Abstract
IL-10-expressing regulatory B cells (B10) play an essential role in immune system balance by suppressing excessive inflammatory responses. In this study, we investigated induction of B 10 cell’s IL-10 competency in vitro and its effect on ligature-induced experimental periodontitis in vivo. Spleen B cells were isolated from C57BL/6J mice and cultured for 48 h under the following conditions: control, CD40L, IL-21, anti-Tim1, CD40L +IL-21, CD40L +anti-Tim1, CD40L +IL-21 +anti-Tim1. Silk ligatures were tied around both maxillary second molars of C57BL/6J mice for two weeks. Optimized combination of CD40L, IL-21 and anti-Tim1 and vehicle were injected into contralateral side of palatal gingiva on days 3, 6 and 9. The palatal gingival tissues and maxillary bone were collected on day 14 to determine expressions of IL-10 and periodontal bone resorption respectively. Our results demonstrated that IL-10 expressions of cultured spleen B cells were significantly increased in the presence of CD40L, IL-21 and anti-Tim1 combination when compared with control groups. Gingival IL-10 mRNA and protein expressions were significantly increased after injection of CD40L, IL-21 and anti-Tim1 combination, when compared to the control side. The gingival RANKL expression and periodontal bone loss were significantly decreased on the combination treatment side, as compared to the control side. These results suggest that combination of IL-21, anti-Tim1 and CD40L treatment induced B10 cell’s IL-10 competency in vitro and inhibited periodontal bone loss in ligature-induced experimental periodontitis.
Keywords: Periodontitis, B10, IL-21, Anti-Tim1, IL-10
1. Introduction
Periodontitis is a common complication of aging population and a leading cause of tooth loss, jaw bone deterioration and risk of developing systemic diseases [1–3]. It is a prevalent chronic inflammatory disease caused by host immune response to pathogenic microorganisms [4,5]. B10 cells are a specific interleukin-10 (IL-10) competent regulatory B cell subset which has been identified in both mice and humans recently [6,7]. B10 cell down-regulates inflammation and immune responses through IL-10 expression [8,9]. The therapeutic potential of secreted IL-10 has been demonstrated by many studies in various autoimmune diseases [10], such as experimental autoimmune encephalomyelitis [11,12]. However, the effect of B10 induction on periodontitis has not been reported.
CD40 is a membrane-associated protein and a member of the tumor necrosis factor (TNF) receptor superfamily [13,14]. The activation of CD40 on B cells not only induces maturation of B cells into antibody-producing B cells [15–17], but also is crucial for the activation of regulatory B cells [18,19]. Interleukin 21 (IL-21) is a cytokine which has potential regulatory effects on immune cells [20,21]. Recent studies showed that IL-21 promoted B 10 cell expansion and induces regulatory B cell differentiation and immunosuppressive effect through cognate interaction with T cells [22]. T cell Ig and mucin-1 (Tim-1) is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation [23–25]. Anti-Tim-1 monoclonal antibody (mAb) treatments enhanced activated B cells proliferation and surface marker expression [26,27]. We hypothesized that combined treatment with co-stimulatory molecules (CD40L, IL-21 and anti-Tim-1 mAb) would enhance IL-10 competency and inhibit periodontal inflammation and bone loss. The purpose of the present study was to investigate induction of B10 function by CD40L, IL-21 and anti-Tim1 combination in vitro and its effect on periodontal inflammation and bone loss in ligature-induced experimental periodontitis in vivo.
2. Materials and methods
2.1. Animal
C57BL/6J mice (Jackson Laboratory) at age of 8–10 weeks old were purchased from the Jackson Laboratory (Bar Harbor, ME). The experimental protocols were approved by the Institutional Animal Care and Use Committee of the Institute.
2.2. B cell isolation and culture
Mice were euthanized in CO2 chamber and spleens were harvested. B cells were separated from single splenic cells by Pan B cell isolation kit (Miltenyi Biotec). Purified B cells (> 95%, 1 × 106 cells/well) were cultured in IMDM + GlutaMAX™ (Life Technologies) complete medium in 96-well plates under the following conditions: CD40L (eBioscience) (1 μg/ml), IL-21 (R & D Systems) (25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml), anti-Tim1 (Abcam) (2.5 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml), CD40L (1 μg/ml) + IL-21(25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml), CD40L (1 μg/ml) +anti-Tim1 (0.2 μg/ml, 1 μg/ml, 5 μg/ml and 10 μg/ml), CD40L (1 μg/ml) +IL-21 (100 ng/ml, 1 μg/ml)+ anti-Tim1 (5 μg/ml). Cells were cultured in a humidified incubator at 37 °C with 5% CO2 for 48 h to determine CD1dhighCD5+ B cell percentages and mRNA expression levels. The culture supernatant was used for the measurement of secreted IL-10 levels. Each in vitro experiment was repeated three times with duplication of each sample.
2.3. Flow cytometry analysis
B cells were staixned and counted by Flow cytometers (BD Biosciences) as described [28]. In addition, the cultured B cells stimulated with CD40L (1 μg/ml) + IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) for 48 h were sorted into CD1dlowCD5−, CD1dhighCD5−, CD1dhighCD5+, CD1dlowCD5+ subsets by FACSAria III (BD Biosciences).
2.4. ELISA assay
The secreted IL-10 levels in the cultured supernatant were measured by Mouse IL-10 ELISA MAX Standard Kit (BioLegend) following the manufacturer’s manual and the detection limit is 15.6 pg/ml.
2.5. Real-time PCR
The total mRNA of total B cells, B cell subsets or gingival tissue was isolated by PureLink RNA Mini Kit (Life technologies) following the manufacturer’s instructions. The real-time quantitative PCR was carried out as described [28]. Briefly, the mRNA expression of IL-10, RANKL and ICAM-1 of sample was detected by real-time qPCR using Light-Cycler® SYBR Green I master and Light-Cycler® 480 Instrument system (Roche). The sequences of primers were used as described at Table 1. GAPDH gene was used as an internal control.
Table 1.
Primers and sequences used for PCR.
| Primers | Sequences |
|---|---|
| TNF-α | Forward: 5′-CAACGCCCTCCTGGCCAACG-3′ Reverse: 5′-TCGGGGCAGCCTTGTCCCTT-3′ |
| IL-10 | Forward: 5′-GACCAGCTGGACAACATACTGCTAA-3′ Reverse: 5′-GATAAGGCTTGGCAACCCAAGTAA-3′ |
| RANKL | Forward: 5′-CAT GTG CCA CTG AGA ACC TTG AA-3′ Reverse: 5′-CAG GTC CCA GCG CAA TGT AAC-3′ |
| ICAM-1 | Forward: 5′-TCGGGAAGGGAGCCAAGTAACT-3′ Reverse: 5′-GATCCTCCGAGCTGGCATT-3′ |
| GAPDH | Forward: 5′-CCCCAGCAAGGACACTGAGCAA-3′ Reverse: 5′-GTGGGTGCAGCGAACTTTATTGATG-3′ |
2.6. Experimental periodontitis
To induce experimental periodontitis in mice, a ligature-induced experimental periodontitis model was used as previously described with slight modification [29]. Briefly, silk ligatures (7-0, Fisher Scientific) were tied around both maxillary second molars on day 0 and remained for 14 days. The combination of CD40L (1 μg/ml) +IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) in PBS (1 μl) was injected into palatal interdental papilla on the left side and PBS (1 μl) was injected into palatal interdental papilla on the right side. Injections were performed on days 3, 6 and 9. All the mice were euthanized by CO2 inhalation on day 14. This experiment was repeated three times with five mice at each time.
2.7. Gingival tissue collection and preparation
Animals were euthanized by CO2 inhalation and the maxilla was removed from each mouse. Gingival tissues at the palatal side were collected under a surgical microscope from maxilla for homogenate preparation. For each gingival tissue, half of the collected gingival tissues were subject to RNA isolation to determine cytokine expressions by real-time PCR. Another half of the gingival tissues were used to measure the IL-10 production by ELISA.
2.8. Bone morphometric analysis
The maxillae were removed and defleshed by a dermestid beetles colony. After bleaching with 3% hydrogen Peroxide, the bone was stained with 1% toluidine blue. Bone resorption measurements were assessed under a Nikon microscope (Nikon SMZ745T, Nikon Instruments Inc., Japan). The polygonal area was measured using Image J (NIH) on buccal and palatal surfaces for each segment and a standard calibrator was used for calibration at the same magnification. The bone resorption area was enclosed coronally by the CEJ of the molars, laterally by the exposed distal root of the first molar and the exposed mesial root of the third molar, and apically by the alveolar crest.
2.9. Tartrate-resistant acid phosphatase (TRAP) staining
Collected maxillae were fixed in 4% formaldehyde overnight followed by decalcification in 10% EDTA for 2 weeks at 4 °C with agitation. Frozen specimens were cut in 8 μm along the long axis of the molars. Tissue sections were stained using acid phosphatase kit (378A, Sigma) following the manufacturer’s instructions. After counterstain with hematoxylin, images of gingival areas mesial and distal to the second molars from each section were acquired under light microscope at total magnification 400×. The number of multi-nucleated TRAP positive cells along alveolar bone surface was counted. For each side of oral cavity per mouse, we had 10–15 consecutive cuts counted.
2.10. Statistical analysis
All of quantitative data were expressed as means ± SD. The samples number n of each group indicates quantity of mice. SD stands for standard deviation of samples from different mice. Paired Student’s t-test was used to analyze differences between two groups. One-way ANOVA was used to analyze differences among groups. Results with probability values p < 0.05 are considered statistically significant.
3. Results
3.1. Effect of IL-21 treatment on CD1dhighCD5+ B cells population and IL-10 protein and mRNA expressions of total splenic B cells
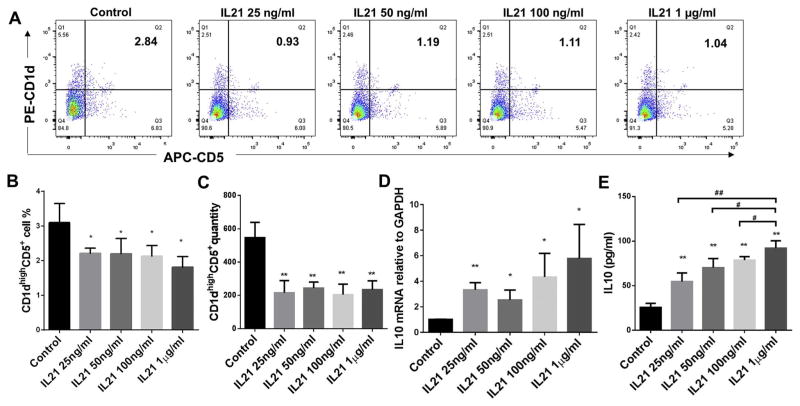
B cells separated from C57/BL6J mice splenocytes were cultured for 48 h under multiple conditions including untreated control, IL-21 treatment at dosages 25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml. The percentage of CD1dhighCD5+ B cells were measured and quantified by flow cytometry for each group (Fig. 1A). Compared to non-treatment control group, all doses of IL-21 treatment significantly reduced percentages (Fig. 1B) and quantities (Fig. 1C) of CD1dhighCD5+ B cells subset; however, the IL-10 mRNA levels (Fig. 1D) and secreted IL-10 (Fig. 1E) were significantly increased by all doses of IL-21 treatment and dosage of 1 μg/ml showed the highest induction effect. Taken together, IL-21 treatments (25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml) alone significantly increased IL-10 protein and mRNA expression in total splenic B cells with a significant decrease of percentage and quantity of CD1dhighCD5+ B cells subset.
Fig. 1.
Effects of different doses of IL-21 treatment on CD1dhighCD5+ B cells frequency, IL-10 protein expression and mRNA level. Splenocyte B cells were separated from C57/BL6J mice and cultured 48 h with IL-21 at dosages 25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml. CD1dhighCD5+ B cells were detected using flow cytometry in control and IL-21 treatment groups (A) (X-axis: CD5 PE staining; Y-axis: CD1d APC staining). The percentage (B) and quantity (C) of CD1dhighCD5+ B cells were quantified and analyzed by FlowJo software (mean ± SD, n =4 mice per group, compared with control group, *p < 0.05, **p < 0.01). IL-10 mRNA levels in total cell lysis were determined by real-time PCR in control and IL-21 treatment groups (D) (mean ± SD, n =4 mice per group, compared with control group, *p < 0.05, **p < 0.01). Medium supernatants were collected and secreted IL-10 protein levels were measured by ELISA in control and IL-21 treatment groups (E) (mean ± SD, n = 4 mice per group, compared with control group, **p < 0.01; compared with 1 μg/ml group, #p < 0.05, ##p < 0.01).
3.2. Effect of anti-Tim1 treatment on CD1dhighCD5+ B cells population and IL-10 protein and mRNA expressions of total splenic B cells
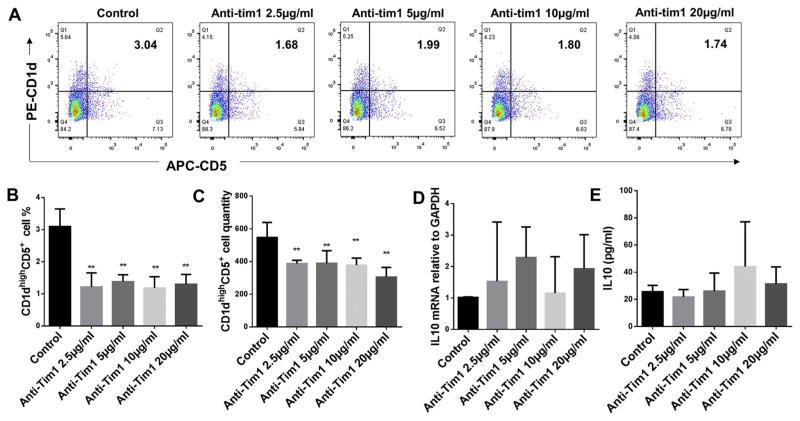
B cells separated from C57/BL6J mice splenocytes were cultured for 48 h under multiple conditions including untreated control, anti-Tim1 treatment at dosages 2.5 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml. The percentage of CD1dhighCD5+ B cells were measured and quantified by flow cytometry for each group (Fig. 2A). Compared to non-treatment control group, all doses of anti-Tim1 treatment significantly reduced percentages (Fig. 2B) and quantities (Fig. 2C) of CD1dhighCD5+ B cells subset; however, the IL-10 mRNA levels (Fig. 2D) and secreted IL-10 (Fig. 2E) showed no significant changes at all doses of anti-Tim1 treatment. These results suggested that anti-Tim1 treatments (2.5 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml) alone significantly decreased percentage and quantity of CD1dhighCD5+ B cells subset but had no effect on IL-10 protein and mRNA expression in total splenic B cells.
Fig. 2.
Effects of different doses of anti-Tim1 treatment on CD1dhighCD5+ B cells frequency, IL-10 protein expression and mRNA level. Splenocyte B cells were separated from C57/BL6J mice and cultured 48 h with anti-Tim1 at dosages 2.5 μg/ml, 5 μg/ml, 10 μg/ml and 20 μg/ml. CD1dhighCD5+ B cells were detected using flow cytometry in control and anti-Tim1 treatment groups (A) (X-axis: CD5 PE staining; Y-axis: CD1d APC staining). The percentage (B) and quantity (C) of CD1dhighCD5+ B cells were quantified and analyzed by FlowJo software (mean ± SD, n= 4 mice per group, compared with control group, **p < 0.01). IL-10 mRNA levels in total cell lysis were determined by real-time PCR in control and anti-Tim1 treatment groups (D) (mean ± SD, n =4 mice per group, compared with control group no significant differences). Medium supernatants were collected and secreted IL-10 protein levels were measured by ELISA in control and anti-Tim1 treatment groups (E) (mean ± SD, n = 4 mice per group, compared with control group no significant differences).
3.3. Effect of CD40L plus IL-21 treatment on CD1dhighCD5+ B cells population and IL-10 protein and mRNA expressions of total splenic B cells
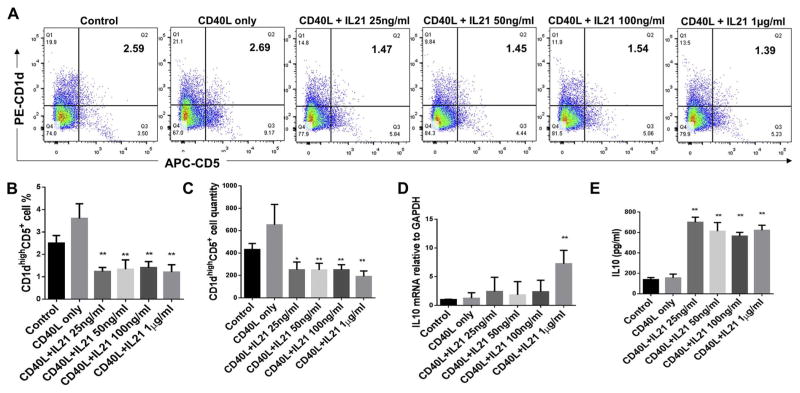
B cells separated from C57/BL6J mice splenocytes were cultured for 48 h under multiple conditions including untreated control, CD40L (1 μg/ml) alone, CD40L (1 μg/ml) +IL-21(25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml). The percentage of CD1dhighCD5+ B cells were measured and quantified by flow cytometry for each group (Fig. 3A). Compared to non-treatment control group, all doses of CD40L + IL-21 treatments significantly reduced percentages (Fig. 3B) and quantities (Fig. 3C) of CD1dhighCD5+ B cells subset; Also, only CD40L + IL-21 (1 μg/ml) group significantly increased IL-10 mRNA levels compared with control group (Fig. 3D). However, the secreted IL-10 expression were significantly increased by all doses of CD40L +IL-21 treatments and CD40L alone treatment showed the highest induction of effect (Fig. 3E). Taken together, CD40L alone and CD40L plus all doses of IL-21 treatments (25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml) significantly increased IL-10 protein expressions and only CD40L + IL-21 (1 μg/ml) group induced IL-10 mRNA expression in total splenic B cells; consistently with IL-21 alone treatments results above, all dosage of IL-21 treatments plus CD40L also significantly reduced percentage and quantity of CD1dhighCD5+ B cells subset.
Fig. 3.
Effects of CD40L plus different doses of IL-21 on CD1dhighCD5+ B cells frequency, IL-10 protein expression and mRNA level. Splenocyte B cells were separated from C57/BL6J mice and cultured 48 h under multiple conditions including untreated control, CD40L (1 μg/ml) alone, CD40L (1 μg/ml) +IL-21(25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml) (A). CD1dhighCD5+ B cells were detected using flow cytometry in control and CD40L +IL-21 treatment groups (X-axis: CD5 PE staining; Y-axis: CD1d APC staining). The percentage (B) and quantity (C) of CD1dhighCD5+ B cells were quantified and analyzed by FlowJo software (mean ± SD, n = 4 mice per group, compared with control group, *p < 0.05, **p < 0.01). IL-10 mRNA levels in total cell lysis were determined by real-time PCR in control and CD40L + IL-21 treatment groups (D) (mean ± SD, n = 4 mice per group, compared with control group, **p < 0.01). Medium supernatants were collected and secreted IL-10 protein levels were measured by ELISA in control and CD40L + IL-21 treatment groups (E) (mean ± SD, n = 4 mice per group, compared with control group, **p < 0.01).
3.4. Effect of CD40L plus anti-Tim1 treatment on CD1dhighCD5+ B cells population and IL-10 protein and mRNA expressions of total splenic B cells
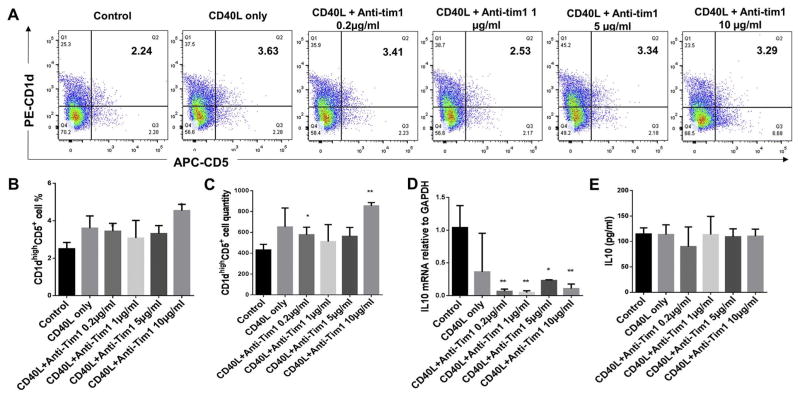
B cells separated from C57/BL6J mice splenocytes were cultured for 48 h under multiple conditions including untreated control, CD40L (1 μg/ml) alone, CD40L (1 μg/ml) + anti-Tim1 (0.2 μg/ml, 1 μg/ml, 5 μg/ml and 10 μg/ml). The percentage of CD1dhighCD5+ B cells were measured and quantified by flow cytometry for each group (Fig. 4A). Compared to non-treatment control group, CD40L + anti-Tim1 at dosage 0.2 μg/ml and 10 μg/ml groups showed significant increase of percentages (Fig. 4B) and quantities (Fig. 4C) of CD1dhighCD5+ B cells subset, and CD40L +anti-Tim1 at dosage 1 μg/ml and 5 μg/ml groups showed no changes; however, CD40L + anti-Tim1 at all doses significantly decreased IL-10 mRNA levels (Fig. 4D) and the secreted IL-10 expression showed no significant changes in these groups (Fig. 4E). These data suggested that CD40L plus anti-Tim1 at dosage 0.2 μg/ml and 10 μg/ml significantly induced CD1dhighCD5+ B cells subset expansion but all combination treatment of CD40L + anti-Tim1 had no effects on IL-10 protein expression and significantly decreased the Il-10 mRNA levels.
Fig. 4.
Effects of different doses of CD40L plus different doses of anti-Tim1 treatment on CD1dhighCD5+ B cells frequency, IL-10 protein expression and mRNA level. Splenocyte B cells were separated from C57/BL6J mice and cultured 48 h under multiple conditions including untreated control, CD40L (1 μg/ml) alone, CD40L (1 μg/ml) +anti-Tim1 (0.2 μg/ml, 1 μg/ml, 5 μg/ml and 10 μg/ml). CD1dhighCD5+ B cells were detected using flow cytometry in control and CD40L + anti-Tim1 treatment groups (A) (X-axis: CD5 PE staining; Y-axis: CD1d APC staining). The percentage (B) and quantity (C) of CD1dhighCD5+ B cells were quantified and analyzed by FlowJo software (mean ± SD, n = 4 mice per group, compared with control group, *p < 0.05, **p < 0.01). Medium supernatants were collected and secreted IL-10 protein levels were measured by ELISA in control and CD40L + anti-Tim1 treatment groups (D) (mean ± SD, n= 4 mice per group, compared with control group no significant differences). IL-10 mRNA levels in total cell lysis were determined by real-time PCR in control and CD40L +anti-Tim1 treatment groups (E) (mean ± SD, n = 4 mice per group, compared with control group, **p < 0.01).
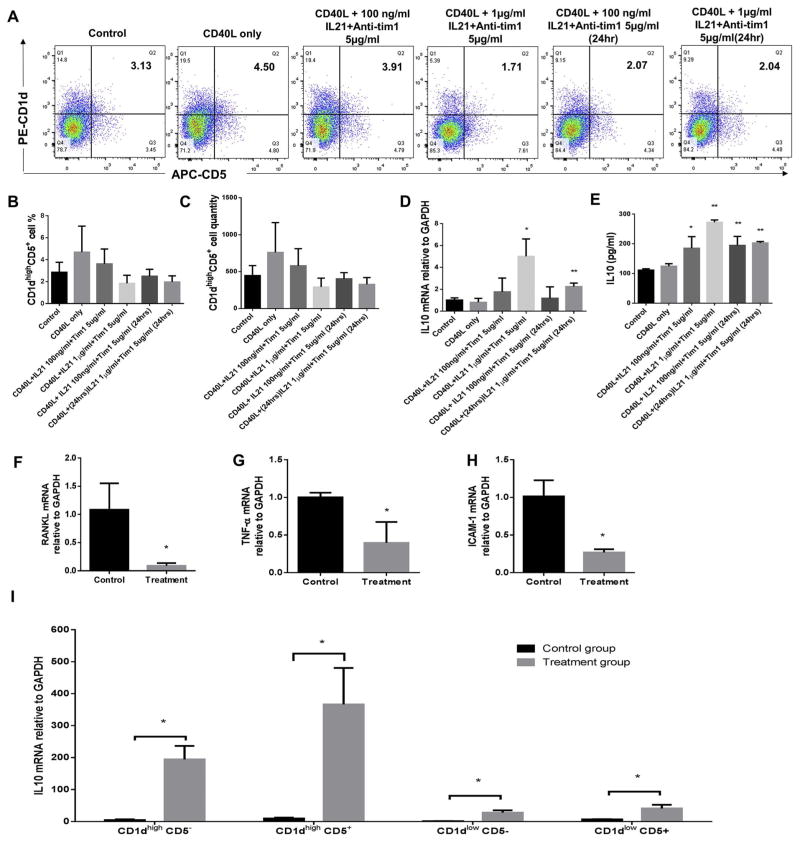
3.5. Effect of CD40L, IL-21 and anti-Tim1 combination treatment on CD1dhighCD5+ B cells population and IL-10 protein and mRNA expressions of total splenic B cells
Based on all the data above (Figs. 1–4), to achieve most B10 cell activation (increasing IL-10 mRNA/protein) and maintain B10 cell population at same time, we selected the optimal concentrations of IL-21 (100 ng/ml and 1 μg/ml) and anti-Tim1 (5 μg/ml) together with CD40L (1 μg/ml) to stimulate splenic B cells. B cells separated from C57/BL6J mice splenocytes were cultured in 6 different groups including (1) untreated control, (2) CD40L (1 μg/ml) alone, (3) 48 h of combination CD40L (1 μg/ml) +IL-21 (100 ng/ml) + anti-Tim1 (5 μg/ml), (4) 48 h of combination CD40L (1 μg/ml) + IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml), (5) 24 h of combination IL-21 (100 ng/ml) + anti-Tim1 (5 μg/ml) plus 48 h of CD40L (1 μg/ml), and (6) 24 h of combination IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) plus 48 h of CD40L (1 μg/ml). The percentage of CD1dhighCD5+ B cells were measured and quantified by flow cytometry for each group (Fig. 5A). Compared to non-treatment control group, all combination of CD40L, IL-21 and anti-Tim1 treatment groups ((3)–(6)) didn’t show significant changes of percentages (Fig. 5B) and quantities (Fig. 5C) of CD1dhighCD5+ B cells subset; Also, group (4) and (6), 48 and 24 h of anti-Tim1 (5 μg/ml) + IL-21 (1 μg/ml) plus 48 h CD40L treatment significantly increased IL-10 mRNA levels (Fig. 5D). However, all combination of CD40L, IL-21 and anti-Tim1 treatment groups significantly increased the secreted IL-10 expression and group 48 h of CD40L (1 μg/ml) +anti-Tim1 (5 μg/ml) + IL-21 (1 μg/ml) treatment had the highest induction (Fig. 5E). Taken together, the optimal combination of CD40L (1 μg/ml) + IL-21 (1 μg/ml) +anti-Tim1 (5 μg/ml) 48 h treatment showed the best induction of IL-10 protein and mRNA expressions with no significant changes of percentage and quantity of CD1dhighCD5+ B cells subset.
Fig. 5.
Effects of combination of IL-21, anti-Tim1 and Cd40L treatment on CD1dhighCD5+ B cells frequency, IL-10 protein expression, and IL-10, RANKL and other cytokines mRNA levels. Splenocyte B cells were separated from C57/BL6J mice and cultured under multiple conditions including untreated control, CD40L (1 μg/ml) alone, CD40L (1 μg/ml) + IL-21 (100 ng/ml) +anti-Tim1 (5 μg/ml) and CD40L (1 μg/ml) +IL-21 (1 μg/ml)+ anti-Tim1 (5 μg/ml) for 48 h, and CD40L (1 μg/ml) 48 h with IL-21 (100 ng/ml) + anti-Tim1 (5 μg/ml) or IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) for 24 h. CD1dhighCD5+ B cells were detected using flow cytometry in control and CD40L + IL-21+ anti-Tim1 combination treatment groups (A) (Xaxis: CD5 PE staining; Y-axis: CD1d APC staining). The percentage (B) and quantity (C) of CD1dhighCD5+ B cells were quantified and analyzed by FlowJo software (mean ± SD, n =4 mice per group, compared with control group no significant differences). IL-10 mRNA levels in total cell lysis were determined by real-time PCR in control and CD40L +IL-21 +anti-Tim1 combination treatment groups (D) (mean ± SD, n = 4 mice per group, compared with control group, *p < 0.05, **p < 0.01). Medium supernatants were collected and secreted IL-10 protein levels were measured by ELISA in control and CD40L + IL-21 +anti-Tim1 combination treatment groups (E) (mean ± SD, n = 4 mice per group, compared with control group, *p < 0.05, **p < 0.01). Splenocyte B cells were separated from C57/BL6J mice and cultured in two groups including untreated control and CD40L (1 μg/ml) +IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) for 48 h. RANKL (F), TNFα (G) and ICAM-1 (H) mRNA levels in total cell lysis were determined by real-time PCR in control and CD40L + IL-21+ anti-Tim1 combination treatment group (mean ± SD, n = 4 mice per group, compared with control group, *p < 0.05). CD1dlowCD5−, CD1dhighCD5−, CD1dhighCD5+, CD1dlowCD5+ B cell subsets were sorted from control and CD40L +IL-21 +anti-Tim1 combination treatment groups respectively. IL-10 mRNA levels of each subsets were determined by real-time PCR in control and CD40L + IL-21+ anti-Tim1 combination treatment group (I) (mean ± SD, n =4 mice per group, compared with control group, *p < 0.05).
3.6. Effect of CD40L, IL-21 and anti-Tim1 combination treatment on mRNA levels of RANKL, TNFα and ICAM-1 of splenic B cells, and IL-10 mRNA levels in B cell subsets
To confirm the effects on bone resorption and inflammatory cytokines of optimal combination dose above, B cells separated from C57/BL6J mice splenocytes were cultured without treatment (control) and with combination treatment (CD40L (1 μg/ml) + IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml)) for 48 h. The mRNA levels of bone resorption cytokine RANKL, proinflammatory cytokines TNFα and proinflammatory membrane protein ICAM-1 were measured by realtime PCR. Compared to control group, combination treatment significantly reduced mRNA levels of RANKL (Fig. 5F), TNFα (Fig. 5G) and ICAM-1 (Fig. 5H). To further investigate which B cell subsets was responsible for the IL-10 mRNA induction by the combination treatment (Fig. 5I), IL-10 mRNA were detected and compared between control and combination treatment in sorted CD1dhighCD5−, CD1dhighCD5+, CD1dlowCD5− and CD1dlowCd5+ B cell subsets. The data showed that combination treatment significantly increased IL-10 mRNA levels in all four subsets and the increased folder of CD1dhighCD5+ B cells was the highest. Taken together, combination of CD40L (1 μg/ml)+ IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) 48 h treatment reduced mRNA levels of bone resorption and inflammation in treated total splenic B cells. Moreover, the up-regulation of IL-10 mRNA expression was mainly derived from the induction of CD1dhighCD5+ B cells subset compared with other three cell subsets. Since CD1dhighCD5+ B cells subset is predominantly enriched in B10 cells [12], this data indicated that B10 cells were activated by the combination treatment.
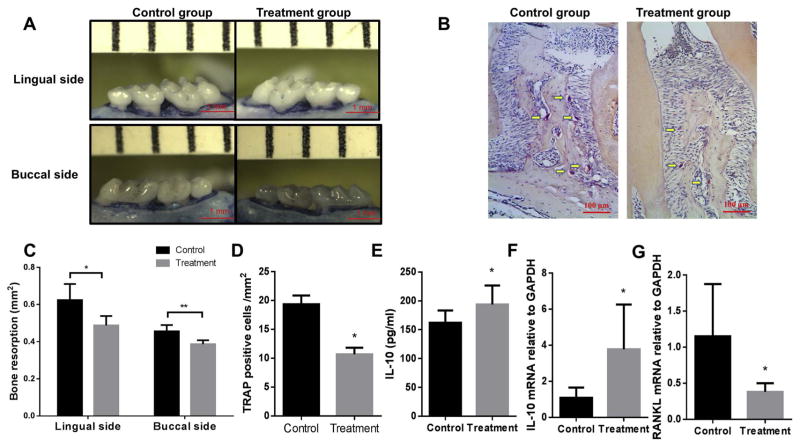
3.7. CD40L, IL-21 and anti-Tim1 combination treatment reduced bone loss in ligature-induced experimental periodontitis mouse model
In order to investigate whether the CD40L, IL-21 and anti-Tim1 combination treatment has the similar induction of IL-10 competency in vivo, the ligature-induced experimental periodontitis mouse model was performed and combination treatment of CD40L (1 μg/ml) +IL-21 (1 μg/ml) + anti-Tim1 (5 μg/ml) and vehicle control were injected into gingival tissues at days 3, 6 and 9 in the total 14 days ligation period. The areas of bone loss around maxillary second molars were measured and quantified in lingual and buccal side in control and treatment teeth (Fig. 6A). As expected, the resorption area on the treatment side was significantly smaller than the control side in both lingual and buccal sides, indicating that CD40L, IL-21 and anti-Tim1 combination treatment significantly decreased periodontal bone loss (Fig. 6C). Moreover, to confirm that, TRAP staining was performed to evaluate the osteoclasogenic activities within periodontal tissues in control and treatment group (Fig. 6B). The quantitative data showed that the number of multinucleated TRAP-positive cells along the alveolar bone surface was decreased significantly after injection of CD40L, IL-21 and anti-Tim1 combination (Fig. 6D). Mice gingival tissues at the palatal side were collected under a surgical microscope from maxilla and homogenized for mRNA and protein expression measurements. Compared to the control group, gingival IL-10 protein expression (Fig. 6E) and mRNA levels (Fig. 6F) were significantly increased after combination treatment, whereas gingival RANKL mRNA levels was significantly decreased in treatment group (Fig. 6G). These data indicated that CD40L, IL-21 and anti-Tim1 combination treatment also induced IL-10 expression and reduced RANKL expression in gingival tissues of ligature-induced experimental periodontitis mouse model, leading to reduction of periodontal bone resorption. Consistent with in vitro studies above, CD40L, IL-21 and anti-Tim1 combination had the induction effect of IL-10 competency in local gingival tissues and resulted the inhibition of bone loss in experimental periodontitis.
Fig. 6.
Combination treatment of CD40L, IL-21 and anti-Tim1 reduced bone loss and increased gingival IL-10 expression and mRNA level and decreased RANKL mRNA level in mouse experimental periodontitis. Silk ligatures were tied around maxillary secondary molars of both sides in C57/BL6 mice on day 0 and CD40L (1 μg/ml)+ IL-21 (1 μg/ml)+ anti-Tim1 (5 μg/ml) combination or PBS was injected on days 3, 6 and 9. Maxilla were collected on day 14 and the lingual and buccal sides alveolar bone resorption areas were pictured (A) and analyzed as bone resorption area/mm2 at the magnification of 30× (C) (means ± SD, n = 5 mice per group, *p < 0.05, **p < 0.01). TRAP staining was performed on tissue sections and images of periodontal tissues were analyzed at the magnification of 200× (B). The number of multinucleated TRAP+ cells along the alveolar bone surface were counted and compared between control group and CD40L +IL-21 + anti-Tim1 combination treatment group (D) (means ± SD, n = 5 mice per group, **p < 0.01). Gingival IL-10 protein expression were measured by ELISA in control and CD40L + IL-21+ anti-Tim1 combination treatment group (E) (mean ± SD, n =5 mice per group, *p < 0.05). Gingival IL-10 (F) and RANKL (G) mRNA levels were determined by real-time PCR in control and CD40L + IL-21+ anti-Tim1 combination treatment group respectively (mean ± SD, n =5 mice per group, *p < 0.05).
4. Discussion
IL-10 producing B cells negatively regulate inflammations in immune disease in both mice and humans [6,9,28,30]. Recent studies showed that IL-21, Tim1 and CD40 signals were involved in functional maturation of B10 cells [27,28,31,32]. However, little is known about the effects of co-stimulation by IL-21, anti-Tim1 and CD40 activator on IL-10 competency in vitro and in vivo. In the present study, we investigated the changes of CD1dhighCD5+ B cells population and IL-10 expression by treatment of IL-21 alone, anti-Tim1 alone, CD40L plus IL-21, CD40L plus anti-Tim1 and combination of IL-21, anti-Tim1 and CD40L. The results showed combination of IL-21, anti-Tim1 and CD40L treatment significantly increased IL-10 secretion and mRNA levels without reducing frequency of CD1dhighCD5+ B cells in splenic B cells; also, combination treatment induced IL-10 competency in gingival tissues and significantly reduce bone loss in experimental periodontitis model in vivo.
Recent study showed that IL-21 promoted B10 cell population expansion using a specific ex vivo culture method, which involved the culture of B cell with IL-4 and NIH-3T3 fibroblast expressing CD40 ligand and B lymphocyte stimulator (BLYS) for 4 days and then IL-21 for 5 days [22]. However, our present data showed that treatments of IL-21 alone at dosage 25 ng/ml, 50 ng/ml, 100 ng/ml and 1 μg/ml for 48 h significantly reduced the population of CD1dhighCD5+ B cells but increased IL-10 expression both in protein and mRNA levels. There are many possible reasons to explain this confliction. Firstly, IL-21 may only have proliferative effect on stimulated mature B10 cells after costimulation of IL-4, CD40L and BLYS [22] and may not induce cell number increase on un-stimulated primary splenic B cells. Secondly, IL-21 alone significantly increased IL-10 expression, suggesting IL-21 treatment may induce IL-10 competency of mature B10 cells instead of increasing overall B10 populations. Also, the combination of IL-21, anti-Tim1 and CD40L treatment failed to expand B10 cells in vitro probably due to the lack of cognate interaction with T cells [22]. These possible explanations need to be investigated in the future work. Moreover, the effect of treatments of IL-21 alone showed no dose dependent effect on reduce of CD1dhighCD5+ B cells population and increase of IL-10 expression (Fig. 1B–E). The possible causes may be due to limited quantity of IL-21 receptors on splenic B cells or the initial dose of IL-21 (25 ng/ml) was too high for these effects, which needs further investigations.
Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and for their regulatory function on inflammations [27,33]. In recent study, Tim-1 identified over 70% of IL-10-producing B cells irrespective of other markers, and alloantigen plus anti-Tim-1 increased B cell TIM-1, IL-10 and IL-4 expression [34]. However, in our present data, anti-Tim1 alone treatment had no effect on IL-10 induction in primary splenic B cells and reduced CD1dhighCD5+ B cells population, whereas CD40L + anti-Tim1 increased CD1dhighCD5+ B cells population but had no effects on IL-10 expression. Only anti-Tim1 combined with CD40L and IL-21 showed induction of IL-10 expression without significant changes in CD1dhighCD5+ B cells population, suggesting the induction effect of B10 by anti-Tim1 treatment needs costimulators. Thus, the detail characterization of Tim1+ B10 cells needs further studies.
In our present study, it is the first time to choose combination of costimulatory molecules IL-21, anti-Tim1 and CD40L to stimulate IL-10 competency both in vitro and in vivo. With IL-21 and CD40L stimulation, the IL-10 expression level was significantly increased only at dosage 1 μg/ml IL-21 but the B10 population was significantly decreased at same time (Fig. 3). Moreover, under anti-Tim1 and CD40L stimulation, the B10 population was significantly increased but IL-10 expression was not significantly different (Fig. 4). Thus, in order to achieve both B10 cells expansion and IL-10 competency, our optimized combination of IL-21, anti-Tim1 and CD40L increased IL-10 expression significantly and didn’t reduced B10 population (Fig. 5), which was more proficient than any single (Figs. 1, 2)/double (Figs. 3, 4) treatment of IL-21, anti-Tim1 and CD40L. This may elute a new insight into the basic biology of B10 cells as well as how to manipulate them for therapeutic purposes.
Based on our results, the combination of IL-21, anti-Tim1 and CD40L significantly induced IL-10 competency from B10 cells in vitro. However, it is not clear whether the induction of gingival IL-10 expression in ligature-induced experimental periodontitis was mainly or partly from B10 activation in the gingival tissue under the combination treatment. Recent studies indicated that IL-10 was secreted not only form B cell s but also from other types of immune cells such as regulator T cells [35,36], dendritic cells [37,38], macrophages [39,40]. Thus, the increase of gingival IL-10 expression may be caused by stimulation on other type of cells as well, suggesting further studies are needed for IL-21, anti-Tim1 and CD40L combination effects on other types of immune cells. Moreover, the changes of IL-10 mRNA levels showed different trend compared with the changes of IL-10 protein expression in our study (Figs. 2–5). It may be caused by post-translational regulation (RNA processing, RNA stability), translation regulation, protein stability and protein post-translation modifications of IL-10 [41].
We used ligature-induced periodontitis mouse model to evaluate the efficacy of combination treatment of IL-21, anti-Tim1 and CD40L in present study. However, there are certain limitations to use this animal model to mimic the natural occurring chronic periodontitis in human. Firstly, ligature-induced periodontitis mouse model only comprises several from hundreds of microbes that form dental plague biofilms are often needed to induce periodontitis in human [42]. Also, the ligature-induced periodontitis mouse model has a much less period (several weeks) which cannot completely mimic the chronic periodontitis conditions in human patients. Additionally, the mouse has different dental anatomical structure from human. Taken together, it is very useful to use ligature-induced periodontitis mouse model to investigate host-microbe interactions and inflammation in periodontitis, but there are certain limitations to mimic human chronic periodontitis.
In summary, IL-21, anti-Tim1 and CD40L combination induced B10 cell’s IL-10 competency in vitro and inhibited periodontal bone loss in ligature-induced experimental periodontitis in vivo.
Acknowledgments
Formatting of funding sources
This study was supported by NIH NIDCR grant R56DE023807 and R01DE025255 to X Han.
All our affiliations, corporate or institutional, and all sources of financial support to this research are properly acknowledged. We certify that we do not have any commercial or associate interest that represents a conflict of interest in connection with the manuscript.
Abbreviations
- IL-10
interleukin 10
- IL-21
interleukin 21
- CD40L
cluster of differentiation 40 ligand
- Tim1
T-cell immunoglobulin and mucin domain 1
- RANKL
receptor activator of nuclear factor kappa-B ligand
Footnotes
Transparency document
The http://dx.doi.org/10.1016/j.bbadis.2017.06.001 associated with this article can be found, in online version.
References
- 1.Barbato L, Francioni E, Bianchi M, Mascitelli E, Marco LB, Tonelli DP. Periodontitis and bone metabolism. Clin Cases Min Bone Metab. 2015;12:174–177. doi: 10.11138/ccmbm/2015.12.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gankovskaya LV, Khelminskaya NM, Molchanova EA, Svitich OA. Role of innate immunity factors in periodontitis pathogenesis. Zh Mikrobiol Epidemiol Immunobiol. 2016:100–107. [PubMed] [Google Scholar]
- 3.Straka M, Straka-Trapezanlidis M, Deglovic J, Varga I. Periodontitis and osteoporosis. Neuro Endocrinol Lett. 2015;36:401–406. [PubMed] [Google Scholar]
- 4.Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol. 2015;98:539–548. doi: 10.1189/jlb.3VMR1014-468R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. The development and function of regulatory B cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 2009;182:7459–7472. doi: 10.4049/jimmunol.0900270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- 8.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lykken JM, Candando KM, Tedder TF. Regulatory B10 cell development and function. Int Immunol. 2015;27:471–477. doi: 10.1093/intimm/dxv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin M, Wang Z, Han X. B cells with regulatory function in animal models of autoimmune and non-autoimmune diseases. Open J Immunol. 2015;5:9–17. doi: 10.4236/oji.2015.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalampokis I, Yoshizaki A, Tedder TF. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res Ther. 2013;15(Suppl 1):S1. doi: 10.1186/ar3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tedder TF. B10 cells: a functionally defined regulatory B cell subset. J Immunol. 2015;194:1395–1401. doi: 10.4049/jimmunol.1401329. [DOI] [PubMed] [Google Scholar]
- 13.Chandel HS, Pandey SP, Roy S, Doyen N, Saha B. TLR-CD40 cross-talk in antileishmanial immune response. Front Immunol. 2014;5:220. doi: 10.3389/fimmu.2014.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirbod-Mobarakeh A, Aghamohammadi A, Rezaei N. Immunoglobulin class switch recombination deficiency type 1 or CD40 ligand deficiency: from bedside to bench and back again. Expert Rev Clin Immunol. 2014;10:91–105. doi: 10.1586/1744666X.2014.864554. [DOI] [PubMed] [Google Scholar]
- 15.Clark EA. A short history of the B-cell-associated surface molecule CD40. Front Immunol. 2014;5:472. doi: 10.3389/fimmu.2014.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop GA, Hostager BS. The CD40–CD154 interaction in B cell-T cell liaisons. Cytokine Growth Factor Rev. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 17.Banchereau J. Generation of human B-cell lines dependent on CD40-ligation and interleukin-4. Front Immunol. 2015;6:55. doi: 10.3389/fimmu.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shao Y, Lo CM, Ling CC, Liu XB, Ng KT, Chu AC, Ma YY, Li CX, Fan ST, Man K. Regulatory B cells accelerate hepatocellular carcinoma progression via CD40/CD154 signaling pathway. Cancer Lett. 2014;355:264–272. doi: 10.1016/j.canlet.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Kaltenmeier C, Gawanbacht A, Beyer T, Lindner S, Trzaska T, van der Merwe JA, Harter G, Gruner B, Fabricius D, Lotfi R, Schwarz K, Schutz C, Honig M, Schulz A, Kern P, Bommer M, Schrezenmeier H, Kirchhoff F, Jahrsdorfer B. CD4+ T cell-derived IL-21 and deprivation of CD40 signaling favor the in vivo development of granzyme B-expressing regulatory B cells in HIV patients. J Immunol. 2015;194:3768–3777. doi: 10.4049/jimmunol.1402568. [DOI] [PubMed] [Google Scholar]
- 20.Gharibi T, Majidi J, Kazemi T, Dehghanzadeh R, Motallebnezhad M, Babaloo Z. Biological effects of IL-21 on different immune cells and its role in autoimmune diseases. Immunobiology. 2016;221:357–367. doi: 10.1016/j.imbio.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 21.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, Petri A, Hansen LT, McArthur GA, Davis ID, Skak K. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–1449. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshizaki A, Tedder TF. IL-21 induces regulatory B cell differentiation and immunosuppressive effect through cognate interaction with T cells. Nihon Rinsho Meneki Gakkai Kaishi. 2015;38:57–64. doi: 10.2177/jsci.38.57. [DOI] [PubMed] [Google Scholar]
- 23.Shi BY, Xiao L, Gao Y, He XY, Xu XG, Huang HY, Zhou WQ, Han Y. Identification and functional study of Tim-1(+)CD19(+) regulatory B cell in kidney transplantation recipients. Zhonghua Yi Xue Za Zhi. 2011;91:3388–3392. [PubMed] [Google Scholar]
- 24.Xiao S, Brooks CR, Zhu C, Wu C, Sweere JM, Petecka S, Yeste A, Quintana FJ, Ichimura T, Sobel RA, Bonventre JV, Kuchroo VK. Defect in regulatory B-cell function and development of systemic autoimmunity in T-cell Ig mucin 1 (Tim-1) mucin domain-mutant mice. Proc Natl Acad Sci U S A. 2012;109:12105–12110. doi: 10.1073/pnas.1120914109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echbarthi M, Zonca M, Mellwig R, Schwab Y, Kaplan G, DeKruyff RH, Roda-Navarro P, Casasnovas JM. Distinct trafficking of cell surface and endosomal TIM-1 to the immune synapse. Traffic. 2015;16:1193–1207. doi: 10.1111/tra.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee KM, Kim JI, Stott R, Soohoo J, O’Connor MR, Yeh H, Zhao G, Eliades P, Fox C, Cheng N, Deng S, Markmann JF. Anti-CD45RB/anti-TIM-1-induced tolerance requires regulatory B cells. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2012;12:2072–2078. doi: 10.1111/j.1600-6143.2012.04055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeung MY, Ding Q, Brooks CR, Xiao S, Workman CJ, Vignali DA, Ueno T, Padera RF, Kuchroo VK, Najafian N, Rothstein DM. TIM-1 signaling is required for maintenance and induction of regulatory B cells. Am J Transplant Off J Am Soc Transplant Am Soc Transplant Surg. 2015;15:942–953. doi: 10.1111/ajt.13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M, Lin J, Wang Y, Bonheur N, Kawai T, Wang Z, Han X. Lipopolysaccharide attenuates CD40 ligand-induced regulatory B10 cell expansion and IL-10 production in mouse splenocytes. Open J Immunol. 2015;5:1–8. doi: 10.4236/oji.2015.51001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Han X. B Cells With Regulatory Function in Human Diseases. Autoimmune Diseases and Therapeutic Approaches: Open Access. 2014:1. [PMC free article] [PubMed] [Google Scholar]
- 31.Leavy O. B cells: IL-21 promotes B10 cell population expansion. Nat Rev Immunol. 2012;12:808–809. doi: 10.1038/nri3346. [DOI] [PubMed] [Google Scholar]
- 32.Poe JC, Smith SH, Haas KM, Yanaba K, Tsubata T, Matsushita T, Tedder TF. Amplified B lymphocyte CD40 signaling drives regulatory B10 cell expansion in mice. PLoS One. 2011;6:e22464. doi: 10.1371/journal.pone.0022464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao S, Brooks CR, Sobel RA, Kuchroo VK. Tim-1 is essential for induction and maintenance of IL-10 in regulatory B cells and their regulation of tissue inflammation. J Immunol. 2015;194:1602–1608. doi: 10.4049/jimmunol.1402632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding Q, Yeung M, Camirand G, Zeng Q, Akiba H, Yagita H, Chalasani G, Sayegh MH, Najafian N, Rothstein DM. Regulatory B cells are identified by expression of TIM-1 and can be induced through TIM-1 ligation to promote tolerance in mice. J Clin Invest. 2011;121:3645–3656. doi: 10.1172/JCI46274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dennis KL, Saadalla A, Blatner NR, Wang S, Venkateswaran V, Gounari F, Cheroutre H, Weaver CT, Roers A, Egilmez NK, Khazaie K. T-cell expression of IL10 is essential for tumor immune surveillance in the small intestine. Cancer Immunol Res. 2015;3:806–814. doi: 10.1158/2326-6066.CIR-14-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakobshagen K, Ward B, Baschuk N, Huss S, Brunn A, Malecki M, Fiolka M, Rappl G, Corogeanu D, Karow U, Schiller P, Abken H, Heukamp LC, Deckert M, Kronke M, Utermohlen O. Endogenous Il10 alleviates the systemic antiviral cellular immune response and T cell-mediated immunopathology in select organs of acutely LCMV-infected mice. Am J Pathol. 2015;185:3025–3038. doi: 10.1016/j.ajpath.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 37.Moreau A, Vandamme C, Segovia M, Devaux M, Guilbaud M, Tilly G, Jaulin N, Le Duff J, Cherel Y, Deschamps JY, Anegon I, Moullier P, Cuturi MC, Adjali O. Generation and in vivo evaluation of IL10-treated dendritic cells in a nonhuman primate model of AAV-based gene transfer. Mol Ther. 2014;1:14028. doi: 10.1038/mtm.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng JC, Abu Bakar S, Richardson MM, Jonsson JJ, Frazer IH, Nielsen LK, Morahan G, Thomas R. IL10 and IL12B polymorphisms each influence IL-12p70 secretion by dendritic cells in response to LPS. Immunol Cell Biol. 2006;84:227–232. doi: 10.1111/j.1440-1711.2006.01419.x. [DOI] [PubMed] [Google Scholar]
- 39.Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, Apte RS. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. 2015;6:7847. doi: 10.1038/ncomms8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang J, Jia T, Gong W, Ning B, Wooley PH, Yang SY. Macrophage polarization in IL-10 treatment of particle-induced inflammation and osteolysis. Am J Pathol. 2016;186:57–66. doi: 10.1016/j.ajpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 42.Oz HS, Puleo DA. Animal models for periodontal disease. J Biomed Biotechnol. 2011;2011:754857. doi: 10.1155/2011/754857. [DOI] [PMC free article] [PubMed] [Google Scholar]