968314562109. [DOI] [PubMed] [Google Scholar]
- 21.Paixao S, Balijepalli A, Serradj N, et al. EphrinB3/EphA4-mediated guidance of ascending and descending spinal tracts. Neuron. 2013;80:1407–20. doi: 10.1016/j.neuron.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serradj N, Paixao S, Sobocki T, et al. EphA4-mediated ipsilateral corticospinal tract misprojections are necessary for bilateral voluntary movements but not bilateral stereotypic locomotion. J Neurosci. 2014;34:5211–21. doi: 10.1523/JNEUROSCI.4848-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch Phys Med Rehabil. 1969;50:311–9. doi: [PubMed] [Google Scholar]
- 24.Charles JR, Wolf SL, Schneider JA, Gordon AM. Efficacy of a child-friendly form of constraint-induced movement therapy in hemiplegic cerebral palsy: a randomized control trial. Dev Med Child Neurol. 2006;48:635–42. doi: 10.1017/S0012162206001356. [DOI] [PubMed] [Google Scholar]
- 25.Taylor N, Sand PL, Jebsen RH. Evaluation of hand function in children. Arch Phys Med Rehabil. 1973;54:129–35. doi: [PubMed] [Google Scholar]
- 26.Krumlinde-Sundholm L, Eliasson AC. Development of the Assisting Hand Assessment: A Rash-built Measure Intented for Children with Unilateral Upper Limb Impairments. Scandinavian Journal of Occupational Therapy. 2003;10:16–26. doi: [Google Scholar]
- 27.Krumlinde-Sundholm L, Holmefur M, Kottorp A, Eliasson AC. The Assisting Hand Assessment: current evidence of validity, reliability, and responsiveness to change. Dev Med Child Neurol. 2007;49:259–64. doi: 10.1111/j.1469-8749.2007.00259.x. [DOI] [PubMed] [Google Scholar]
- 28.Holmefur M, Krumlinde-Sundholm L, Eliasson AC. Interrater and intrarater reliability of the Assisting Hand Assessment. Am J Occup Ther. 2007;61:79–84. doi: 10.5014/ajot.61.1.79. doi: [DOI] [PubMed] [Google Scholar]
- 29.Krumlinde-Sundholm L. Reporting outcomes of the Assisting Hand Assessment: what scale should be used? Dev Med Child Neurol. 2012;54:807–8. doi: 10.1111/j.1469-8749.2012.04361.x. [DOI] [PubMed] [Google Scholar]
- 30.Arnould C, Penta M, Renders A, Thonnard JL. ABILHAND-Kids: a measure of manual ability in children with cerebral palsy. Neurology. 2004;63:1045–52. doi: 10.1212/01.wnl.0000138423.77640.37. doi: [DOI] [PubMed] [Google Scholar]
- 31.Law M, Baptiste S, McColl M, Opzoomer A, Polatajko H, Pollock N. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57:82–7. doi: 10.1177/000841749005700207. doi: [DOI] [PubMed] [Google Scholar]
- 32.Wallen M, Ziviani J, Naylor O, Evans R, Novak I, Herbert RD. Modified constraint-induced therapy for children with hemiplegic cerebral palsy: a randomized trial. Dev Med Child Neurol. 2011;53:1091–9. doi: 10.1111/j.1469-8749.2011.04086.x. [DOI] [PubMed] [Google Scholar]
- 33.Verkerk GJ, Wolf MJ, Louwers AM, Meester-Delver A, Nollet F. The reproducibility and validity of the Canadian Occupational Performance Measure in parents of children with disabilities. Clin Rehabil. 2006;20:980–8. doi: 10.1177/0269215506070703. [DOI] [PubMed] [Google Scholar]
- 34.Holmefur M, Aarts P, Hoare B, Krumlinde-Sundholm L. Test-retest and alternate forms reliability of the assisting hand assessment. J Rehabil Med. 2009;41:886–91. doi: 10.2340/16501977-0448. [DOI] [PubMed] [Google Scholar]
- 35.Holmstrom L, Vollmer B, Tedroff K, et al. Hand function in relation to brain lesions and corticomotor-projection pattern in children with unilateral cerebral palsy. Dev Med Child Neurol. 2010;52:145–52. doi: 10.1111/j.1469-8749.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- 36.Staudt M, Gerloff C, Grodd W, Holthausen H, Niemann G, Krageloh-Mann I. Reorganization in congenital hemiparesis acquired at different gestational ages. Ann Neurol. 2004;56:854–63. doi: 10.1002/ana.20297. [DOI] [PubMed] [Google Scholar]
- 37.Mackey A, Stinear C, Stott S, Byblow WD. Upper limb function and cortical organization in youth with unilateral cerebral palsy. Front Neurol. 2014;5:117. doi: 10.3389/fneur.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzzetta A, Bonanni P, Biagi L, et al. Reorganisation of the somatosensory system after early brain damage. Clin Neurophysiol. 2007;118:1110–21. doi: 10.1016/j.clinph.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Staudt M, Braun C, Gerloff C, Erb M, Grodd W, Krageloh-Mann I. Developing somatosensory projections bypass periventricular brain lesions. Neurology. 2006;67:522–5. doi: 10.1212/01.wnl.0000227937.49151.fd. [DOI] [PubMed] [Google Scholar]
- 40.Staudt M, Berweck S. Is constraint-induced movement therapy harmful in unilateral spastic cerebral palsy with ipsilateral cortico-spinal projections? Dev Med Child Neurol. 2014;56:202–3. doi: 10.1111/dmcn.12372. [DOI] [PubMed] [Google Scholar]
- 41.Eyre JA, Taylor JP, Villagra F, Smith M, Miller S. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–54. doi: 10.1212/WNL.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 42.Jaspers E, Byblow WD, Feys H, Wenderoth N. The corticospinal tract: A biomarker to categorize upper limb functional potential in unilateral cerebral palsy. Frontiers in Pediatrics. 2016;3:112. doi: 10.3389/fped.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]


