Abstract
Background
Hispanics/Latinos are at increased risk for cardiovascular disease and cognitive decline and dementias. High blood pressure (BP) has been implicated in both stroke and dementias. Associations between BP and cognition among diverse Latinos are still unpublished.
Objective
We examined associations between cognition and four BP based measures among diverse Hispanics/Latinos. We hypothesized that higher BP, particularly systolic pressure, and increased arterial stiffness (i.e., pulse pressure), would be associated with lower cognitive function.
Methods
We used baseline (2008–2011) Hispanic Community Health Study/Study of Latinos (HCHS/SOL; n = 9,019; ages 45–74 years) data to examine cognition in relation to BP measures.
Results
In age, sex, and education adjusted models, systolic, pulse, and mean arterial pressure were consistently negatively associated with executive function, psychomotor speed and sustained attention, verbal episodic learning and memory, speech fluency, and mental status measures. These associations were attenuated but remained statistically significant in fully adjusted models.
Conclusion
Among middle-aged and older diverse Hispanics/Latinos, we found modest but consistent associations between indicators of arterial stiffness, and compromised blood flow and lower cognitive function. Clinical management and public health interventions to raise awareness and enhance BP management beginning in midlife could reduce disparities and improve population health by reducing cognitive decline burdens.
Keywords: Arterial stiffness, blood pressure, cardiovascular health, cognitive, Hispanic/Latino, hypertension, neurocognitive
INTRODUCTION
Proper blood pressure (BP) is vital for brain health [1, 2]. Compromised BP is a major risk for acute and subclinical cerebrovascular disease [3–5] and affects several cognitive domains, including executive function, attention, processing speed, and memory [6]. Substantial epidemiologic evidence identifies hypertension as a risk factor for dementia [1]. Still, while acknowledging the link, the American Heart Association has found current data to be insufficient to make specific “evidence-based recommendations” relating to hypertension and cognition [7]. The mechanism underlying the association of BP and cognitive function is complex, but largely centers on compromised cerebral circulation resulting from deregulation in perfusion pressure [3]. This mechanism is complicated by the confounding effects of age on aortic compliance, arterial stiffness, and resistance. The differential needs for normal regulation and arterial compromise lead to variation in risk over the age spectrum and have led to competing hypotheses about the shape (linear, curvilinear, or J-shape) of the relationship between BP and cognitive function, as well as the determination of optimal points of inversion [8, 9]. Most research evidence on the relationship between BP and neurocognitive (NC) function is derived from non-Hispanic White samples. Replication studies using data from large and well-characterized racial/ethnic groups, particularly Hispanics/Latinos (henceforth Latino will be used), remain largely unavailable. The current study addresses this shortcoming.
Prior studies have focused largely on older population cohorts with hypertension becoming a well-described risk factor for cognitive impairment and dementia [6]. Elevated BP, below hypertension criteria, has also been associated with lower cognitive performance [10, 11]. A limited number of studies have explicitly examined cognitive function and concurrent BP in middle-age [6, 8]. This body of work provides evidence that BP impacts cognition before older age and support the hypothesis that its effects could become clearly pronounced in midlife [10, 12–15].
No previous studies, to our knowledge, have comprehensively examined cognitive function and BP in middle-aged and older, diverse Latinos. Latinos are reportedly at increased risk for cognitive decline and dementias compared to non-Hispanic Whites [16]. Currently, published studies on the association between BP and cognition among Latinos have mostly relied on data from older cohorts of restricted backgrounds (e.g., Mexican origin). Evidence from this work show largely null or marginal associations [17–20]. Recently, Levin and colleagues, using data from the Northern Manhattan Study, which is largely Caribbean Latinos, examined a more comprehensive battery of cognitive tests and reported significant inverse associations between higher BP and measures of language, executive function, memory, and visual motor function [21]. Yet, Gardener and colleagues in more recent work reported no association between ideal BP and cognitive function [22].
These inconsistent results are significant given that Latinos are excessively burdened by cardiovascular disease (CVD) risk factors, and have lower access to care and BP clinical control compared to non-Hispanic Whites [23]. Lower awareness, control, and treatment of hypertension among Latinos compared to other race/ethnic groups present an important public health challenge [23–26]. Untreated and uncontrolled hypertension, particularly in middle-age, presents long-term risks for adverse CVD events and vascular cognitive health.
The purpose of this study is to examine associations between BP and cognitive function among diverse middle-aged and older Latinos. BP dysregulation [27] and arterial stiffness [28] have been specifically linked to executive dysfunction. The latter is implicated in cognitive impairment secondary to vascular disease [6] rather than decline in memory, which is more notable in Alzheimer’s disease [29]. Mechanistically, impaired cerebral blood flow has been linked to insidious white matter changes, and reduction in brain volume particularly in regions associated with executive function [30]. We anticipate that lower cognitive performance, associated with higher BP, will be more pronounced for executive measures.
METHODS
Data
The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) (baseline 2008–2011) is a multisite (San Diego, Chicago, Bronx, and Miami) prospective cohort sample (n = 16,415) of diverse Latinos. HCHS/SOL characteristics and sampling design and strategies are discussed in details elsewhere [31]. HCHS/SOL collected data on five cognitive tests in middle-aged and older participants (ages 45–74 years; n = 9,623). The study protocol was reviewed and approved by the institutional review boards of all participating sites and all participants gave written informed consent.
Outcomes
Outcomes included the Digit symbol substitution (DSS; executive function, psychomotor speed and sustained attention) test; the Brief-Spanish English Verbal learning test (B-SEVLT [32, 33]; verbal episodic learning and memory); the word fluency (WF; speech production); and the Six-Item Screener (SIS; mental status). A detailed discussion of these tests as applied to HCHS/SOL data is published elsewhere [34]. All continuous measures were z-score transformed ((Score-Mean)/Standard Deviation (SD); using the tests’ probability weighted means and SDs) to facilitate score comparisons across tests using a common metric. SIS was dichotomized with a score ≤4 (range 0–6) indicating low mental status [35].
Main predictors
BP was measured using an automated sphygmomanometer (OMRON HEM-907 XL, Omron Healthcare, Inc., Lake Forest, IL) on seated participants using their right resting arm following a rest period of 5-min. Cuff sizes were matched to participants using upper arm circumference measurement and three sets of 1-min spaced measurements were taken from each person (99% completion rate). Our analyses accounts for two direct measures of BP: 1) systolic (SBP), and 2) diastolic (DBP), and two derived measures representing pulsatile and steady components of BP [36]: 3) pulse pressure (PP; measured as the difference between average systolic and diastolic), and 4) mean arterial pressure (MAP; DBP+ 1/3*(SBP-DBP)). Although closely linked, these four measures represent different BP hemodynamic patterns and could reflect independent cardio-and cerebrovascular pathologies [9, 37–41]. Each variable was constructed based on the average of three measurements or using all available data for the 1% of participants that did not complete all three measures [23]. Comprehensive medical examinations and histories were conducted in clinical facilities at each field center. BP testing was standardized across sites and conducted by trained and certified research staff.
Covariates
Previous work indicates that social, behavioral, and cardiovascular and metabolic factors are associated with both blood pressure and cognitive function which could confound reported associations [23, 34, 42, 43]. We control for demographic factors including Latino background (Mexican, Puerto Rican, Cuban, Dominican, Central and South American), age (continuous and categorical scales; 45–54, 55–64, and 65 years and older), education (3 categories: <high school (<HS); HS or equivalent (HS); and >HS), sex, marital status (1 = Single, 2 = Married or living with a partner, and 3 = Separated, divorced, or widower), and language preference (English/Spanish). Health behavior covariates included: 1) body mass index (weight in kg/height in m squared), and 2) self-reported physical activity (low, moderate, and high) assessed using the Global Physical Activity Questionnaire (available from http://www.who.int/chp/steps/GPAQ/en/), which included work, transportation, and recreational activities. CVD and risk factors included: 1) self-reported stroke/transient ischemic attack, as they could potentially be on the causal pathway between BP and cognition; 2) coronary heart disease measured based on a combination of self-reported medical history and electrocardiogram reports of possible old myocardial infarction, angina, heart attack, or procedure (angioplasty, stent, bypass); and 3) glycemic control (HbA1c), over the 3-months preceding the clinical visit, measured using a Tosoh G7 Automated HPLC Analyzer (Tosoh Bioscience) [44]. We also accounted for antihypertensive medication use based on scanned medication used during the 4-weeks preceding the participants’ clinic visit. Finally, we adjusted for field sites to control for possible cross-center variations.
Analytic subpopulation
We performed all analyses using the complex survey commands in Stata 14.1 software. We used appropriate methods for the analyses of subpopulations to generate our estimates and corresponding standard errors [45]. In particular, design adjustment for variance estimation and correct standard errors calculation were done using a Taylor Series Linearization approach [46]. Additionally, all analyses accounted for the HCHS/SOL probability weights to ensure appropriate generalizations to the target population. To ensure appropriate inferences to Latino background groups, we excluded participants that did not report a specific background (n = 227). In addition, we excluded respondents with incomplete data on the model covariates (n = 377). The number of participants ages 45–74 years with complete data on the covariates and the main predictors were n = 9,012 and n = 9,009 for diastolic and systolic BP, respectively. Analytic sample sizes, however, varied by outcome measures (n = 8,991 to n = 8,742 for DSS, respectively). Adjusted for age and sex, participants with missing values on the outcome measures were not statistically distinct compared to their complete records counterparts. Details on inclusion criteria and specific Ns are presented in Supplementary Figure 1).
Analytic plan
The analytic plan consisted of five steps. First, we provide descriptive statistics to characterize the HCHS/SOL target population (Table 1). Additionally, we provide the weighted means and SDs for the cognitive tests—used to generate the z-scores and primary exposure variables and include the prevalence of individuals with low mental health status scores (i.e., SIS ≤ 4) in HCHS/SOL target population. Second, we use survey linear regression models to estimate 1) crude, 2) age, sex, and education adjusted, and 3) full covariates adjusted associations between our BP measures and cognitive function. We use ordinary least squares regression for the continuous outcomes and logistic regression for the binary SIS. We present the regression results, including the beta coefficients and standard errors for the z-scored tests and odds ratios and 95% confidence intervals for the dichotomous mental status outcome, in Table 2. We present exact p-values for the estimated parameters for all primary analyses in Supplementary Table 1, to provide the readers with needed information to weigh the inferences. To facilitate the interpretation of these estimated parameters we calculated? and plot average marginal means (for continuous outcomes) and probabilities (for the binary SIS) and their 95% confidence intervals across the BP exposure continuum in Figs. 1 and 2. To ensure the robustness of our inferences, in sensitivity analyses, we adjusted the p-values of the estimates generated in sex, age, and education adjusted models using methods advanced by Simes-Benjamini-Hochberg [47, 48], as developed for Stata by Newson [49]. Our inferences were robust to multiple (n = 20) testing (results available from authors). Third, we estimate three sets of interaction models to examine the modifying effects of 1) age (45–54, 55–64, and 65 years and over), 2) sex, and 3) Latino background on the associations between our BP measures and cognitive function. For all interaction models, we present unadjusted and fully adjusted (for the set of covariates described above) Wald tests for the significance of the considered interaction (Supplementary Tables 2–4). Fourth, we generate splines for each of the BP exposures to correspond with multiple clinical guidelines, to test for differential associations between BP and cognitive function [50]. We consider two scenarios. First, we fit our models using two linear (first order) splines to estimate local slopes for BP values under/over 140 mmHg for SBP, under/over 90 mmHg for DBP, under/over 60 mmHg for PP, and under/over 100 mmHg for MAP. Secondly, we fit our models using four linear splines to estimate differences in slopes in the intervals below 120, 120–140, 140–160, and 160 and over and below 80 mmHg, 80–90 mmHg, 90–100 mmHg, and 100 mmHg and over for SBP, and DBP respectively. The tested differences in slopes for the considered measures—including the estimated differences, standard errors for continuous measures, 95% confidence intervals for the SIS, and p-values of the tests— are included in Supplementary Tables 5 and 6. Survey sampling procedures were used in all analyses.
Table 1.
Descriptive statistics to characterize the outcomes, primary exposures, and covariates of the HCHS/SOL target population (unweighted n = 9,019)
| Mean [se]/% [95% CI] | |
|---|---|
| Outcomes | |
| B-SEVLT-sum | 22.3 [0.11] |
| B-SEVLT-recall | 8.0 [0.05] |
| Word fluency | 18.3 [0.16] |
| Digit symbol substitution | 33.8 [0.31] |
| Six item screener (≤4) (%) | 16.1 [14.9;17.4] |
| Primary exposures | |
| SBP (mm/Hg) | 129.1 [0.31] |
| DBP (mm/Hg) | 75.4 [0.19] |
| PP (mm/Hg) | 53.8 [0.23] |
| MAP (mm/Hg) | 93.3 [0.21] |
| Covariates | |
| Education (%) | |
| Less than high school (HS) | 40.1 [38.2;42.1] |
| HS or equivalent | 21.0 [19.7;22.3] |
| More than HS | 38.9 [37.1;40.6] |
| Sex (%) | |
| Female | 54.7 [53.3;56.1] |
| Hispanic/Latino background (%) | |
| Dominican | 9.4 [8.0;11.0] |
| Central American | 6.8 [5.9; 77] |
| Cuban | 28.3 [24.4;32.6] |
| Mexican | 31.5 [28.2;35.1] |
| Puerto Rican | 18.3 [16.3;20.4] |
| South American | 5.7 [5.0;6.4] |
| Age (in years) | 56.5 [0.15] |
| Age (%) | |
| 45–54 | 46.7 [45.0;48.3] |
| 55–64 | 31.9 [30.4;33.5] |
| 65+ | 21.4 [19.9;22.9] |
| Marital status (%) | |
| Single | 16.7 [15.4;18.0] |
| Married/living with partner | 53.5 [51.4;55.6] |
| Separated/divorced/widowed | 29.8 [28.2;31.5] |
| Field center (%) | |
| Bronx | 26.2 [23.1;29.4] |
| Chicago | 12.7 [11.1;14.5] |
| Miami | 37.0 [32.3;41.8] |
| San Diego | 24.2 [20.9;27.8] |
| Language preference (%) | |
| Spanish | 86.1 [84.4;87.7] |
| Stroke/TIA (%) | |
| Yes | 4.2 [3.6;4.8] |
| CHD/Angina (%) | |
| Yes | 10.4 [9.4;11.4] |
| % glycosylated hemoglobin | 6.1 [0.02] |
| BMI (%) | |
| Underweight | 0.5 [0.3;0.8] |
| Normal | 16.4 [15.2;17.6] |
| Overweight | 40.6 [39.1;42.1] |
| Obese | 42.5 [40.8;44.1] |
| Physical activity (%) | |
| High | 7.8 [7.0;8.7] |
| Moderate | 41.9 [40.3;43.5] |
| Low | 50.3 [48.7;51.9] |
| Antihypertensive (%) | |
| Yes | 27.4 [25.9;28.9] |
Mean [se] are provided for continuous measures. % [95% CI] are provided for categorical measures. SEVLT, Spanish English Verbal Learning Test; Physical activity levels are based on the Global Physical Activity Questionnaire instrumentation; SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure. Pulse pressure was measured as the difference between average SBP and DBP, and MAP was measured as DBP+ 1/3*(SBP-DBP).
Table 2.
Associations between blood pressure measures (in 10 mm/Hg units) and cognitive function (z-scores). Results are derived from survey linear regression models and include 1) crude, 2) age, sex, and education adjusted, and 3) full covariates adjusted estimates (unweighted n = 9,019)
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
|
|
|||
| (b,OR)/(se,95% CI) | (b,OR)/(se,95% CI) | (b,OR)/(se,95% CI) | |
| B-SEVLT-sum | |||
| SBP | −0.08*** (0.01) | −0.03*** (0.01) | −0.02*** (0.01) |
| DBP | −0.02 (0.01) | −0.03** (0.01) | −0.02 (0.01) |
| PP | −0.11*** (0.01) | −0.03*** (0.01) | −0.03*** (0.01) |
| MAP | −0.07*** (0.01) | −0.04*** (0.01) | −0.03** (0.01) |
| B-SEVLT-recall | |||
| SBP | −0.07*** (0.01) | −0.02*** (0.01) | −0.01* (0.01) |
| DBP | −0.02 (0.01) | −0.03** (0.01) | −0.01 (0.01) |
| PP | −0.11*** (0.01) | −0.03** (0.01) | −0.02** (0.01) |
| MAP | −0.06*** (0.01) | −0.03*** (0.01) | −0.01 (0.01) |
| WF | |||
| SBP | −0.07*** (0.01) | −0.05*** (0.01) | −0.04*** (0.01) |
| DBP | −0.06*** (0.01) | −0.08*** (0.01) | −0.05*** (0.01) |
| PP | −0.08*** (0.01) | −0.05*** (0.01) | −0.04*** (0.01) |
| MAP | −0.08*** (0.01) | −0.08*** (0.01) | −0.05*** (0.01) |
| DSS | |||
| SBP | −0.10*** (0.01) | −0.04*** (0.01) | −0.02*** (0.01) |
| DBP | −0.01 (0.01) | −0.04*** (0.01) | −0.02* (0.01) |
| PP | −0.16*** (0.01) | −0.04*** (0.01) | −0.03*** (0.01) |
| MAP | −0.08*** (0.01) | −0.05*** (0.01) | −0.03** (0.01) |
| SIS ≤ 4 | |||
| SBP | 1.14*** [1.09;1.18] | 1.06* [1.01;1.10] | 1.05* [1.00;1.10] |
| DBP | 1.01 [0.93;1.09] | 1.06 [0.98;1.15] | 1.05 [0.96;1.14] |
| PP | 1.12*** [1.05;1.19] | 1.07* [1.00;1.15] | 1.06 [0.99;1.14] |
| MAP | 1.23*** [1.16;1.30] | 1.07* [1.00;1.14] | 1.06 [1.00;1.14] |
p < 0.001;
p < 0.01;
p < 0.05.
Model 1: Crude; Model 2: Age, sex, education; Model 3: Model 2 + Hispanic/Latino background, marital status, center, language preference, stroke/TIA, CHD/angina, % hemoglobin, BMI, physical activity, antihypertensive medications. Note 1: B-SEVLT, Brief Spanish English Verbal Learning Test; WF, Word Fluency; DSS, Digit Symbol Substitution; SIS, Six Item Screener. Note 2: b, Beta Coefficient; OR, Odds Ratio; se, standard error; CI, confidence interval. Note 3: BP measures are tested independently. The grouping and table formatting is to facilitate presentation of results. SBP, systolic blood pressure; DBP, diastolic blood pressure; PP, pulse pressure; MAP, mean arterial pressure. Pulse pressure was measured as the difference between average SBP and DBP, and was measured as DBP+ 1/3*(SBP-DBP).
Fig. 1.
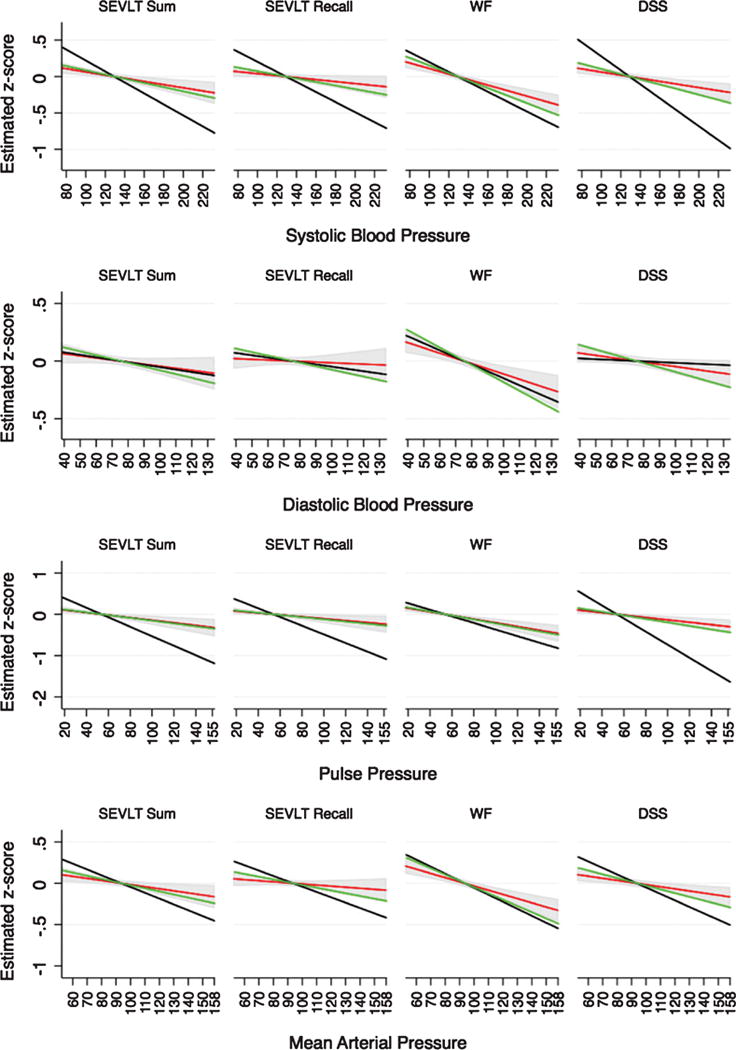
Estimated marginal means and their 95% confidence intervals across the blood pressure measures continuums. Results are derived from survey linear regression models and include 1) crude (black), 2) age, sex, and education adjusted (green), and 3) full covariates adjusted (red) estimates. Results for Hispanic/Latino adults ages 45–74 years from the HCHS/SOL (2008–2011). Model 1: Crude; Model 2: Age, sex, education; Model 3: Model 2 + Hispanic/Latino background, marital status, center, language preference, stroke/TIA, CHD/angina, % hemoglobin, BMI, physical activity, antihypertensive medications. Note 1: B-SEVLT, Brief Spanish English Verbal Learning Test; WF, Word Fluency; DSS, Digit Symbol Substitution; SIS, Six Item Screener. Note 2: Pulse pressure was measured as the difference between average systolic (SBP) and diastolic (DBP) pressure, and mean arterial pressure was measured as DBP+ 1/3*(SBP-DBP).
Fig. 2.
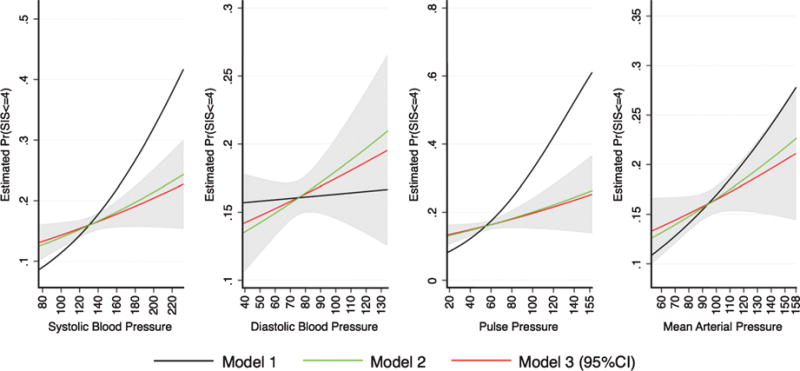
Average marginal probabilities for impaired mental status based on the Six Item Screener (SIS ≤ 4), and their 95% confidence intervals across the blood pressure measures continuums. Results are derived from survey logistic regression models and include 1) crude, 2) age, sex, and education adjusted, and 3) full covariates adjusted estimates. Results for Hispanic/Latino adults ages 45–74 years from the HCHS/SOL (2008–2011). Model 1: Crude. Model 2: Age, sex, education. Model 3: Model 2 + Hispanic/Latino background, marital status, center, language preference, stroke/TIA, CHD/angina, % hemoglobin, BMI, physical activity, antihypertensive medications. Note 1: The figures represent estimated marginal probabilities. Higher values indicate higher likelihood of impairment. Note 2: B-SEVLT, Brief Spanish English Verbal Learning Test; WF, Word Fluency; DSS, Digit Symbol Substitution; SIS, Six Item Screener. Note 3: Pulse pressure was measured as the difference between average systolic (SBP) and diastolic (DBP) pressure, and mean arterial pressure was measured as DBP+ 1/3*(SBP-DBP).
RESULTS
Descriptive statistics
Characteristics for the HCHS/SOL target population are included in Table 1. Slightly more than half the target population was female (54.7%), and the mean age was 56.5 years. Close to a third of individuals had a Mexican background (31.5%), followed by Cuban (28.3%), Puerto Rican (18.3%), Dominican (9.4%), Central American (6.8%), and South American (5.7%). Two-fifth of individuals (40.1%) had less than high school education, the majority (86.1%) had Spanish as their language of preference, and slightly more than half (53.5%) were married or living with a partner. The prevalence of stroke/transient ischemic attack was 4.2% and the prevalence of coronary heart disease/angina was 10.4%. More than four-in-five individuals (83.1%) were overweight (40.6%) or obese (42.5%), yet 50.3% satisfied criteria for a high level of physical activity. The average glycosylated hemoglobin level was 6.1%. Finally, 27.4% of individuals used antihypertensive medications.
The estimated means for the cognitive tests were B-SEVLT Sum, 22.3 (SD = 6.9), B-SEVLT Recall, 8.0 (SD = 3.5), WF, 18.3 (SD = 8.8), and DSS, 33.8 (SD = 16.4). Additionally, 16.1% of participants satisfied criteria for low mental status on the SIS (≤4). The estimated means for the BP measures were: SBP, 129.1 (SD = 22.7), DBP, 75.4 (SD = 75.4), PP, 53.8 (SD = 17.5), and MAP 93.3 (SD = 14.8).
Crude associations
Unadjusted associations are provided in Table 2. We found that SBP was negatively and significantly associated with B-SEVLT-sum, B-SEVLT-recall, WF, DSS, and SIS. A 10 mmHg increase in SBP was consistently associated with about one-tenth of a standard deviation decrease in cognitive function irrespective of the considered continuous measured test, and increased the odds of low mental status by close to 14%. DBP (per 10 mmHg) was negatively associated with WF (β= −0.06; SE = 0.01; p < 0.001), but no other statistically distinct association with the other cognitive outcomes were evidenced. PP (per 10 mmHg) associations were similar to our SBP results with negative relationships documented with B-SEVLT-sum (β = −0.11; SE = 0.01; p < 0.001), B-SEVLT-recall (β = −0.11; SE = 0.01), WF (β = −0.08; SE = 0.01), DSS (β = −0.16; SE = 0.01), and SIS (OR = 1.12; 95% CI = 1.05–1.19), all p < 0.001. MAP (per 10 mmHg) was also negatively associated with B-SEVLT-sum (β = −0.07; SE = 0.01), B-SEVLT-recall (β = −0.06; SE = 0.01), WF (β = −0.08; SE = 0.01), DSS (β = −0.08; SE = 0.01), and SIS (OR = 1.23; 95% CI = 1.16–1.30), all p < 0.001.
Adjusted associations
Age, sex, and education adjustment reduced the reported associations between SBP and cognitive function by, 61.7% for B-SEVLT-sum, 64.5% for B-SEVLT-recall, 23.6% for WF, 63.1% for DSS, and 57.8% for low mental status. Higher DBP (per 10 mmHg) became significantly associated with B-SEVLT-sum (β = −0.03**; p < 0.01), B-SEVLT-recall (β = −0.03**; p < 0.01), WF (β = −0.08***; p < 0.001), and DSS (β = −0.04***; p < 0.001) with control for these variables. We also found reductions in associations, similar to SBP, between PP and B-SEVLT-sum, B-SEVLT-recall, WF, DSS, and low mental status; equivalent to 70.7%, 74.6%, 39.7%, 73.3%, and 68.2%, respectively. The associations between MAP, B-SEVLT-sum, B-SEVLT-recall, and DSS were also decreased by 46.4%, 48.4%, and 41.9%, respectively.
Full adjustment to model covariates further attenuated the relationship between SBP and B-SEVLT-sum, B-SEVLT-recall, WF, DSS, and low mental status by 24.5%, 44.4%, 26.8%, and 39.8%, and 14.0%, respectively. DBP remained negatively associated with WF and DSS, but these associations were also attenuated by 39.7%, and 49.8%, respectively. PP remained negatively associated with B-SEVLT-sum, B-SEVLT-recall, WF, and the DSS, but the associations were reduced by 13.2%, 7.6%, and 31.2% for the latter three tests, respectively. Finally, MAP remained negatively associated with B-SEVLT-sum, WF, and DSS but the associations decreased by 33.2%, 33.1%, and 43.7%, respectively. Figures 1 and 2 provide representations of the associations (and incremental attenuations) between cognitive performance and the four BP measures.
Interaction effects
Tests of interactions for our BP measures with age-groups, sex, and Latino background indicated that none of these three indicators modified the BP effects on the cognitive outcomes detailed in our regression results above (Supplementary Tables 2–4).
Tests of splines
Test of splines (Supplementary Tables 5 and 6) showed that the differences in slopes were consistently insignificant for all BP markers and across all NC tests considered. In additional models, we considered two polynomial functions for our BP measures. We found that neither the quadratic nor the cubic terms in those tested models were statistically linked to cognitive outcomes (available from authors).
DISCUSSION
In this large community-based study of middle-aged and older diverse Latinos, we found that higher systolic, pulse, mean arterial, and, to a lesser extent, diastolic BP measures were modestly, but consistently, associated with lower cognitive function. Our findings indicate that these associations are apparent beginning in midlife and into older age. In the absence of pharmacological substitutes for treating neurodegenerative disorders, efforts to prevent and appropriately manage BP dysregulations in midlife can prove valuable for enhancing public health in an increasingly diverse and older US population.
Overall, this study extends the growing literature linking CVD and cognitive health in middle-aged and older Latinos. Exposure to high BP raised the risk for lower cognitive performance uniformly across sex and Latino backgrounds. This consistency is noteworthy given the mixed reports in the literature [17, 18, 22, 51], and the differential risk distribution of both cardiovascular health [23, 42, 52, 53] and NC function and disorders [42, 54, 55] by sex and background.
All our BP measures were inversely and diffusely linked to cognitive performance across all cognitive domains in age, sex, and education adjusted models. These findings are compelling given that previous studies suggest that abnormalities in different hemodynamic patterns (e.g., through pulsatile versus steady BP components) are independently associated with, arguably, divergent cardiovascular and cerebrovascular outcomes [38–41]. Our results are concordant with mechanistic suggestions that both the progressive stiffening of arteries and vessels, as suggested by higher SBP and PP [9, 56], as well as the compromise of vascular resistance and blood flow [57], indicated by higher MAP, have the potential to damage brain microcirculation, disrupt cerebral autoregulation, impair brain perfusion, and eventually lead to cognitive dysfunction [9, 58].
We found no crude associations between diastolic pressure and cognitive performance. Furthermore, adjusting for social, behavioral, and cardio-metabolic factors explained the age, sex, and education adjusted inverse associations between higher DBP and performance on tests of episodic memory and learning. It is not clear from our results what mechanism could have led to this complete attenuation. Previous studies have linked higher DBP to cognitive dysfunction and decline, and argued that it is implicated in small cerebral vessels damage and disease and brain atrophy [6, 59]. Some, however, have argued that higher DBP poses little unique (independent of SBP) risk for cardiovascular and cerebrovascular events (e.g., stroke) [60] or advanced cerebral disease (e.g., white matter lesions) [61] Yet others suggest that the effects of higher DBP on cognition are limited, protective with older age [1, 8], or might be restricted to individuals with higher risk of cerebral small vessels damage (e.g., through diabetes) [62]. Most existing studies, however, have focused on older non-Hispanic populations and conceivably different mediating or moderating social (e.g., healthcare access) and biological (e.g., CVD risk clusters) factors could be at work. While our study shows largely consistent associations between higher BP and lower cognitive function in Latinos more detailed investigations of the complex pathway between BP dysregulations and cognitive outcomes and brain disorders are needed.
We were unable to identify cognitively optimal or “protective” BP ranges among middle-aged and older Latinos. Our BP measures were linearly inversely related to cognitive performance and we found no evidence to suggest age moderation or optimal points of inversion. The target population of HCHS/SOL is relatively young which could have masked nonlinear (e.g., u-shaped) relationships; likely because the requisite need for higher BP, secondary to vascular damage, does not manifest until much later in life (e.g., among the oldest-old). Longitudinal data and clinical trials such as the ongoing Systolic Blood Pressure Intervention Trial (SPRINT) which was designed to explore whether, at what levels, and how better BP control reduces CVD outcomes and preserves cognition remain invaluable for growing the evidence base [63].
Several limitations merit emphasis. First, our results were based on cross-sectional data and as such do not allow causal inferences about BP measures and cognition, or to test associations with cognitive decline or disorders (e.g., dementias). Secondly, our findings may not apply to adults over age 74 years. This is particularly important since extant findings among older adults have reported differential effects of BP; where higher BP may be neuroprotective [64]. Thirdly, we were limited to clinic-based, daytime, seated BP readings. Timing (daytime versus nighttime), setting (seated versus supine), locale (office versus ambulatory), and position (central versus brachial) of BP measurement can be differentially related to cognition. Finally, despite the rich Latino background represented within the HCHS/SOL cohort, our findings are limited to Latinos in our targeted sampling areas, and we were unable to make comparisons to ethnic/racial groups.
Conclusion
Among middle-aged and older, diverse Latinos, we found consistent associations between higher SBP, PP, and MAP and lower cognitive function. BP dysregulations have been implicated in both stroke and dementia. As the US population ages and diversifies, CVD and neurodegenerative disorders (e.g., Alzheimer’s disease) are expected to continue to exact extensive personal, economic and social costs [65]. Health education and promotion efforts to raise awareness, improve access to better BP management would likely promote cognitive health, reduce stroke, and potentially could reduce cognitive decline and disorders among diverse populations, including Latinos.
Supplementary Material
Acknowledgments
Dr. Tarraf, Dr. González, and Mr. Conceicao receive support for this work from R01-AG48642. Drs. Tarraf and Gonzalez also receive support from P30-AG053760 and previously received support from NHLBI HC-65233. The Hispanic Community Health Study/Study of Hispanic/Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
Footnotes
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/17-0017r1).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-170017.
References
- 1.Kennelly S, Collins O. Walking the cognitive “minefield” between high and low blood pressure. J Alzheimers Dis. 2012;32:609–621. doi: 10.3233/JAD-2012-120748. [DOI] [PubMed] [Google Scholar]
- 2.de la Torre JC. Cerebral hemodynamics and vascular risk factors: Setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32:553–567. doi: 10.3233/JAD-2012-120793. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rigaud AS, Seux ML, Staessen JA, Birkenhager WH, Fore-tte F. Cerebral complications of hypertension. J Hum Hypertens. 2000;14:605–616. doi: 10.1038/sj.jhh.1001118. [DOI] [PubMed] [Google Scholar]
- 5.Faraco G, Iadecola C. Hypertension: A harbinger of stroke and dementia. Hypertension. 2013;62:810–817. doi: 10.1161/HYPERTENSIONAHA.113.01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias MF, Goodell AL, Dore GA. Hypertension and cognitive functioning: A perspective in historical context. Hypertension. 2012;60:260–268. doi: 10.1161/HYPERTENSIONAHA.111.186429. [DOI] [PubMed] [Google Scholar]
- 7.Iadecola C, Yaffe K, Biller J, Bratzke LC, Faraci FM, Gore-lick PB, Gulati M, Kamel H, Knopman DS, Launer LJ, Saczynski JS, Seshadri S, Zeki Al Hazzouri A, An Hypertension; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Quality of Care and Outcomes Research; and Stroke Council Im pact of hypertension on cognitive function: A scientific statement from the American Heart Association. Hypertension. 2016;68:e67–e94. doi: 10.1161/HYP.0000000000000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 9.O’Rourke MF. Arterial aging: Pathophysiological principles. Vasc Med. 2007;12:329–341. doi: 10.1177/1358863X07083392. [DOI] [PubMed] [Google Scholar]
- 10.Knecht S, Wersching H, Lohmann H, Bruchmann M, Duning T, Dziewas R, Berger K, Ringelstein EB. High-normal blood pressure is associated with poor cognitive performance. Hypertension. 2008;51:663–668. doi: 10.1161/HYPERTENSIONAHA.107.105577. [DOI] [PubMed] [Google Scholar]
- 11.Launer LJ, Hughes T, Yu BB, Masaki K, Petrovitch H, Ross GW, White LR. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: Perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension. 2010;55:1352–1359. doi: 10.1161/HYPERTENSIONAHA.109.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohler S, Baars MA, Spauwen P, Schievink S, Verhey FR, van Boxtel MJ. Temporal evolution of cognitive changes in incident hypertension: Prospective cohort study across the adult age span. Hypertension. 2014;63:245–251. doi: 10.1161/HYPERTENSIONAHA.113.02096. [DOI] [PubMed] [Google Scholar]
- 13.Elias PK, Elias MF, Robbins MA, Budge MM. Blood pressure-related cognitive decline does age make a difference? Hypertension. 2004;44:631–636. doi: 10.1161/01.HYP.0000145858.07252.99. [DOI] [PubMed] [Google Scholar]
- 14.Singh-Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle-age in the Whitehall II study. J Clin Epidemiol. 2005;58:1308–1315. doi: 10.1016/j.jclinepi.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Yaffe K, Vittinghoff E, Pletcher MJ, Hoang TD, Launer LJ, Whitmer R, Coker LH, Sidney S. Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 2014;129:1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- 17.Yaffe K, Haan M, Blackwell T, Cherkasova E, Whitmer RA, West N. Metabolic syndrome and cognitive decline in elderly Latinos: Findings from the Sacramento Area Latino Study of Aging study. J Am Geriatr Soc. 2007;55:758–762. doi: 10.1111/j.1532-5415.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeki Al Hazzouri A, Haan MN, Neuhaus JM, Pletcher M, Peralta CA, Lopez L, Perez Stable EJ. Cardiovascular risk score, cognitive decline, and dementia in older Mexican Americans: The role of sex and education. J Am Heart Assoc. 2013;2:e004978. doi: 10.1161/JAHA.113.004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downer B, Raji MA, Markides KS. Relationship between metabolic and vascular conditions and cognitive decline among older Mexican Americans. Int J Geriatr Psychiatry. 2016;31:213–221. doi: 10.1002/gps.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Insel KC, Palmer RF, Stroup-Benham CA, Markides KS, Espino DV. Association between change in systolic blood pressure and cognitive decline among elderly Mexican Americans: Data from the Hispanic established population for epidemiology study of the elderly. Exp Aging Res. 2005;31:35–54. doi: 10.1080/03610730590882837. [DOI] [PubMed] [Google Scholar]
- 21.Levin BE, Llabre MM, Dong C, Elkind MS, Stern Y, Rundek T, Sacco RL, Wright CB. Modeling metabolic syndrome and its association with cognition: The Northern Manhattan study. J Int Neuropsychol Soc. 2014;20:951–960. doi: 10.1017/S1355617714000861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gardener H, Wright CB, Dong C, Cheung K, DeRosa J, Nannery M, Stern Y, Elkind MS, Sacco RL. Ideal cardiovascular health and cognitive aging in the Northern Manhattan Study. J Am Heart Assoc. 2016;5:e002731. doi: 10.1161/JAHA.115.002731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorlie PD, Allison MA, Aviles-Santa ML, Cai J, Daviglus ML, Howard AG, Kaplan R, Lavange LM, Raij L, Schneiderman N, Wassertheil-Smoller S, Talavera GA. Prevalence of hypertension, awareness, treatment, and control in the Hispanic Community Health Study/Study of Latinos. Am J Hypertens. 2014;27:793–800. doi: 10.1093/ajh/hpu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bersamin A, Stafford RS, Winkleby MA. Predictors of hypertension awareness, treatment, and control among Mexican American women and men. J Gen Intern Med. 2009;24(Suppl 3):521–527. doi: 10.1007/s11606-009-1094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52:818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- 26.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 27.Oveisgharan S, Hachinski V. Hypertension, executive dysfunction, and progression to dementia: The canadian study of health and aging. Arch Neurol. 2010;67:187–192. doi: 10.1001/archneurol.2009.312. [DOI] [PubMed] [Google Scholar]
- 28.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of arterial stiffness and blood pressure in hypertension-associated cognitive decline in healthy adults. Hypertension. 2016;67:171–175. doi: 10.1161/HYPERTENSIONAHA.115.06277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–698. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behav Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- 31.Lavange LM, Kalsbeek WD, Sorlie PD, Aviles-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16:439–451. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez HM, Mungas D, Reed BR, Marshall S, Haan MN. A new verbal learning and memory test for English-and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–555. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Arguelles W, Choca JP, Catellier DJ, Mosley TH. Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Darne B, Girerd X, Safar M, Cambien F, Guize L. Pulsatile versus steady component of blood pressure: A cross-sectional analysis and a prospective analysis on cardiovascular mortality. Hypertension. 1989;13:392–400. doi: 10.1161/01.hyp.13.4.392. [DOI] [PubMed] [Google Scholar]
- 37.Franklin SS, Gustin Wt, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 38.Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Loster M, Kloch-Badelek M, Wilinski J, Curylo AM, Dudek D, Aortic Blood P, Survival Study G Pulsatile but not steady component of blood pressure predicts cardiovascular events in coronary patients. Hypertension. 2008;51:848–855. doi: 10.1161/HYPERTENSIONAHA.107.101725. [DOI] [PubMed] [Google Scholar]
- 39.Jankowski P, Bilo G, Kawecka-Jaszcz K. The pulsatile component of blood pressure: Its role in the pathogenesis of atherosclerosis. Blood Press. 2007;16:238–245. doi: 10.1080/08037050701428166. [DOI] [PubMed] [Google Scholar]
- 40.Gutierrez J, Elkind MS, Cheung K, Rundek T, Sacco RL, Wright CB. Pulsatile and steady components of blood pressure and subclinical cerebrovascular disease: The Northern Manhattan Study. J Hypertens. 2015;33:2115–2122. doi: 10.1097/HJH.0000000000000686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Boxtel MP, Henskens LH, Kroon AA, Hofman PA, Gronenschild EH, Jolles J, de Leeuw PW. Ambulatory blood pressure, asymptomatic cerebrovascular damage and cognitive function in essential hypertension. J Hum Hyper-tens. 2006;20:5–13. doi: 10.1038/sj.jhh.1001934. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez HM, Tarraf W, Gouskova N, Rodriguez CJ, Rundek T, Grober E, Pirzada A, Gonzalez P, Lutsey PL, Camacho A, Daviglus ML, Wright C, Mosley TH. Life’s Simple 7’s cardiovascular health metrics are associated with Hispanic/Latino neurocognitive function: HCHS/SOL results. J Alzheimers Dis. 2016;53:955–965. doi: 10.3233/JAD-151125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qiu CX, Fratiglioni L. A major role for cardiovascular burden in age-related cognitive decline. Nat Rev Cardiol. 2015;12:267–277. doi: 10.1038/nrcardio.2014.223. [DOI] [PubMed] [Google Scholar]
- 44.Fortmann AL, Roesch SC, Penedo FJ, Isasi CR, Carnethon MR, Corsino L, Schneiderman N, Daviglus ML, Teng Y, Giachello A. Glycemic control among US Hispanics/Latinos with diabetes from the HCHS/SOL Sociocultural Ancillary Study: Do structural and functional social support play a role? J Behav Med. 2015;38:153–159. doi: 10.1007/s10865-014-9587-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heeringa SG, West BT, Berglund PA. Applied survey data analysis. CRC Press; 2010. [Google Scholar]
- 46.Rust K. Variance estimation for complex estimators in sample surveys. J Off Stat. 1985;1:381–397. [Google Scholar]
- 47.Simes RJ. An improved bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- 48.Benjamini Y, Hochberg Y. Controlling the false discovery rate - a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 49.Newson RB. Frequentist q-values for multiple-test procedures. Stata J. 2010;10:568–584. [Google Scholar]
- 50.Touyz RM, Dominiczak AF. Hypertension guidelines: Is it time to reappraise blood pressure thresholds and targets? Hypertension. 2016;67:688–689. doi: 10.1161/HYPERTENSIONAHA.116.07090. [DOI] [PubMed] [Google Scholar]
- 51.Lamar M, Wu D, Durazo-Arvizu RA, Brickman AM, Gonzalez HM, Tarraff W, Daviglus ML. Cognitive associates of current and more intensive control of hypertension: Findings from the Hispanic Community Health Study/Study of Latinos. Cognitive Aging Conference; Atlanta, Georgia: 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daviglus ML, Pirzada A, Talavera GA. Cardiovascular disease risk factors in the Hispanic/Latino population: Lessons from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Prog Cardiovasc Dis. 2014;57:230–236. doi: 10.1016/j.pcad.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Daviglus ML, Talavera GA, Aviles-Santa ML, Allison M, Cai J, Criqui MH, Gellman M, Giachello AL, Gouskova N, Kaplan RC, LaVange L, Penedo F, Perreira K, Pirzada A, Schneiderman N, Wassertheil-Smoller S, Sorlie PD, Stamler J. Prevalence of major cardiovascular risk factors and cardiovascular diseases among Hispanic/Latino individuals of diverse backgrounds in the United States. JAMA. 2012;308:1775–1784. doi: 10.1001/jama.2012.14517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.González HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Argüelles W, Choca JP, Catellier DJ. Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehta KM, Yeo GW. Systematic review of dementia prevalence and incidence in US race/ethnic populations. Alzheimers Dement. 2017;13:72–83. doi: 10.1016/j.jalz.2016.06.2360. [DOI] [PubMed] [Google Scholar]
- 56.Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. doi: 10.1161/01.hyp.36.5.801. [DOI] [PubMed] [Google Scholar]
- 57.Lefferts WK, Heffernan KS, Barreira TV. Association between pulsatile blood pressure and cognitive performance among older adults: Insight from the National Health and Nutrition Examination Survey 1999–2002. Int J Cardiol. 2016;223:981–984. doi: 10.1016/j.ijcard.2016.08.287. [DOI] [PubMed] [Google Scholar]
- 58.Deverdun J, Akbaraly TN, Charroud C, Abdennour M, Brickman AM, Chemouny S, Steffener J, Portet F, Bonafe A, Stern Y, Ritchie K, Molino F, Le Bars E, Menjot de Champfleur N. Mean arterial pressure change associated with cerebral blood flow in healthy older adults. Neurobiol Aging. 2016;46:49–57. doi: 10.1016/j.neurobiolaging.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 59.Tsivgoulis G, Alexandrov AV, Wadley VG, Unverzagt FW, Go RC, Moy CS, Kissela B, Howard G. Association of higher diastolic blood pressure levels with cognitive impairment. Neurology. 2009;73:589–595. doi: 10.1212/WNL.0b013e3181b38969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tin LL, Beevers DG, Lip GY. Systolic vs diastolic blood pressure and the burden of hypertension. J Hum Hypertens. 2002;16:147–150. doi: 10.1038/sj.jhh.1001373. [DOI] [PubMed] [Google Scholar]
- 61.Kim KW, MacFall JR, Payne ME. Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol Psychiatry. 2008;64:273–280. doi: 10.1016/j.biopsych.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spauwen PJ, van Boxtel MP, Verhey FR, Kohler S, Sep SJ, Koster A, Dagnelie PC, Henry RM, Schaper NC, van der Kallen CJ, Schram MT, Kroon AA, Stehouwer CD. Both low and high 24-hour diastolic blood pressure are associated with worse cognitive performance in type 2 diabetes: The Maastricht Study. Diabetes Care. 2015;38:1473–1480. doi: 10.2337/dc14-2502. [DOI] [PubMed] [Google Scholar]
- 63.Group SR. Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC, Jr, Fine LJ, Cutler JA, Cushman WC, Cheung AK, Ambrosius WT. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kennelly S, Collins O. Walking the cognitive “minefield” between high and low blood pressure. J Alzheimers Dis. 2012;32:609–621. doi: 10.3233/JAD-2012-120748. [DOI] [PubMed] [Google Scholar]
- 65.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


