Abstract
Heparin and heparan sulfate glycosaminoglycans are long, linear polysaccharides that are made up of alternating dissacharide sequences of highly sulfated uronic acid and amino sugars. Unlike heparin, which is only found in mast cells, heparan sulfate is ubiquitously expressed on the cell surface and in the extracellular matrix of all animal cells. These negatively-charged carbohydrate chains play essential roles in important cellular functions such as cell growth, adhesion, angiogenesis, and blood coagulation. However, these biomolecules are also involved in pathophysiological conditions such as pathogen infection and human disease. This review discusses past and current methods for targeting these complex biomolecules as a novel therapeutic strategy to treating disorders such as cancer, neurodegenerative diseases, and infection.
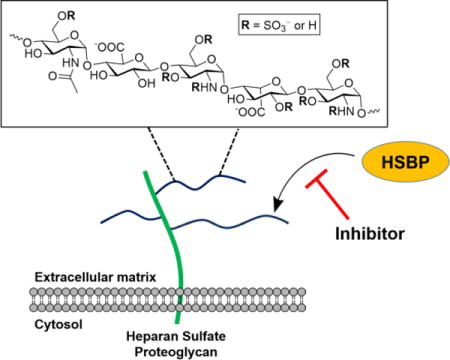
Graphical Abstract
Heparan sulfate is ubiquitously expressed on the cell surface and in the extracellular matrix of all animal cells. These negatively-charged carbohydrate chains play essential roles in important cellular functions such as cell growth, adhesion, angiogenesis, and blood coagulation by interacting with various heparan sulfate binding proteins (HSBP). This review discusses methods for targeting these complex biomolecules, as a strategy for treating disorders such as cancer, neurodegenerative diseases, and infection.
Introduction
Heparan sulfate proteoglycans (HSPGs) are glycoconjugates found in the glycocalyx that surround virtually all mammalian cells.1 Each HSPG consists of a core protein linked to one or more linear heparan sulfate (HS) chains. The chains are composed of alternating D-glucosamine and uronic acids (D-glucuronic and L-iduronic acids) that can be variably N- and O-sulfated (Figure 1). The presence of sulfate groups at specific positions in HS imparts an overall high negative charge and their arrangement in short segments of the chain create binding sites for protein ligands.2 HSPGs are assembled in the endoplasmic reticulum and Golgi apparatus by glucosyltransferases, sulfotransferases and an epimerase and can be processed further by plasma membrane bound endosulfatases that remove sulfate groups from specific sites.3 The composition of HS varies spatially and temporally in different cell types and during development, but the factors involved in regulating its biosynthesis remain poorly defined.2 Nevertheless, the HSPG’s expressed on the cell surface and in the surrounding extracellular matrix tend to be heritable and impart the capacity for selective cell signaling and cell-to-cell crosstalk.4
Figure 1.
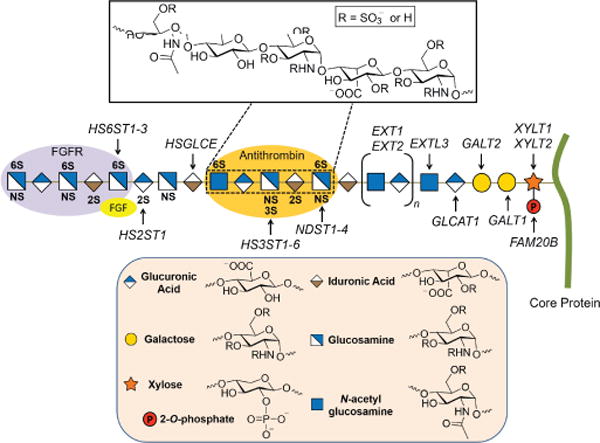
Heparan sulfate structure and biosynthesis. Respective O- and N-sulfotransferases (HS2ST, HS3ST, HS6ST, and NDST) sulfate specific sites along the HS chain (shown in bold). The purple and orange oval shapes depict protein binding sites along the HS chain. Inset: Pentasaccharide antithrombin (AT) binding sequence.
There are at least 300 known HS-binding proteins (HSBPs) with varying affinity, specificity, and function.5 The family of HS-binding proteins includes growth factors, cytokines, chemokines, enzymes, enzyme inhibitors, and extracellular matrix proteins.6 Binding to HS can have profound effects on deposition and presentation of HSBPs at their sites of production, protection of HSBPs against proteolysis, functioning of complexes with signaling receptors, protein oligomerization, and allosteric effects on enzyme activity.4b
There has been a considerable amount of research investigating the nature of HS–protein interactions.5, 7 Certain proteins preferentially bind spatial arrangements of the sulfated sugar subunits of HS rather than specific sequences. In other cases, a lock and key fit occurs, such as found for antithrombin-heparin.4b Over two decades ago, HS was shown to act as a co-receptor for the binding of growth factors to receptor tyrosine kinases, such as fibroblast growth factor 2 (FGF2) and vascular endothelial growth factor (VEGF) binding to FGF and VEGF receptors, respectively.8 For example, a complex is formed between HS, FGF2 and the FGF2 receptor (Figure 2), resulting in FGF/FGFR dimerization and activation of the tyrosine kinase domain and signal transduction.9 Whereas FGF2 binding to HS depends on N-sulfation of glucosamine residues and 2-O-sulfation of iduronic acids, additional 6-O-sulfated sites on glucosamine HS are essential for its interaction with the FGF receptor.4b, 9–10 Thus, the analysis of HS–protein interactions are often complex and require different contiguous sequences of sulfated domains.
Figure 2.
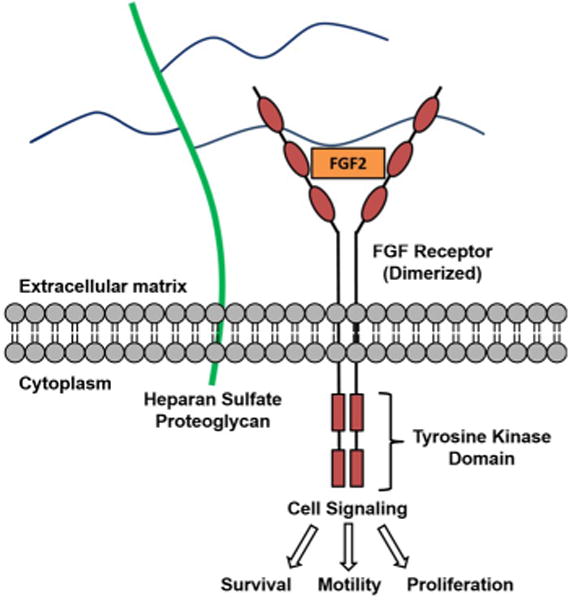
FGF2 binds to HS (blue lines) and forms a ternary complex with its receptor.
HSPGs have also been implicated in pathophysiological conditions.11 For example, changes in HS composition occur in tumors, and tumor growth and angiogenesis depend on HS– growth factor interactions.11b, 12 Changes in the expression level and composition of HS have been shown to correlate with tumor cell transformation and invasiveness.12a The HSPG syndecan-1 is expressed abundantly on the cell surface of epithelial tumors13 and multiple myeloma cells14 and is up regulated in pancreatic cancer.15 Additionally, HS has been implicated in neurodegenerative disorders including prion disease (e.g., mad cow disease),16 Parkinson’s,17 and Alzheimer’s disease.18 Many pathogens, including viruses and bacteria, are able to display HS-binding proteins that help facilitate their attachment to host cells, mediate cell entry, and increase infectivity.4b, 30 Some examples are illustrated in Table 1. In most cases, HSPGs on the plasma membrane act as initial, low affinity co-receptors that concentrate the pathogen on the cell surface and promote binding to secondary receptors, which stimulates infection. Due to the apparent role of these complex polysaccharides in infection and disease, HS is a viable drug target.
Table 1.
Examples of heparin/HS-binding proteins and their biological activity.
| Class | Examples | Physiological Function | Pathophysiology | Refs |
|---|---|---|---|---|
| Growth factors | FGF2, VEGF165, neuropilin-1 | mitogenesis, development, wound healing, angiogenesis, axon guidance | cancer | 8b, 19 |
| ECM proteins | collagen, fibrinogen, laminin | cell adhesion, migration, differentiation, blood coagulation | cancer, Knobloch syndrome | 20 |
| Cell adhesion proteins | P-selectin, L-selectin, integrins | cell adhesion, inflammation | cancer | 21 |
| Morphogens | activin, BMP-2, sonic hedgehog | development, regeneration, bone formation | multiple hereditary exostoses, osteoarthritis | 22 |
| Chemokines | platelet factor 4, IL-8, TNF-α, CXCL12 | chemotaxis, cell migration, immune response, angiogenesis | inflammation, arteriosclerosis, cancer | 23 |
| Blood coagulation factors | antithrombin III, factor Xa, leuserpin-2 | regulation of clotting cascade | heparin-induced thrombocytopenia | 24 |
| Lipoproteins | ApoE, ApoB, lipoprotein lipase | lipid metabolism, cell membrane functions | atherosclerosis, Alzheimer’s disease, AA amyloidosis | 25 |
| Nuclear proteins | histones, transcription factors | unknown | cancer | 26 |
| Amyloid proteins | APP, Aβ, tau protein, α-synuclein, PrPSc | synapse organization, brain development, memory, circadian rhythm | Alzheimer’s/Parkinson’s disease, prion disease | 27 |
| Viral proteins | gB, gC, gD, gp120, Tat, E protein, L1 capsid protein | ----- | HIV-1, HPV, HSV-1, HCMV, Dengue virus | 28 |
| Microbial proteins | M protein, PfEMP1, Opa, circumsporozoite protein | ----- | bacterial/parasite infection, inflammation | 29 |
Heparin, a fractionated form of HS from porcine entrails or horse lungs, is a powerful anticoagulant used in surgery and for treatment of clotting disorders.31 The purification of heparin involves a series of steps that enrich for highly sulfated oligosaccharide fragments with high anticoagulant activity. Its anticoagulant activity is due to its high affinity to the serine protease inhibitor antithrombin III (AT).31b–d Heparin has the highest negative charge density of any known biomolecule (~3.3 negative charges per disaccharide) and is related in structure to HS (2.3 sulfate groups per disaccharide in heparin vs. ~0.8 sulfate groups per disaccharide in typical HS). While HS is ubiquitously expressed in all animal cells, heparin is produced and stored selectively in the secretory granules of connectivetissue mast cells.32 Low molecular weight (e.g. enoxaparin) and ultralow molecular weight heparins (fondaparinux) are also in clinical use.33 Progress has also been made in making non-anticoagulant forms of heparin that might be useful as competitive ligands for treating other types of disorders.34
While excellent reviews have been published on HS structure, biosynthesis, and function,1, 4a, 7b, 35 and heparin/HS binding proteins,5, 7a, 36 the purpose of this contribution is to provide an overview of various approaches and therapeutic agents used to target heparin and heparan sulfate.
Targeting heparan sulfate-protein interactions
Several different strategies have been explored to target HS– protein interactions including enzymatic methods to remove or modify HS, the use of glycosaminoglycan (GAG) mimetics to competitively block HS–protein interactions, and the utilization of large cationic proteins or small molecules to antagonize HS-protein interactions. The following sections will provide an overview of each strategy and will give details of diverse agents that have been designed over recent years.
Enzymatic targeting of HS
Enzymes such as bacterial heparinases and mammalian endosulfatases have been explored as potential agents for the treatment of disorders involving HS–protein interactions. Heparinases I (Hep I) and III (Hep III) from Flavobacterium heparinum specifically cleave the highly sulfated (Hep I) and poorly sulfated (Hep III) regions of the HS polysaccharide backbone (Hep II cleaves both regions),37 while endosulfatases remove specific sulfate residues located in HS chains (Figure 3).3, 38 These enzymes serve as useful tools for biologists probing the role of HS in homeostasis and disease. Some groups have looked at their effect on preventing infection and other processes dependent on the interaction with cell-surface HS. Treatment of cells with heparinases inhibits the attachment or entry of several HS-binding pathogens including viruses,39 bacteria,40 and parasites.41 Heparinase treatment has also been explored in tumor growth/metastasis42 and amyloid-related diseases in mice.25f, 43 Early clinical trials demonstrated that a single intravenous injection of recombinant heparinase-I (Neutralase) could dose-dependently neutralize anticoagulant heparin in heart surgery patients.44 However, later trials were terminated due to ineffectiveness and safety concerns. Endosulfatases are important enzymes that edit the sulfated domains of HS by removing the 6-O-sulfate groups.45 Modulation of endosulfatases in cells has been shown to limit Chlamydia muridarum bacterial infection.46 Sulfatase 1 (Sulf1) might prove useful as a tool to modulate the capacity of HSPGs to bind to growth factors up regulated in tumors such as FGF247 and hepatocyte growth factor.48 However, targeting these agents to selectively modulate HS on tumor cells without affecting normal tissues remains a challenge.
Figure 3.
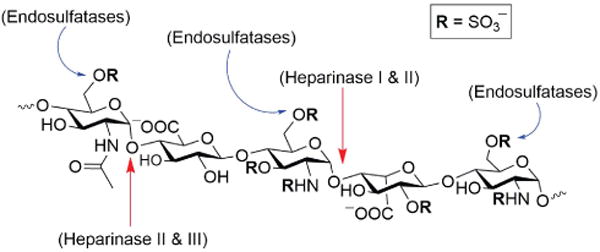
Heparinase (I-III) and endosulfatase (Sulf1, Sulf2) activity on a pentasaccharide region of heparin/HS.
GAG mimetics
Another approach being explored for inhibiting HS–protein interactions is the use of exogenous heparin/HS or synthetic GAG mimetics as competitive inhibitors. Exogenously added heparin and HS chains inhibit infection of host cells by HS-binding pathogens including HSV,49 HPV,39b hepatitis B,39c, 50 and various bacteria.51 Additionally, exogenous HS and heparin have been used to block cancer cell growth and metastasis.52 However, there are major drawbacks associated with using heparin due to its anticoagulant activity and the risk of heparin-induced thrombocytopenia.53 The use of soluble HS and heparin also can result in undesirable effects on normal cells and physiology. To circumvent some of these issues, GAG derivatives have been generated (Figure 4). Some of these polyanionic molecules can be synthesized by digestion of heparin polysaccharides. In one example, a nonsulfated K5 polysaccharide from E. coli was engineered to inhibit viral infection.54 Another study examined a synthetic 3-O-sulfated octasaccharide to block infection by HSV, which depends on a 3-O-sulfated domain in HS.55 Chemically modified heparin derivatives were shown to inhibit influenza H5N1 virus attachment.56 Selective desulfation of heparin minimized its anticoagulant effect while maintaining inhibitory activity. Partially depolymerized GAGs have been made for the treatment of malaria.57
Figure 4.

Examples of GAG mimetic compounds.
Low molecular weight heparin and HS mimetics, such as rhamnan sulfate,58 PG545,59 and PI-88 (1),60 have displayed potent antiviral and anticancer activity. HS-imitative sulfated polysaccharides isolated from seaweed known as carrageenans (2) have also shown the ability to inhibit dengue virus61 and HSV infection.62
Sulfated small molecules have been constructed from commercially available starting materials to avoid the heterogeneity implicit in using naturally occurring GAGs and GAG fragments (Figure 4). GAG mimetics such as 3-amino-1-propanesulfonic acid (3)63 and eprodisate disodium (4)64 have been tested as anti-amyloid agents for the treatment of Alzheimer’s disease and amyloidosis. Polysulfonated compounds such as suramin (5),65 poly(sodium-4-styrene sulfonate) (6),66 and sulfonate polymers have been tested as well.67
GAG mimetic compounds have shown promise as therapeutics when tested in vitro, but translation of these findings in vivo have not yet met with success. Several of these compounds, such as PI-88 and PG545, are currently in clinical trials for blocking tumor growth.68 PG545 exhibited tolerability and a long plasma half-life when administered by intravenous infusion for treatment of advanced solid tumors (ClinicalTrials.gov NCT02042781). However, later clinical trials were terminated due to negative reactions upon injection.69 Daily injections of PI-88 have shown preliminary efficacy as an adjuvant therapy for hepatocellular carcinoma and melanoma in Phase I and II clinical trials.70 Further studies are still ongoing to determine its safety and efficacy.71 Additionally, carrageenan has been formulated as a prophylactic microbicidal gel to block HIV and HPV infection.72 Unfortunately, it failed in a Phase III trial and has been discontinued.
Cationic proteins and polymers as HS antagonists
Other types of agents used as antagonists of HS–protein interactions include cationic proteins, foldamers, and small molecules. These molecules rely on electrostatic interactions between their positively charged functional groups and the highly anionic sulfate and carboxylate moieties of heparin and HS. Lactoferrin, a heparin- and iron-binding protein found in the secretory granules of neutrophils, has been shown to neutralize heparin and antagonize certain HS–protein interactions.73 Lactoferrin has proven to be an effective antimicrobial agent74 and inhibitor of HSV,75 hepatitis C (HCV),76 HIV, and human cytomegalovirus (HCMV) infection.77 However, clinical trials observing the oral treatment of HCV with a combination of lactoferrin and interferon78 or interferon alpha-2b and ribavirin79 showed no added benefits compared to treatments without lactoferrin. Other proteins have been tested as potent inhibitors of heparin and its derivatives, including inactive recombinant antithrombin (AT) variants designed to bind heparin.80 These modified proteins have shown promise in vitro and in vivo in mice, but they may prove expensive to produce in large quantities for clinical use.
Other cationic macromolecules have proven to be potent antagonists of GAG–protein interactions. Positively-charged arginine-rich proteins isolated from the sperm of salmon and other fish, known as protamine, have long been used clinically to reverse the anticoagulant activity of heparin, despite undesired side effects and allergic reactions observed in some patients.81 Protamine binds to heparin and blocks its interaction with antithrombin in the coagulation cascade. Protamine was also shown to inhibit hepatitis B viral infection through blocking viral interaction with cell-surface HSPGs.50 Protamine inhibits host cell infection of Pseudomonas aeruginosa by preventing bacterial-enhanced HSPG shedding.82 Low molecular weight protamine (LMWP) has also been explored as a safer alternative to protamine in vitro83 and in vivo in dogs.84 An arginine-rich protamine variant (VSRRRRRRGGRRRR) is currently being investigated as a nontoxic and non-immunogenic protamine substitute for neutralization of heparin and LMWH.85
Protamine, despite potential undesirable side effects, is currently the only heparin reversal agent used clinically.86 Many other polycationic agents have therefore been investigated as alternatives to protamine for heparin neutralization. Hexadimethrine bromide (i.e. polybrene) (7) was one of the first protamine alternatives investigated in vivo.87 This fully synthetic cationic polymer showed promise, but was eventually abandoned due to toxicity and lower efficacy compared to protamine. Histones, polycationic proteins that normally function in DNA packaging, and synthetic poly-DL-lysine (8), have also been explored as heparin neutralization agents, but proved to be inferior to protamine or exhibited high toxicity (Figure 5).88 A more recent study utilized poly-L-lysine dendrimers with glycine cores [Gly–Lys63(NH2)64] as neutralization agents. These dendrimers neutralized heparin activity in rats.89 Another study utilized arginine-substituted poly(allylamine hydrochloride) derivatives (9) for potent reversal of heparin in vitro and in vivo.90 The polycationic macrocyclic calix[8]arenes (10), also have been shown to rapidly neutralize heparin in blood through tight electrostatic interactions.91 A subsequent study showed these molecules, immobilized onto polymer matrix filters, were able to remove heparin from blood, therefore minimizing side effects associated with administration of heparin reversal agents.92
Figure 5.
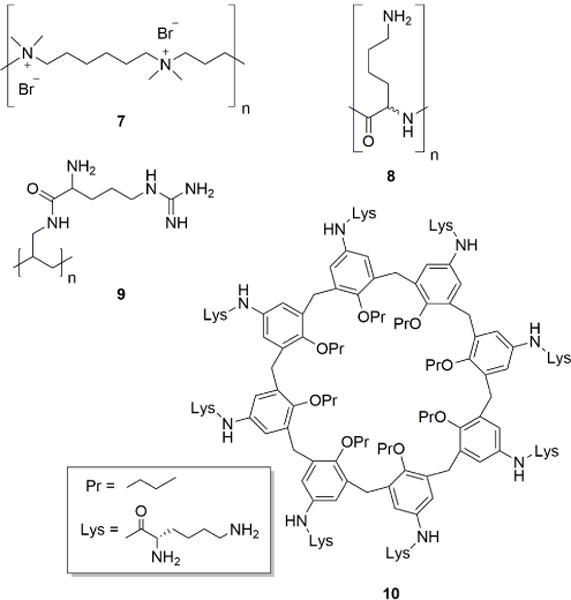
Examples of cationic antagonists of heparin and HS.
Polyvalent peptide-derivatized dendrimers that display multiple cationic motifs have also been investigated for interaction with heparin and HS. Arginine-rich polycationic virus-like particles neutralize the heparin-antithrombin interaction in human plasma,93 branched dendrimer-based peptides inhibited HSV-1 and 2 infection,94 and a large peptide isolated from human hemofiltrate blocked HCMV infection in vitro through a HS-dependent pathway.95 Synthetic ligands (e.g., 11) that form micelles can bind heparin through electrostatic interactions of their protonated amines (Figure 6).96 A universal synthetic heparin reversal agent (UHRA) was developed based on a branched dendritic polyglycerol core containing multiple hexamethylated tris(2-aminoethylamine) binding groups (12).97 These UHRA molecules show great promise as safer alternatives to protamine for neutralization of heparin and its analogs; however, their application and safety in humans need to be explored.
Figure 6.
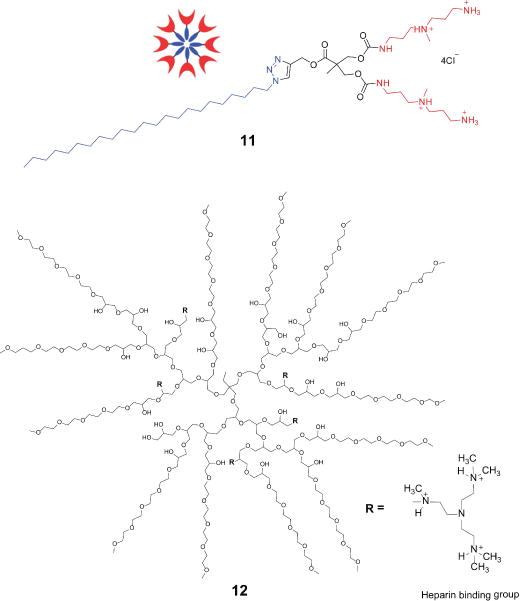
Synthetic self-assembling ligands (11) and UHRA (12) for multivalent binding to heparin. Adapted from Ref. 96 with permission from the Royal Society of Chemistry.
Small molecule antagonists of heparin and HS
Small, low molecular weight compounds make up 90% of approved drugs and have the advantage of versatility, simplified manufacturing, formulation, delivery, and stability when compared to biologics.98 As such, small molecules (MW < 900 Da) have also been exploited as antagonists of HS and heparin. A dispirotripiperazine derivative, (DSTP 27) (13), was discovered to bind to HS on the cell surface and inhibit attachment, absorption, and replication of a wide range of viruses (Figure 7).99 This compound proved to be a potent antiviral in a structure-activity relationship study with a novel class of antiviral compounds containing the N,N’-bis-5-pyrimidyl moiety.100 A subsequent study demonstrated the ability of DSTP 27 to block HS-dependent viral attachment of an HPV virus with long-term efficacy.101
Figure 7.

Small molecule antagonists of heparin and HS-protein interactions.
A water soluble small molecule heparin antagonist, ciraparantag (PER977) (14), was recently found to potently bind and neutralize the activity of multiple anticoagulants including unfractionated heparin and the LMWH enoxaparin.102 In an early phase I/II trial, a single dose of ciraparantag restored baseline homeostasis within 10–30 minutes and the reversal effects were sustained for up to 24 hours in patients.103 This promising compound is currently being studied in phase III clinical trials as an intravenously administered universal antagonist of heparinoids and other novel oral anticoagulants.
A new family of aromatic cationic small molecule antagonists of HS were synthesized and probed as anti-inflammatory agents (e.g. 15, 16).104 They were first investigated as inhibitors of protein binding to HS in a 96-well ELISA-based assay. The most potent compounds were also evaluated for their ability to block acute inflammation in mice with positive results. A catechin extract from green tea, epigallocatechin gallate (EGCG) (17), was shown to inhibit virion attachment of many unrelated viruses to HS and sialic acid, another type of charged glycan found on the cell surface.105 This broad-spectrum antiviral is one of the first small molecules effective against enveloped and nonenveloped viruses such as SV-1, HCV, and influenza A virus (IAV). EGCG blocked the binding of HSV-1 and HCV to HS, while also inhibiting attachment of IAV to sialic acid.
Synthetic small molecule peptide mimics known as foldamers (e.g., 18), based on amine and guanidinium-substituted arylamides, have also been developed for in vitro neutralization of heparin (Figure 8). These molecules self-assemble to form β-like sheets with enhanced association to fondaparinux, a synthetic pentasaccharide analog of heparin.106 A novel salicylamide derivative, known as delparantag (19), was also developed as an alternative to protamine administration.107 This compound, displaying multiple cationic lysines and aromatic amino acid units, neutralized heparin and enoxaparin, a low molecular weight heparin (LMWH), in rats and later showed promise in a small trial in humans.108 However, phase II clinical trials revealed unsafe toxicity profiles and hypotension in patients upon intravenous infusion of delparantag (plasma half-life of 3–5 minutes), thus halting further development of this compound.109
Figure 8.
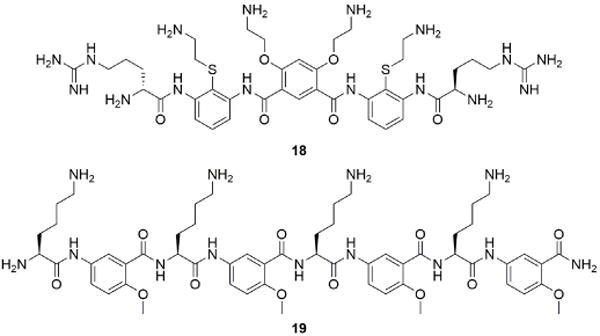
Foldamers: small molecule peptide mimetics for neutralizing heparin.
A high-throughput screen of the National Cancer Institute’s (NCI) small molecule diversity set identified bis-2-methyl-4-amino-quinolyl-6-carbamide (20), a dimeric aminoquinoline called surfen (NSC 12155), as a potent small molecule antagonist of HS (Figure 9).110 Surfen binds to multiple types of GAGs in solution, but most potently to heparin and HS. Surfen neutralized the ability of heparin to activate antithrombin, blocked the sulfation and degradation of GAG chains by bacterial lyases in vitro, and inhibited angiogenesis initiated by the binding of HS-dependent growth factors. In addition, surfen blocked HS-mediated HSV-1 infection and the enhancement of HIV-1 infection by amyloid fibrils found in semen (known as SEVI).111 Interestingly, surfen not only inhibited HS-mediated viral attachment but also directly bound to SEVI fibrils and disrupted their interaction with the virus.
Figure 9.
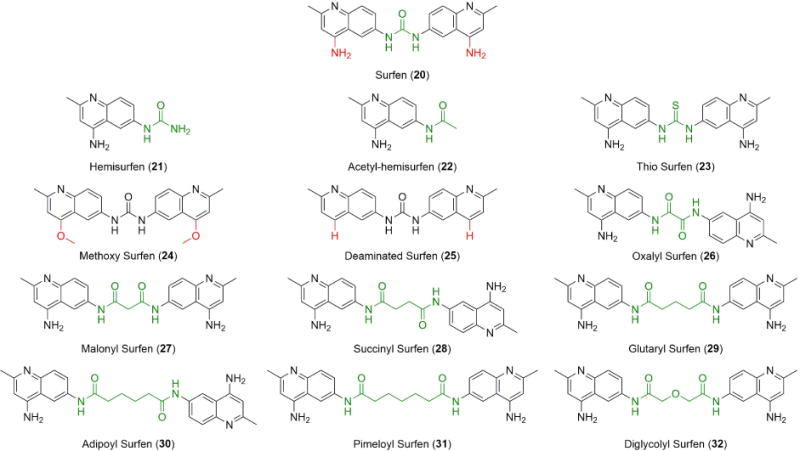
Structures of surfen (20) and its analogs. Modifications are highlighted in red and green. Reproduced from Ref. 118 with permission from the Royal Society of Chemistry.
Other studies have utilized surfen’s ability to antagonize HS as a tool to study HS interactions in biological systems. Surfen has been shown to regulate murine T-cell activation and proliferation,112 stimulate chondrogenesis in vitro,113 inhibit highly anionic polyphosphates involved in the inflammatory process,114 and reduce lesion formation in demyelination of a multiple sclerosis mouse model.115 Surfen also acts as an inhibitor of vascular endothelial growth factor receptor (VEGFR) phosphorylation and vascular hyperpermeability in mice.116
The biocompatibility of surfen, based on early studies of surfen as an excipient for depot insulin,117 encourages further research of its neutralizing activity. However, due to this compound’s relatively low efficacy (micromolar range), the design and synthesis of more selective and potent analogs was undertaken (Figure 9). A structure-activity relationship was established by measuring the inhibition of HS-dependent FGF binding to CHO cells.118 In addition, their activity as inhibitors of HS binding to receptor for advanced glycation endproducts (RAGE) in vitro and neutralization of the anticoagulant activity of unfractionated heparin and low molecular weight heparins were investigated. The dimeric molecular structure of surfen and its aminoquinoline ring systems were found to be essential for its interaction with HS. Dimeric analogs displayed higher inhibitory potency than surfen and blocked downstream FGF signaling in mouse embryonic fibroblast cells.118 Importantly, oxalyl surfen (26) was shown to neutralize both in vitro and in mice the synthetic heparin analog fondaparinux, for which no antidote exists.118 These findings illustrate the therapeutic potential of small molecules as antagonists of HS and raise the possibility of using surfen-type compounds as biochemical tools and as potential effectors in disorders that involve GAG-protein interactions.
Summary and Prospects
GAGs are vital components of the glycocalyx and are involved in many important biological processes that are essential for maintaining homeostasis. HSPGs, in particular, perform vital roles in cell signaling and cell-to-cell interactions. The heterogeneity and anionic nature of HS polysaccharides allow them to interact with a large number of proteins, including viral and bacterial proteins. Their apparent pathophysiological roles in disease and infection have stimulated research into the development of agents that can antagonize HS–protein and HS–pathogen interactions. As mentioned above, different methods for blocking these interactions have been explored including enzymatic alteration of HS, GAG mimetic compounds as competitive protein binders, and various types of cationic molecules that bind competitively to HS and inhibit its binding to proteins.
There is still much to uncover about HS–protein interactions, as illustrated by the discovery of new HS-binding proteins.5 As in all areas of medicinal chemistry, there are numerous challenges associated with the development of novel therapeutics that target these types of biomolecules and their biomolecular interactions. Most of the agents developed so far have failed to yield approved treatments, in part because it has been difficult to identify agents that target only undesirable interactions while leaving intact other vital HS-protein interactions.
Currently, the only clinically approved drug known to block GAG–protein interactions is the heparin-neutralizing agent protamine.119 The success of protamine stems from its potent activity and acute application in patients to reverse heparin overdose. Nevertheless, protamine can exhibit adverse side effects in some patients due to anaphylactic reaction to foreign protein.120 Protamine also lacks the ability to neutralize newly developed ultra-low molecular weight heparin derivatives, such as the synthetic pentasaccharide fondaparinux.121 Thus, finding safer alternatives to protamine that can neutralize heparin and LMWHs is an important area of research. It remains to be seen whether the answer to this challenge will be found in small molecules or larger heparin-neutralizing oligomeric materials.
Developing effective agents to target GAG–protein interactions in disease by focusing on GAG-selective agents is a formidable challenge due to the ubiquitous expression of GAGs in different tissues and the likelihood of adverse effects. Most GAG inhibitors rely on blocking electrostatic interactions that dominate GAG–protein interactions, without any sequence specificity. The dearth of structural information available about the nature of these binding sites makes it difficult to model and identify selective inhibitors. Advances should be forthcoming as new schemes for synthesizing chemically defined HS oligosaccharides emerge,122 as glycan array technology becomes refined,123 and with the development of new top down methods for sequencing HS oligosaccharides.124 Up to now, the discovery of novel inhibitors of heparin– and HS–protein interactions has been an exciting area of research. We hope that continued development of drugs targeting these complex carbohydrates and the proteins they interact with will lead to new therapeutic approaches for treating human disorders.
Supplementary Material
Acknowledgments
The authors acknowledge grants CA199292 and HL107150 (to J.D.E.) and GM077471 (to Y.T.) from the National Institutes of Health.
Notes and references
- 1.Bishop JR, Schuksz M, Esko JD. Nature. 2007;446:1030–1037. doi: 10.1038/nature05817. [DOI] [PubMed] [Google Scholar]
- 2.Esko JD, Lindahl U. J Clin Invest. 2001;108:169–173. doi: 10.1172/JCI13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, Emerson CP., Jr Science. 2001;293:1663–1666. doi: 10.1126/science.293.5535.1663. [DOI] [PubMed] [Google Scholar]
- 4.(a) Sarrazin S, Lamanna WC, Esko JD. Csh Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Varki A. Essentials of glycobiology. 2nd. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, N.Y.: 2009. [PubMed] [Google Scholar]
- 5.Xu D, Esko JD. Annual Review of Biochemistry. 2014;83:129–157. doi: 10.1146/annurev-biochem-060713-035314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarrazin S, Lamanna WC, Esko JD. Cold Spring Harbor Perspectives in Biology. 2011;3:a004952. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Kreuger J, Spillmann D, Li J-p, Lindahl U. The Journal of Cell Biology. 2006;174:323–327. doi: 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Annual Review of Biochemistry. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]; (c) Kjellen L, Lindahl U. Annual Review of Biochemistry. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]; (d) Lindahl U, Li Jp. In: International Review of Cell and Molecular Biology. Kwang WJ, editor. Vol. 276. Academic Press; 2009. pp. 105–159. [DOI] [PubMed] [Google Scholar]; (e) Proudfoot AEI. Biochemical Society Transactions. 2006;34:422–426. doi: 10.1042/BST0340422. [DOI] [PubMed] [Google Scholar]
- 8.(a) Grünewald FS, Prota AE, Giese A, Ballmer-Hofer K. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 2010;1804:567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]; (b) Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadi M, Olsen SK, Ibrahimi OA. Cytokine & Growth Factor Reviews. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Yayon A, Klagsbrun M. Cancer and Metastasis Reviews. 1990;9:191–202. doi: 10.1007/BF00046360. [DOI] [PubMed] [Google Scholar]
- 11.(a) Lindahl U, Kjellen L. Journal of Internal Medicine. 2013;273:555–571. doi: 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]; (b) Knelson EH, Nee JC, Blobe GC. Trends in biochemical sciences. 2014;39:277–288. doi: 10.1016/j.tibs.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Blackhall FH, Merry CLR, Davies EJ, Jayson GC. British Journal of Cancer. 2001;85:1094–1098. doi: 10.1054/bjoc.2001.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Okolicsanyi RK, van Wijnen AJ, Cool SM, Stein GS, Griffiths LR, Haupt LM. Journal of cellular biochemistry. 2014;115:967–976. doi: 10.1002/jcb.24746. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Nat Rev Cancer. 2002;2:521–528. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 13.Szatmári T, Dobra K. Frontiers in Oncology. 2013;3:310. doi: 10.3389/fonc.2013.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ridley RC, Xiao H, Hata H, Woodliff J, Epstein J, Sanderson RD. Blood. 1993;81:767–774. [PubMed] [Google Scholar]
- 15.Conejo JR, Kleeff J, Koliopanos A, Matsuda K, Zhu ZW, Goecke H, Bicheng N, Zimmermann A, Korc M, Friess H, Büchler MW. International Journal of Cancer. 2000;88:12–20. doi: 10.1002/1097-0215(20001001)88:1<12::aid-ijc3>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 16.(a) Ben-Zaken O, Tzaban S, Tal Y, Horonchik L, Esko JD, Vlodavsky I, Taraboulos A. Journal of Biological Chemistry. 2003;278:40041–40049. doi: 10.1074/jbc.M301152200. [DOI] [PubMed] [Google Scholar]; (b) Warner RG, Hundt C, Weiss S, Turnbull JE. Journal of Biological Chemistry. 2002;277:18421–18430. doi: 10.1074/jbc.M110406200. [DOI] [PubMed] [Google Scholar]; (c) Horonchik L, Tzaban S, Ben-Zaken O, Yedidia Y, Rouvinski A, Papy-Garcia D, Barritault D, Vlodavsky I, Taraboulos A. Journal of Biological Chemistry. 2005;280:17062–17067. doi: 10.1074/jbc.M500122200. [DOI] [PubMed] [Google Scholar]
- 17.(a) Cohlberg JA, Li J, Uversky VN, Fink AL. Biochemistry. 2002;41:1502–1511. doi: 10.1021/bi011711s. [DOI] [PubMed] [Google Scholar]; (b) Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Proceedings of the National Academy of Sciences. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Horssen J, Wesseling P, van den Heuvel LPWJ, de Waal RMW, Verbeek MM. The Lancet Neurology. 2003;2:482–492. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 19.(a) Ellis LM. American Association for Cancer Research. 2006;5:1099–1107. [Google Scholar]; (b) Turner N, Grose R. Nature Reviews Cancer. 2010;10:116–129. doi: 10.1038/nrc2780. [DOI] [PubMed] [Google Scholar]; (c) Rapraeger A, Krufka A, Olwin B. Science. 1991;252:1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]; (d) Thacker BE, Seamen E, Lawrence R, Parker MW, Xu Y, Liu J, Vander Kooi CW, Esko JD. ACS Chemical Biology. 2016;11:971–980. doi: 10.1021/acschembio.5b00897. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Krilleke D, Ng YS, Shima DT. Biochemical Society transactions. 2009;37:1201–1206. doi: 10.1042/BST0371201. [DOI] [PubMed] [Google Scholar]
- 20.(a) Sertie AL, Sossi V, Camargo AA, Zatz M, Brahe C, Passos-Bueno MR. Human molecular genetics. 2000;9:2051–2058. doi: 10.1093/hmg/9.13.2051. [DOI] [PubMed] [Google Scholar]; (b) Sherman-Baust CA, Weeraratna AT, Rangel LB, Pizer ES, Cho KR, Schwartz DR, Shock T, Morin PJ. Cancer cell. 2003;3:377–386. doi: 10.1016/s1535-6108(03)00058-8. [DOI] [PubMed] [Google Scholar]; (c) Harisi R, Jeney A. OncoTargets and therapy. 2015;8:1387–1398. doi: 10.2147/OTT.S48883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(a) Bendas G, Borsig L. International Journal of Cell Biology. 2012;2012:10. doi: 10.1155/2012/676731. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ley K. Trends in molecular medicine. 2003;9:263–268. doi: 10.1016/s1471-4914(03)00071-6. [DOI] [PubMed] [Google Scholar]
- 22.(a) Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong CH, Lotz MK. Proc Natl Acad Sci U S A. 2010;107:10202–10207. doi: 10.1073/pnas.0913897107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huegel J, Sgariglia F, Enomoto-Iwamoto M, Koyama E, Dormans JP, Pacifici M. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:1021–1032. doi: 10.1002/dvdy.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zak BM, Schuksz M, Koyama E, Mundy C, Wells DE, Yamaguchi Y, Pacifici M, Esko JD. Bone. 2011;48:979–987. doi: 10.1016/j.bone.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Baggiolini M. J Intern Med. 2001;250:91–104. doi: 10.1046/j.1365-2796.2001.00867.x. [DOI] [PubMed] [Google Scholar]; (b) Monneau Y, Arenzana-Seisdedos F, Lortat-Jacob H. Journal of leukocyte biology. 2016;99:935–953. doi: 10.1189/jlb.3MR0915-440R. [DOI] [PubMed] [Google Scholar]
- 24.(a) Kelton JG, Warkentin TE. Blood. 2008;112:2607–2616. doi: 10.1182/blood-2008-02-078014. [DOI] [PubMed] [Google Scholar]; (b) Jin L, Abrahams JP, Skinner R, Petitou M, Pike RN, Carrell RW. Proceedings of the National Academy of Sciences. 1997;94:14683–14688. doi: 10.1073/pnas.94.26.14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.(a) Gonzales JC, Gordts PLSM, Foley EM, Esko JD. The Journal of Clinical Investigation. 2013;123:2742–2751. doi: 10.1172/JCI67398. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gordts PLSM, Foley E, Lawrence R, Sinha R, Lameda-Diaz C, Deng L, Nock R, Glass CK, Erbilgin A, Lusis AJ, Witztum JL, Esko JD. Cell metabolism. 2014;20:813–826. doi: 10.1016/j.cmet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. The Journal of Clinical Investigation. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) MacArthur JM, Bishop JR, Stanford KI, Wang L, Bensadoun A, Witztum JL, Esko JD. Journal of Clinical Investigation. 2007;117:153–164. doi: 10.1172/JCI29154. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Noborn F, Ancsin JB, Ubhayasekera W, Kisilevsky R, Li JP. J Biol Chem. 2012;287:25669–25677. doi: 10.1074/jbc.M112.363895. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Li JP, Galvis MLE, Gong F, Zhang X, Zcharia E, Metzger S, Vlodavsky I, Kisilevsky R, Lindahl U. P Natl Acad Sci USA. 2005;102:6473–6477. doi: 10.1073/pnas.0502287102. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Eisenberg S, Sehayek E, Olivecrona T, Vlodavsky I. J Clin Invest. 1992;90:2013–2021. doi: 10.1172/JCI116081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.(a) Freeman CG, Parish CR, Knox KJ, Blackmore JL, Lobov SA, King DW, Senden TJ, Stephens RW. Biomaterials. 2013;34:5670–5676. doi: 10.1016/j.biomaterials.2013.03.091. [DOI] [PubMed] [Google Scholar]; (b) Stewart MD, Sanderson RD. Matrix Biology. 2014;35:56–59. doi: 10.1016/j.matbio.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Brockstedt U, Dobra K, Nurminen M, Hjerpe A. Experimental cell research. 2002;274:235–245. doi: 10.1006/excr.2002.5477. [DOI] [PubMed] [Google Scholar]
- 27.(a) Small DH, Nurcombe V, Reed G, Clarris H, Moir R, Beyreuther K, Masters CL. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1994;14:2117–2127. doi: 10.1523/JNEUROSCI.14-04-02117.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Small DH, Williamson T, Reed G, Clarris H, Beyreuther K, Masters CL, Nurcombe V. Annals of the New York Academy of Sciences. 1996;777:316–321. doi: 10.1111/j.1749-6632.1996.tb34439.x. [DOI] [PubMed] [Google Scholar]; (c) Zhang X, Li JP. Progress in molecular biology and translational science. 2010;93:309–334. doi: 10.1016/S1877-1173(10)93013-5. [DOI] [PubMed] [Google Scholar]; (d) Holmes BB, DeVos SL, Kfoury N, Li M, Jacks R, Yanamandra K, Ouidja MO, Brodsky FM, Marasa J, Bagchi DP, Kotzbauer PT, Miller TM, Papy-Garcia D, Diamond MI. Proc Natl Acad Sci U S A. 2013;110:E3138–3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.(a) Spear PG, Shieh MT, Herold BC, WuDunn D, Koshy TI. Adv Exp Med Biol. 1992;313:341–353. doi: 10.1007/978-1-4899-2444-5_33. [DOI] [PubMed] [Google Scholar]; (b) Shukla D, Liu J, Blaiklock P, Shworak NW, Bai X, Esko JD, Cohen GH, Eisenberg RJ, Rosenberg RD, Spear PG. Cell. 1999;99:13–22. doi: 10.1016/s0092-8674(00)80058-6. [DOI] [PubMed] [Google Scholar]; (c) Chen Y, Maguire T, Hileman RE, Fromm JR, Esko JD, Linhardt RJ, Marks RM. Nature medicine. 1997;3:866–871. doi: 10.1038/nm0897-866. [DOI] [PubMed] [Google Scholar]; (d) Patel M, Yanagishita M, Roderiquez G, Bou-Habib DC, Oravecz T, Hascall VC, Norcross MA. AIDS Research and Human Retroviruses. 1993;9:167–174. doi: 10.1089/aid.1993.9.167. [DOI] [PubMed] [Google Scholar]; (e) Vivès RR, Imberty A, Sattentau QJ, Lortat-Jacob H. J Biol Chem. 2005;280:21353–21357. doi: 10.1074/jbc.M500911200. [DOI] [PubMed] [Google Scholar]; (f) Connell BJ, Lortat-Jacob H. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00385. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, Sands JA, Jansen KU, Keller PM. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]; (h) Boyle KA, Compton T. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(a) Freissler E, Meyer auf der Heyde A, David G, Meyer TF, Dehio C. Cellular Microbiology. 2000;2:69–82. doi: 10.1046/j.1462-5822.2000.00036.x. [DOI] [PubMed] [Google Scholar]; (b) Wuppermann FN, Hegemann JH, Jantos CA. Journal of Infectious Diseases. 2001;184:181–187. doi: 10.1086/322009. [DOI] [PubMed] [Google Scholar]; (c) Frick IM, Schmidtchen A, Sjobring U. European journal of biochemistry/FEBS. 2003;270:2303–2311. doi: 10.1046/j.1432-1033.2003.03600.x. [DOI] [PubMed] [Google Scholar]; (d) Vogt AM, Barragan A, Chen Q, Kironde F, Spillmann D, Wahlgren M. Blood. 2003;101:2405–2411. doi: 10.1182/blood-2002-07-2016. [DOI] [PubMed] [Google Scholar]; (e) Pinzon-Ortiz C, Friedman J, Esko J, Sinnis P. J Biol Chem. 2001;276:26784–26791. doi: 10.1074/jbc.M104038200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Bishop JR, Crawford BE, Esko JD. Infection and Immunity. 2005;73:5395–5401. doi: 10.1128/IAI.73.9.5395-5401.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Oliveira FO, Jr, Alves CR, Calvet CM, Toma L, Boucas RI, Nader HB, Castro Cortes LM, Krieger MA, Meirelles Mde N, Souza Pereira MC. Microbial pathogenesis. 2008;44:329–338. doi: 10.1016/j.micpath.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 30.Bartlett AH, Park PW. Expert Reviews in Molecular Medicine. 2010;12 doi: 10.1017/S1462399409001367. 1-null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(a) Hirsh J, Anand SS, Halperin JL, Fuster V. Circulation. 2001;103:2994–3018. doi: 10.1161/01.cir.103.24.2994. [DOI] [PubMed] [Google Scholar]; (b) Wardrop D, Keeling D. British Journal of Haematology. 2008;141:757–763. doi: 10.1111/j.1365-2141.2008.07119.x. [DOI] [PubMed] [Google Scholar]; (c) McLean J. Circulation. 1959;19:75–78. doi: 10.1161/01.cir.19.1.75. [DOI] [PubMed] [Google Scholar]; (d) Björk I, Lindahl U. Mol Cell Biochem. 1982;48:161–182. doi: 10.1007/BF00421226. [DOI] [PubMed] [Google Scholar]
- 32.Guyton AC, Hall JE. Textbook of Medical Physiology. Elsevier Saunders; 2006. [Google Scholar]
- 33.Kakkar AK. Best Practice & Research Clinical Haematology. 2004;17:77–87. doi: 10.1016/j.beha.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.(a) Alekseeva A, Casu B, Cassinelli G, Guerrini M, Torri G, Naggi A. Analytical and bioanalytical chemistry. 2014;406:249–265. doi: 10.1007/s00216-013-7446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Naggi A, Casu B, Perez M, Torri G, Cassinelli G, Penco S, Pisano C, Giannini G, Ishai-Michaeli R, Vlodavsky I. J Biol Chem. 2005;280:12103–12113. doi: 10.1074/jbc.M414217200. [DOI] [PubMed] [Google Scholar]; (c) Casu B, Naggi A, Torri G. Matrix biology : journal of the International Society for Matrix Biology. 2010;29:442–452. doi: 10.1016/j.matbio.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Babazada H, Yamashita F, Yanamoto S, Hashida M. Journal of controlled release : official journal of the Controlled Release Society. 2014;194:332–340. doi: 10.1016/j.jconrel.2014.09.011. [DOI] [PubMed] [Google Scholar]; (e) Liu J, Linhardt RJ. Nat Prod Rep. 2014;31:1676–1685. doi: 10.1039/c4np00076e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Mousa SA, Linhardt R, Francis JL, Amirkhosravi A. Thromb Haemost. 2006;96:816–821. doi: 10.1160/th06-05-0289. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Chen J, Avci FY, Munoz EM, McDowell LM, Chen M, Pedersen LC, Zhang L, Linhardt RJ, Liu J. J Biol Chem. 2005;280:42817–42825. doi: 10.1074/jbc.M504338200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Xu Y, Masuko S, Takieddin M, Xu H, Liu R, Jing J, Mousa SA, Linhardt RJ, Liu J. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Hu YP, Lin SY, Huang CY, Zulueta MM, Liu JY, Chang W, Hung SC. Nature chemistry. 2011;3:557–563. doi: 10.1038/nchem.1073. [DOI] [PubMed] [Google Scholar]
- 35.(a) Casu B, Lindahl U. Advances in carbohydrate chemistry and biochemistry. 2001;57:159–206. doi: 10.1016/s0065-2318(01)57017-1. [DOI] [PubMed] [Google Scholar]; (b) Hacker U, Nybakken K, Perrimon N. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]; (c) Esko JD, Selleck SB. Annual Review of Biochemistry. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]; (d) Sasisekharan R, Venkataraman G. Current Opinion in Chemical Biology. 2000;4:626–631. doi: 10.1016/s1367-5931(00)00145-9. [DOI] [PubMed] [Google Scholar]
- 36.Capila I, Linhardt RJ. Angewandte Chemie (International ed in English) 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.(a) Ernst S, Langer R, Cooney CL, Sasisekharan R. Critical reviews in biochemistry and molecular biology. 1995;30:387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]; (b) Pojasek K, Shriver Z, Hu Y, Sasisekharan R. Biochemistry. 2000;39:4012–4019. doi: 10.1021/bi992514k. [DOI] [PubMed] [Google Scholar]; (c) Ernst S, Venkataraman G, Winkler S, Godavarti R, Langer R, Cooney CL, Sasisekharan R. Biochemical Journal. 1996;315:589–597. doi: 10.1042/bj3150589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.(a) Morimoto-Tomita M, Uchimura K, Werb Z, Hemmerich S, Rosen SD. J Biol Chem. 2002;277:49175–49185. doi: 10.1074/jbc.M205131200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Freeman SD, Keino-Masu K, Masu M, Ladher RK. Developmental dynamics : an official publication of the American Association of Anatomists. 2015;244:168–180. doi: 10.1002/dvdy.24223. [DOI] [PubMed] [Google Scholar]
- 39.(a) de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TBH, Gallay P. Proceedings of the National Academy of Sciences. 2007;104:19464–19469. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Giroglou T, Florin L, Schäfer F, Streeck RE, Sapp M. Journal of Virology. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Leistner CM, Gruen-Bernhard S, Glebe D. Cell Microbiol. 2008;10:122–133. doi: 10.1111/j.1462-5822.2007.01023.x. [DOI] [PubMed] [Google Scholar]; (d) Tiwari V, Clement C, Xu D, Valyi-Nagy T, Yue BY, Liu J, Shukla D. J Virol. 2006;80:8970–8980. doi: 10.1128/JVI.00296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Broutian TR, Brendle SA, Christensen ND. The Journal of General Virology. 2010;91:531–540. doi: 10.1099/vir.0.012732-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Nasimuzzaman M, Persons DA. Mol Ther. 2012;20:1158–1166. doi: 10.1038/mt.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.(a) Alvarez-Dominguez C, Vazquez-Boland JA, Carrasco-Marin E, Lopez-Mato P, Leyva-Cobian F. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) de Vries FP, Cole R, Dankert J, Frosch M, van Putten JP. Mol Microbiol. 1998;27:1203–1212. doi: 10.1046/j.1365-2958.1998.00763.x. [DOI] [PubMed] [Google Scholar]; (c) Moelleken K, Hegemann JH. Mol Microbiol. 2008;67:403–419. doi: 10.1111/j.1365-2958.2007.06050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogt AM, Barragan A, Chen Q, Kironde F, Spillmann D, Wahlgren M. Heparan sulfate on endothelial cells mediates the binding ofPlasmodium falciparum–infected erythrocytes via the DBL1α domain of PfEMP1. 2003 doi: 10.1182/blood-2002-07-2016. [DOI] [PubMed] [Google Scholar]
- 42.(a) Liu D, Shriver Z, Venkataraman G, El Shabrawi Y, Sasisekharan R. Proceedings of the National Academy of Sciences. 2002;99:568–573. doi: 10.1073/pnas.012578299. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sasisekharan R, Moses MA, Nugent MA, Cooney CL, Langer R. P Natl Acad Sci USA. 1994;91:1524–1528. doi: 10.1073/pnas.91.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu D, Pojasek K, Shriver Z, Holley K, El-Shabrawi Y, Venkataraman G, Sasisekharan R. Journal. 2011 [Google Scholar]
- 43.(a) Bateman D, McLaurin J, Chakrabartty A. BMC Neuroscience. 2007;8:29. doi: 10.1186/1471-2202-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Kanekiyo T, Zhang J, Liu Q, Liu CC, Zhang L, Bu G. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:1644–1651. doi: 10.1523/JNEUROSCI.5491-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heres EK, Horrow JC, Gravlee GP, Tardiff BE, Luber J, Jr, Schneider J, Barragry T, Broughton R. Anesthesia and analgesia. 2001;93:1446–1452. doi: 10.1097/00000539-200112000-00019. table of contents. [DOI] [PubMed] [Google Scholar]
- 45.Ai X, Do AT, Kusche-Gullberg M, Lindahl U, Lu K, Emerson CP., Jr J Biol Chem. 2006;281:4969–4976. doi: 10.1074/jbc.M511902200. [DOI] [PubMed] [Google Scholar]
- 46.Kim JH, Chan C, Elwell C, Singer MS, Dierks T, Lemjabbar-Alaoui H, Rosen SD, Engel JN. Cell Microbiol. 2013;15:1560–1571. doi: 10.1111/cmi.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S, Ai X, Freeman SD, Pownall ME, Lu Q, Kessler DS, Emerson CP. P Natl Acad Sci USA. 2004;101:4833–4838. doi: 10.1073/pnas.0401028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lai J, Chien J, Staub J, Avula R, Greene EL, Matthews TA, Smith DI, Kaufmann SH, Roberts LR, Shridhar V. J Biol Chem. 2003;278:23107–23117. doi: 10.1074/jbc.M302203200. [DOI] [PubMed] [Google Scholar]
- 49.(a) Feyzi E, Trybala E, Bergström T, Lindahl U, Spillmann D. J Biol Chem. 1997;272:24850–24857. doi: 10.1074/jbc.272.40.24850. [DOI] [PubMed] [Google Scholar]; (b) Laquerre S, Argnani R, Anderson DB, Zucchini S, Manservigi R, Glorioso JC. J Virol. 1998;72:6119–6130. doi: 10.1128/jvi.72.7.6119-6130.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schulze A, Gripon P, Urban S. Hepatology (Baltimore, Md) 2007;46:1759–1768. doi: 10.1002/hep.21896. [DOI] [PubMed] [Google Scholar]
- 51.(a) Henry-Stanley M, Hess DJ, Erickson E, Garni RM, Wells C. Medical microbiology and immunology. 2003;192:107–115. doi: 10.1007/s00430-002-0165-7. [DOI] [PubMed] [Google Scholar]; (b) Henry-Stanley MJ, Hess DJ, Erlandsen SL, Wells CL. Shock (Augusta, Ga) 2005;24:571–576. doi: 10.1097/01.shk.0000184286.95493.78. [DOI] [PubMed] [Google Scholar]; (c) Yan Y, Silvennoinen-Kassinen S, Leinonen M, Saikku P. Microbes and infection/Institut Pasteur. 2006;8:866–872. doi: 10.1016/j.micinf.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 52.(a) Zacharski LR, Lee AY. Expert opinion on investigational drugs. 2008;17:1029–1037. doi: 10.1517/13543784.17.7.1029. [DOI] [PubMed] [Google Scholar]; (b) Lim HC, Multhaupt HA, Couchman JR. Molecular Cancer. 2015;14:15. doi: 10.1186/s12943-014-0279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Mousa SA, Petersen LJ. Thromb Haemost. 2009;102:258–267. doi: 10.1160/TH08-12-0832. [DOI] [PubMed] [Google Scholar]; (d) Nagy Z, Turcsik V, Blasko G. Pathology oncology research : POR. 2009;15:689–692. doi: 10.1007/s12253-009-9204-7. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed I, Majeed A, Powell R. Postgraduate Medical Journal. 2007;83:575–582. doi: 10.1136/pgmj.2007.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rusnati M, Vicenzi E, Donalisio M, Oreste P, Landolfo S, Lembo D. Pharmacology & Therapeutics. 2009;123:310–322. doi: 10.1016/j.pharmthera.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Copeland R, Balasubramaniam A, Tiwari V, Zhang F, Bridges A, Linhardt RJ, Shukla D, Liu J. Biochemistry. 2008;47:5774–5783. doi: 10.1021/bi800205t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skidmore MA, Kajaste-Rudnitski A, Wells NM, Guimond SE, Rudd TR, Yates EA, Vicenzi E. MedChemComm. 2015 doi: 10.1039/C4MD00516C. [DOI] [Google Scholar]
- 57.Vogt AM, Pettersson F, Moll K, Jonsson C, Normark J, Ribacke U, Egwang TG, Ekre H-P, Spillmann D, Chen Q, Wahlgren M. PLoS Pathog. 2006;2:e100. doi: 10.1371/journal.ppat.0020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JB, Hayashi K, Hayashi T, Sankawa U, Maeda M. Planta Med. 1999;65:439–441. doi: 10.1055/s-2006-960804. [DOI] [PubMed] [Google Scholar]
- 59.(a) Ferro V, Liu L, Johnstone KD, Wimmer N, Karoli T, Handley P, Rowley J, Dredge K, Li CP, Hammond E, Davis K, Sarimaa L, Harenberg J, Bytheway I. Journal of Medicinal Chemistry. 2012;55:3804–3813. doi: 10.1021/jm201708h. [DOI] [PubMed] [Google Scholar]; (b) Dredge K, Hammond E, Davis K, Li CP, Liu L, Johnstone K, Handley P, Wimmer N, Gonda TJ, Gautam A, Ferro V, Bytheway I. Investigational New Drugs. 2010;28:276–283. doi: 10.1007/s10637-009-9245-5. [DOI] [PubMed] [Google Scholar]; (c) Said JS, Trybala E, Görander S, Ekblad M, Liljeqvist J-Å, Jennische E, Lange S, Bergström T. Antimicrobial agents and chemotherapy. 2016;60:1049–1057. doi: 10.1128/AAC.02132-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.(a) Nyberg K, Ekblad M, Bergström T, Freeman C, Parish CR, Ferro V, Trybala E. Antiviral Research. 2004;63:15–24. doi: 10.1016/j.antiviral.2004.01.001. [DOI] [PubMed] [Google Scholar]; (b) Lee E, Pavy M, Young N, Freeman C, Lobigs M. Antiviral Research. 2006;69:31–38. doi: 10.1016/j.antiviral.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 61.Talarico LB, Damonte EB. Virology. 2007;363:473–485. doi: 10.1016/j.virol.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 62.Carlucci MJ, Scolaro LA, Damonte EB. Chemotherapy. 1999;45:429–436. doi: 10.1159/000007236. [DOI] [PubMed] [Google Scholar]
- 63.Tremblay P, Aisen P, Garceau D. Alzheimer’s & Dementia. 2005;1:S2. [Google Scholar]
- 64.Rumjon A, Coats T, Javaid MM. International Journal of Nephrology and Renovascular Disease. 2012;5:37–43. doi: 10.2147/IJNRD.S19165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garson JA, Lubach D, Passas J, Whitby K, Grant PR. Journal of Medical Virology. 1999;57:238–242. [PubMed] [Google Scholar]
- 66.(a) Herold BC, Bourne N, Marcellino D, Kirkpatrick R, Strauss DM, Zaneveld LJ, Waller DP, Anderson RA, Chany CJ, Barham BJ, Stanberry LR, Cooper MD. The Journal of infectious diseases. 2000;181:770–773. doi: 10.1086/315228. [DOI] [PubMed] [Google Scholar]; (b) Kisilevsky R, Lemieux LJ, Fraser PE, Kong X, Hultin PG, Szarek WA. Nature medicine. 1995;1:143–148. doi: 10.1038/nm0295-143. [DOI] [PubMed] [Google Scholar]
- 67.(a) Cheshenko N, Keller MJ, MasCasullo V, Jarvis GA, Cheng H, John M, Li JH, Hogarty K, Anderson RA, Waller DP, Zaneveld LJ, Profy AT, Klotman ME, Herold BC. Antimicrobial agents and chemotherapy. 2004;48:2025–2036. doi: 10.1128/AAC.48.6.2025-2036.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Keller MJ, Zerhouni-Layachi B, Cheshenko N, John M, Hogarty K, Kasowitz A, Goldberg CL, Wallenstein S, Profy AT, Klotman ME, Herold BC. The Journal of infectious diseases. 2006;193:27–35. doi: 10.1086/498533. [DOI] [PubMed] [Google Scholar]; (c) Taylor DL, Brennan TM, Bridges CG, Mullins MJ, Tyms AS, Jackson R, Cardin AD. Antiviral Res. 1995;28:159–173. doi: 10.1016/0166-3542(95)00046-o. [DOI] [PubMed] [Google Scholar]
- 68.(a) Liu CJ, Lee PH, Lin DY, Wu CC, Jeng LB, Lin PW, Mok KT, Lee WC, Yeh HZ, Ho MC, Yang SS, Lee CC, Yu MC, Hu RH, Peng CY, Lai KL, Chang SSC, Chen PJ. Journal of Hepatology. 2009;50:958–968. doi: 10.1016/j.jhep.2008.12.023. [DOI] [PubMed] [Google Scholar]; (b) Kudchadkar R, Gonzalez R, Lewis KD. Expert opinion on investigational drugs. 2008;17:1769–1776. doi: 10.1517/13543784.17.11.1769. [DOI] [PubMed] [Google Scholar]; (c) Basche M, Gustafson DL, Holden SN, O’Bryant CL, Gore L, Witta S, Schultz MK, Morrow M, Levin A, Creese BR, Kangas M, Roberts K, Nguyen T, Davis K, Addison RS, Moore JC, Eckhardt SG. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:5471–5480. doi: 10.1158/1078-0432.CCR-05-2423. [DOI] [PubMed] [Google Scholar]
- 69.Winterhoff B, Freyer L, Hammond E, Giri S, Mondal S, Roy D, Teoman A, Mullany SA, Hoffmann R, von Bismarck A, Chien J, Block MS, Millward M, Bampton D, Dredge K, Shridhar V. European journal of cancer (Oxford, England : 1990) 2015;51:879–892. doi: 10.1016/j.ejca.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu CJ, Chang J, Lee PH, Lin DY, Wu CC, Jeng LB, Lin YJ, Mok KT, Lee WC, Yeh HZ, Ho MC, Yang SS, Yang MD, Yu MC, Hu RH, Peng CY, Lai KL, Chang SSC, Chen PJ. World Journal of Gastroenterology : WJG. 2014;20:11384–11393. doi: 10.3748/wjg.v20.i32.11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gnoni A, Santini D, Scartozzi M, Russo A, Licchetta A, Palmieri V, Lupo L, Faloppi L, Palasciano G, Memeo V, Angarano G, Brunetti O, Guarini A, Pisconti S, Lorusso V, Silvestris N. Expert opinion on therapeutic targets. 2015;19:1623–1635. doi: 10.1517/14728222.2015.1071354. [DOI] [PubMed] [Google Scholar]
- 72.(a) Singh O, Garg T, Rath G, Goyal AK. Journal of Pharmaceutics. 2014;2014:18. doi: 10.1155/2014/352425. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Marais D, Gawarecki D, Allan B, Ahmed K, Altini L, Cassim N, Gopolang F, Hoffman M, Ramjee G, Williamson AL. Antiviral therapy. 2011;16:1219–1226. doi: 10.3851/IMP1890. [DOI] [PubMed] [Google Scholar]
- 73.(a) Metz-Boutigue MH, Jolles J, Mazurier J, Schoentgen F, Legrand D, Spik G, Montreuil J, Jolles P. European journal of biochemistry/FEBS. 1984;145:659–676. doi: 10.1111/j.1432-1033.1984.tb08607.x. [DOI] [PubMed] [Google Scholar]; (b) Wu H, Lundblad R, Church F. Neutralization of heparin activity by neutrophil lactoferrin. 1995 [PubMed] [Google Scholar]; (c) Sánchez L, Calvo M, Brock JH. Archives of Disease in Childhood. 1992;67:657–661. doi: 10.1136/adc.67.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farnaud S, Evans RW. Molecular Immunology. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- 75.(a) Fujihara T, Hayashi K. Archives of Virology. 1995;140:1469–1472. doi: 10.1007/BF01322673. [DOI] [PubMed] [Google Scholar]; (b) Andersen JH, Jenssen H, Sandvik K, Gutteberg TJ. J Med Virol. 2004;74:262–271. doi: 10.1002/jmv.20171. [DOI] [PubMed] [Google Scholar]
- 76.EL-Fakharany E, Sanchez L, Al-Mehdar H, Redwan E. Virology Journal. 2013;10:199. doi: 10.1186/1743-422X-10-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harmsen MC, Swart PJ, Béthune M-Pd, Pauwels R, Clercq ED, The TB, Meijer DKF. Journal of Infectious Diseases. 1995;172:380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 78.Hirashima N, Orito E, Ohba K, Kondo H, Sakamoto T, Matsunaga S, Kato A, Nukaya H, Sakakibara K, Ohno T, Kato H, Sugauchi F, Kato T, Tanaka Y, Ueda R, Mizokami M. Hepatology Research. 2004;29:9–12. doi: 10.1016/j.hepres.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Ishibashi Y, Takeda K, Tsukidate N, Miyazaki H, Ohira K, Dosaka-Akita H, Nishimura M. Hepatology Research. 2005;32:218–223. doi: 10.1016/j.hepres.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 80.(a) Bianchini EP, Fazavana J, Picard V, Borgel D. Development of a recombinant antithrombin variant as a potent antidote to fondaparinux and other heparin derivatives. 2011 doi: 10.1182/blood-2010-06-288522. [DOI] [PubMed] [Google Scholar]; (b) Fazavana J, Bianchini EP, Saller F, Smadja C, Picard V, Taverna M, Borgel D. Journal of Thrombosis and Haemostasis. 2013;11:1128–1136. doi: 10.1111/jth.12249. [DOI] [PubMed] [Google Scholar]
- 81.Carr JA, Silverman N. The Journal of cardiovascular surgery. 1999;40:659–666. [PubMed] [Google Scholar]
- 82.Park PW, Pier GB, Hinkes MT, Bernfield M. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 83.Chang LC, Liang JF, Lee HF, Lee LM, Yang VC. AAPS pharmSci. 2001;3:E18. doi: 10.1208/ps030318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.(a) Chang LC, Wrobleski S, Wakefield TW, Lee LM, Yang VC. AAPS pharmSci. 2001;3:24–31. doi: 10.1208/ps030319. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Byun Y, Singh VK, Yang VC. Thrombosis Research. 1999;94:53–61. doi: 10.1016/s0049-3848(98)00201-1. [DOI] [PubMed] [Google Scholar]
- 85.He H, Ye J, Liu E, Liang Q, Liu Q, Yang VC. Journal of Controlled Release. 2014;193:63–73. doi: 10.1016/j.jconrel.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 86.Sokolowska E, Kalaska B, Miklosz J, Mogielnicki A. Expert Opinion on Drug Metabolism & Toxicology. 2016;12:897–909. doi: 10.1080/17425255.2016.1194395. [DOI] [PubMed] [Google Scholar]
- 87.(a) Weiss WA, Gilman JS, Catenacci AJ, Osterberg AE. Journal of the American Medical Association. 1958;166:603–607. doi: 10.1001/jama.1958.02990060041010. [DOI] [PubMed] [Google Scholar]; (b) Lillehei CW, Sterns LP, Long DM, Lepley D. Annals of Surgery. 1960;151:11–16. [PMC free article] [PubMed] [Google Scholar]
- 88.Fabian I, Aronson M. Thrombosis Research. 17:239–247. doi: 10.1016/0049-3848(80)90310-2. [DOI] [PubMed] [Google Scholar]
- 89.Al-Jamal KT, Al-Jamal WT, Kostarelos K, Turton JA, Florence AT. Results in Pharma Sciences. 2012;2:9–15. doi: 10.1016/j.rinphs.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaminski K, Kalaska B, Koczurkiewicz P, Michalik M, Szczubialka K, Mogielnicki A, Buczko W, Nowakowska M. MedChemComm. 2014;5:489–495. [Google Scholar]
- 91.Mecca T, Consoli GML, Geraci C, La Spina R, Cunsolo F. Organic & Biomolecular Chemistry. 2006;4:3763–3768. doi: 10.1039/b608887b. [DOI] [PubMed] [Google Scholar]
- 92.Mecca T, Cunsolo F. Polymers for Advanced Technologies. 2010;21:752–757. [Google Scholar]
- 93.Udit AK, Everett C, Gale AJ, Kyle JR, Ozkan M, Finn MG. Chembiochem. 2009;10:503–510. doi: 10.1002/cbic.200800493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Luganini A, Nicoletto SF, Pizzuto L, Pirri G, Giuliani A, Landolfo S, Gribaudo G. Antimicrobial agents and chemotherapy. 2011;55:3231–3239. doi: 10.1128/AAC.00149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Borst EM, Ständker L, Wagner K, Schulz TF, Forssmann W-G, Messerle M. Antimicrobial agents and chemotherapy. 2013;57:4751–4760. doi: 10.1128/AAC.00854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.(a) Bromfield SM, Wilde E, Smith DK. Chemical Society Reviews. 2013;42:9184–9195. doi: 10.1039/c3cs60278h. [DOI] [PubMed] [Google Scholar]; (b) Rodrigo AC, Barnard A, Cooper J, Smith DK. Angewandte Chemie International Edition. 2011;50:4675–4679. doi: 10.1002/anie.201100019. [DOI] [PubMed] [Google Scholar]
- 97.(a) Shenoi RA, Kalathottukaren MT, Travers RJ, Lai BFL, Creagh AL, Lange D, Yu K, Weinhart M, Chew BH, Du C, Brooks DE, Carter CJ, Morrissey JH, Haynes CA, Kizhakkedathu JN. Science Translational Medicine. 2014;6:260ra150. doi: 10.1126/scitranslmed.3009427. [DOI] [PubMed] [Google Scholar]; (b) Kalathottukaren MT, Abraham L, Kapopara PR, Lai BF, Shenoi RA, Rosell FI, Conway EM, Pryzdial EL, Morrissey JH, Haynes CA, Kizhakkedathu JN. Blood. 2017;129:1368–1379. doi: 10.1182/blood-2016-10-747915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Overington JP, Al-Lazikani B, Hopkins AL. Nat Rev Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- 99.Schmidtke M, Karger A, Meerbach A, Egerer R, Stelzner A, Makarov V. Virology. 2003;311:134–143. doi: 10.1016/s0042-6822(03)00166-1. [DOI] [PubMed] [Google Scholar]
- 100.Schmidtke M, Riabova O, Dahse HM, Stelzner A, Makarov V. Antiviral Res. 2002;55:117–127. doi: 10.1016/s0166-3542(02)00014-1. [DOI] [PubMed] [Google Scholar]
- 101.Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, Makarov VA, Sapp M. Journal of Virology. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smythe MA, Trujillo T, Fanikos J. American Journal of Health-System Pharmacy. 2016;73:S27–S48. doi: 10.2146/ajhp150959. [DOI] [PubMed] [Google Scholar]
- 103.Ansell JE, Laulicht BE, Bakhru SH, Hoffman M, Steiner SS, Costin JC. Thrombosis Research. 146:113–118. doi: 10.1016/j.thromres.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Harris N, Kogan F, Il’kova G, Juhas S, Lahmy O, Gregor YI, Koppel J, Zhuk R, Gregor P. Biochimica Et Biophysica Acta-General Subjects. 2014;1840:245–254. doi: 10.1016/j.bbagen.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 105.Colpitts CC, Schang LM. Journal of Virology. 2014;88:7806–7817. doi: 10.1128/JVI.00896-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.(a) Choi S, Clements DJ, Pophristic V, Ivanov I, Vemparala S, Bennett JS, Klein ML, Winkler JD, DeGrado WE. Angewandte Chemie-International Edition. 2005;44:6685–6689. doi: 10.1002/anie.200501279. [DOI] [PubMed] [Google Scholar]; (b) Montalvo GL, Zhang Y, Young TM, Costanzo MJ, Freeman KB, Wang J, Clements DJ, Magavern E, Kavash RW, Scott RW, Liu D, DeGrado WF. Acs Chemical Biology. 2014;9:967–975. doi: 10.1021/cb500026x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kuziej J, Litinas E, Hoppensteadt DA, Liu Dahui, Walenga JM, Fareed J, Jeske W. Clinical and Applied Thrombosis/Hemostasis. 2010;16:377–386. doi: 10.1177/1076029610366439. [DOI] [PubMed] [Google Scholar]
- 108.McAllister RE. Thrombosis Research. 125:S162–S163. [Google Scholar]
- 109.Mahan CE. Journal of thrombosis and thrombolysis. 2014;37:271–278. doi: 10.1007/s11239-013-0927-7. [DOI] [PubMed] [Google Scholar]
- 110.Schuksz M, Fuster MM, Brown JR, Crawford BE, Ditto DP, Lawrence R, Glass CA, Wang L, Tor Y, Esko JD. Proceedings of the National Academy of Sciences. 2008;105:13075–13080. doi: 10.1073/pnas.0805862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.(a) Roan NR, Sowinski S, Muench J, Kirchhoff F, Greene WC. Journal of Biological Chemistry. 2010;285:1861–1869. doi: 10.1074/jbc.M109.066167. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Castellano LM, Shorter J. Biology. 2012;1:58–80. doi: 10.3390/biology1010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Warford J, Doucette CD, Hoskin DW, Easton AS. Biochemical and Biophysical Research Communications. 2014;443:524–530. doi: 10.1016/j.bbrc.2013.11.119. [DOI] [PubMed] [Google Scholar]
- 113.(a) Huegel J, Mundy C, Sgariglia F, Nygren P, Billings PC, Yamaguchi Y, Koyama E, Pacifici M. Developmental biology. 2013;377:100–112. doi: 10.1016/j.ydbio.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huegel J, Sgariglia F, Enomoto-Iwamoto M, Koyama E, Dormans JP, Pacifici M. Developmental dynamics : an official publication of the American Association of Anatomists. 2013;242:1021–1032. doi: 10.1002/dvdy.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Smith SA, Choi SH, Collins JNR, Travers RJ, Cooley BC, Morrissey JH. Inhibition of polyphosphate as a novel strategy for preventing thrombosis and inflammation. 2012 doi: 10.1182/blood-2012-07-444935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Warford J, Madera L, Hoskin D, Easton A. Journal of Neuroimmunology. 2014;275:73. [Google Scholar]
- 116.Xu D, Fuster MM, Lawrence R, Esko JD. Journal of Biological Chemistry. 2011;286:737–745. doi: 10.1074/jbc.M110.177006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Umber F, Störring FK, Föllmer W. Klinische Wochenschrift. 1938;17:443–446. [Google Scholar]
- 118.Weiss RJ, Gordts PLSM, Le D, Xu D, Esko JD, Tor Y. Chemical Science. 2015 doi: 10.1039/C5SC01208B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pai M, Crowther M. In: Heparin – A Century of Progress. Lever R, Mulloy B, Page CP, editors. Vol. 207. Springer; Berlin Heidelberg: 2012. pp. 265–277. ch. 11. [Google Scholar]
- 120.(a) Portmann AF, Holden WD. Journal of Clinical Investigation. 1949;28:1451–1458. doi: 10.1172/JCI102210. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Weiler JM, Freiman P, Sharath MD, Metzger WJ, Smith JM, Richerson HB, Ballas ZK, Halverson PC, Shulan DJ, Matsuo S, et al. J Allergy Clin Immunol. 1985;75:297–303. doi: 10.1016/0091-6749(85)90061-2. [DOI] [PubMed] [Google Scholar]
- 121.(a) Nagler M, Haslauer M, Wuillemin WA. Thrombosis Research. 2012;129:407–417. doi: 10.1016/j.thromres.2011.10.037. [DOI] [PubMed] [Google Scholar]; (b) van Veen JJ, Maclean RM, Hampton KK, Laidlaw S, Kitchen S, Toth P, Makris M. Blood Coagul Fibrinolysis. 2011;22:565–570. doi: 10.1097/MBC.0b013e3283494b3c. [DOI] [PubMed] [Google Scholar]; (c) Schroeder M, Hogwood J, Gray E, Mulloy B, Hackett AM, Johansen KB. Anal Bioanal Chem. 2011;399:763–771. doi: 10.1007/s00216-010-4220-8. [DOI] [PubMed] [Google Scholar]
- 122.(a) Arungundram S, Al-Mafraji K, Asong J, Leach FE, Amster IJ, Venot A, Turnbull JE, Boons GJ. Journal of the American Chemical Society. 2009;131:17394–17405. doi: 10.1021/ja907358k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu R, Xu Y, Chen M, Weïwer M, Zhou X, Bridges AS, DeAngelis PL, Zhang Q, Linhardt RJ, Liu J. J Biol Chem. 2010;285:34240–34249. doi: 10.1074/jbc.M110.159152. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chen Y, Li Y, Yu H, Sugiarto G, Thon V, Hwang J, Ding L, Hie L, Chen X. Angewandte Chemie International Edition. 2013;52:11852–11856. doi: 10.1002/anie.201305667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.(a) Dulaney SB, Xu Y, Wang P, Tiruchinapally G, Wang Z, Kathawa J, El-Dakdouki MH, Yang B, Liu J, Huang X. The Journal of Organic Chemistry. 2015;80:12265–12279. doi: 10.1021/acs.joc.5b02172. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yang J, Hsieh PH, Liu X, Zhou W, Zhang X, Zhao J, Xu Y, Zhang F, Linhardt RJ, Liu J. Chemical Communications. 2017;53:1743–1746. doi: 10.1039/c6cc08204a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Puvirajesinghe Tania M, Ahmed Yassir A, Powell Andrew K, Fernig David G, Guimond Scott E, Turnbull Jeremy E. Chemistry & Biology. 2012;19:553–558. doi: 10.1016/j.chembiol.2012.03.011. [DOI] [PubMed] [Google Scholar]; (d) de Paz JL, Noti C, Seeberger PH. Journal of the American Chemical Society. 2006;128:2766–2767. doi: 10.1021/ja057584v. [DOI] [PubMed] [Google Scholar]; (e) Noti C, Seeberger PH. Chemistry & Biology. 2005;12:731–756. doi: 10.1016/j.chembiol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 124.(a) Guimond SE, Puvirajesinghe TM, Skidmore MA, Kalus I, Dierks T, Yates EA, Turnbull JE. J Biol Chem. 2009;284:25714–25722. doi: 10.1074/jbc.M109.032755. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thanawiroon C, Rice KG, Toida T, Linhardt RJ. J Biol Chem. 2004;279:2608–2615. doi: 10.1074/jbc.M304772200. [DOI] [PubMed] [Google Scholar]; (c) Hu H, Huang Y, Mao Y, Yu X, Xu Y, Liu J, Zong C, Boons GJ, Lin C, Xia Y, Zaia J. Molecular & Cellular Proteomics. 2014;13:2490–2502. doi: 10.1074/mcp.M114.039560. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Huang R, Zong C, Venot A, Chiu Y, Zhou D, Boons GJ, Sharp JS. Analytical Chemistry. 2016;88:5299–5307. doi: 10.1021/acs.analchem.6b00519. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Li L, Zhang F, Zaia J, Linhardt RJ. Analytical Chemistry. 2012;84:8822–8829. doi: 10.1021/ac302232c. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


