Abstract
Natural killer (NK) cells play a critical role in host defense against viruses. Here, we investigated the role of NKG2D in the expansion of NK cells after mouse cytomegalovirus (MCMV) infection. Wild-type and NKG2D-deficient (Klrk1−/−) Ly49H+ NK cells robustly proliferated when infected with MCMV strains engineered to allow expression of NKG2D ligands, which enhanced the response of wild-type NK cells. Naïve NK cells exclusively express NKG2D-L, which pairs only with DAP10, whereas NKG2D-S expressed by activated NK cells pairs with both DAP10 or DAP12, similar to Ly49H. However, NKG2D alone was unable to drive robust expansion of Ly49H− NK cells when mice were infected with these MCMV strains likely because NKG2D-S was only transiently expressed after infection. These findings demonstrate that NKG2D augments Ly49H-dependent proliferation of NK cells; however, NKG2D signaling alone is inadequate for expansion of NK cells, likely due to only transient expression of a NKG2D-DAP12 complex.
Introduction
Natural killer (NK) cells recognize abnormal or allogeneic cells by using a variety of receptors that regulate their activation, cytotoxicity, and cytokine production (1, 2). NK cells have adaptive immune features, which include antigen-specific proliferation after exposure to mouse cytomegalovirus (MCMV) and alloantigens (3–6). We have previously demonstrated that mouse NK cells expressing Ly49H, which specifically recognizes the m157 MCMV glycoprotein on infected cells (7), robustly expand after MCMV infection (4). The activating signals though Ly49H, the costimulatory molecule DNAM-1, and cytokines including IL-12, IL-18, and IL-33 are required for the optimal expansion of effector Ly49H+ NK cells (4, 6, 8–11). Ly49H associates with the DAP12 and DAP10 adapter molecules to transmit the activating signaling (12). DAP12-mediated signaling is essential for the clonal expansion of antigen-specific NK cells, whereas DAP10 augments this response (4, 5, 13).
NKG2D is an activating receptor that is expressed by essentially all NK cells, human CD8+ T cells, mouse activated CD8+ T cells, γδ T cells, subsets of iNKT cells, and a minor population of human CD4+ T cells under certain conditions (14–16). NKG2D recognizes a family of stress-induced ligands that are distantly related to MHC class I molecules and are expressed frequently on infected and tumor cells in both mice and humans (16, 17). Human NKG2D, which has no signaling motif within its short intracellular domain, associates with DAP10 via charged residues in its transmembrane domain (14, 18). Mouse NKG2D has two isoforms with a long (NKG2D-L) or short (NKG2D-S) cytoplasmic domain, which are generated by alternative RNA splicing (19, 20). As in humans, mouse NKG2D-L associates exclusively with DAP10, whereas mouse NKG2D-S associates both DAP10 and DAP12 (19, 20). In mice, naïve NK cells exclusively express NKG2D-L. Stimulation of NK cells with IL-2 in vitro or by treatment with polyinosinic:polycytidylic acid in vivo induces NKG2D-S, which pairs with DAP12, as demonstrated by the expression of NKG2D on the cell surface of DAP10-deficient NK cells after activation (19, 20). NKG2D triggers IFN-γ production from NK cells and induces NK cell-mediated cytotoxicity against target cells expressing its ligands (16, 17, 21–23). However, the role of NKG2D signaling in expansion of effector NK cells remains to be elucidated. Here, we addressed whether an activating signal through NKG2D promotes the expansion of NK cells during MCMV infection.
Materials and Methods
Mice
C57BL/6 and congenic CD45.1+ C57BL/6 mice were purchased from the National Cancer Institute and Charles River. NKG2D-deficient (Klrk1−/−) mice (Supplemental Fig. 1), Ly49H-deficient (Klra8−/−) mice (24) (generously provided by Dr. S. Vidal, McGill University, Canada), DAP10 (Hcst) and DAP12 (Tyrobp) double-deficient mice (25) (generously provided by T. Takai, Tohoku University, Japan), DAP10-deficient (Hcst−/−) mice (26), and DAP12-deficient (Tyrobp−/−) mice (27) on the C57BL/6 background were maintained at the University California, San Francisco, in accordance with the guidelines of the Institutional Animal Care and Use Committee. CD45.1+ wild-type (WT) and CD45.2+ Klrk1−/− mixed bone marrow (BM) chimeric mice were generated as described (4).
MCMV
Smith strain WT MCMV, Δm151-m158 mutant MCMV (MC96.73) (28), Δm152 mutant MCMV (29), MCMV expressing RAE-1γ (RAE-1γ-MCMV) (30), and MCMV expressing MULT1 which was inserted into a place of its viral inhibitor m145 gene locus (MULT1-MCMV) were prepared by infecting BALB/c 3T3 cells or mouse embryonic fibroblasts in cell culture as described (31). Mice were infected by intraperitoneal injection of 1–10 × 104 plaque forming unit (PFU) WT or mutant MCMV strains. In some experiments, Smith strain WT MCMV was prepared by homogenizing salivary glands harvested from infected BALB/c mice as described (32). Mice were infected by intraperitoneal injection of 1 × 104 PFU salivary gland WT MCMV. For in vitro experiments, C57BL/6 3T3 cells were infected with tissue-cultured MCMV strains at 1 PFU/cell.
NK cell enrichment and adoptive transfer
NK cells were enriched by incubating splenocytes with rat monoclonal antibodies (mAbs) against mouse CD4, CD5, CD8, CD19, Gr-1, and Ter119, followed by anti-rat IgG antibodies conjugated to magnetic beads (QIAGEN), as described (8). Two hundred thousand NK cells or one hundred thousand Ly49H+ NK cells from donor mice were injected intravenously into recipient mice on the day before infection. In some experiments Ly49H− NK cells were purified by using a FACSAria III and 5 x 104 Ly49H− NK cells were injected intravenously into recipient mice on the day before infection. In some experiments, NK cells were cultured in the presence of 1000–2500 U/ml recombinant human IL-2 (generously provided by Prometheus Laboratories, Inc.) for 3 days.
Flow cytometry
Fc receptors (CD16 and CD32) were blocked with 2.4G2 mAb before staining with the indicated fluorochrome-conjugated mAbs or isotype-matched control mAbs (BD Biosciences, eBioscience, BioLegend, or TONBO Biosciences). NK cells were gated as NK1.1+ and T cell receptor β chain− lymphocytes. Samples were acquired on a LSRII or a LSR Fortessa (BD Biosciences) and data were analyzed with FlowJo software (FlowJo)
Statistical methods
The Student’s t test or the one-way ANOVA were used to compare results. Error bars represent S.E.M.
Results and Discussion
NKG2D enhances expansion of Ly49H+ NK cells after MCMV infection
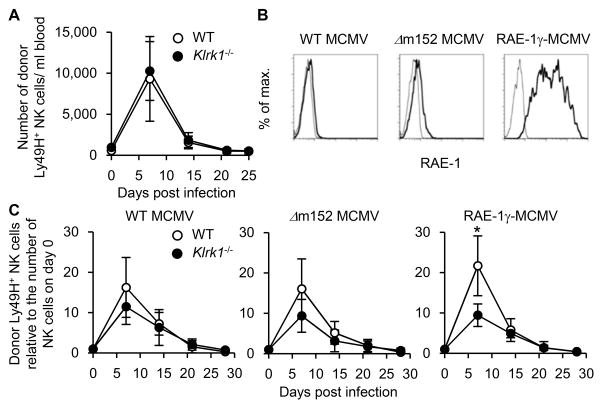
To determine whether an intrinsic deficiency of NKG2D affects NK cell responses during MCMV infection, CD45.1+ WT C57BL/6 NK cells and CD45.2+ NKG2D-deficient (Klrk1−/−) C57BL/6 NK cells were adoptively co-transferred into syngeneic DAP10- and DAP12-double deficient recipient mice, which lack functional surface expression of Ly49H and NKG2D, and then infected with WT MCMV. Klrk1−/− Ly49H+ NK cells and WT Ly49H+ NK cells equivalently proliferated after MCMV infection (Figure 1A). As MCMV possesses several proteins that block expression of NKG2D ligands in infected cells (16, 17), a comparable response by WT and NKG2D-deficient NK cells was anticipated. To determine whether NKG2D has the potential to augment a Ly49H+ NK cells response or to directly induce Ly49H-independent, NKG2D-mediated NK cell expansion, we infected mice with mutant strains of MCMV that were engineered to permit expression of NKG2D ligands on infected cells. A MCMV mutant strain (Δm152 MCMV) lacking m152, which downregulates the cell surface expression of RAE-1 proteins (33, 34), and a MCMV strain engineered to express the mouse NKG2D ligand, RAE-1γ (RAE-1γ-MCMV), were used. These mutant MCMV-infected cells expressed higher amounts of RAE-1 proteins on their surface as compared with those infected with WT MCMV (Figure 1B). A prior report has demonstrated that viral burdens in mice infected with the Δm152 MCMV and RAE-1γ-MCMV strains are lower than with WT MCMV in an NKG2D-dependent manner (30). We reconstituted lethally irradiated recipient mice with CD45.1+ WT and CD45.2+ Klrk1−/− bone marrow cells and allowed NK cells to reconstitute in the recipient mice for more than 5 weeks so that both WT and NKG2D-deficient NK cells would develop in the same environment. Ly49H+ NK cells were isolated from the mixed BM chimeric mice, adoptively transferred into DAP10- and DAP12-double deficient mice, and then infected with WT MCMV, Δm152 MCMV, and RAE-1γ-MCMV. When infected with RAE-1γ-MCMV, WT Ly49H+ NK cells proliferated more robustly than Klrk1−/− Ly49H+ NK cells, with a peak NK cell expansion in the blood on day 7 p.i. (Figure 1C), although RAE-1γ-MCMV is attenuated and results in reduced viral load when infected in vivo (30). These results demonstrate that NKG2D is dispensable for MCMV-specific expansion of Ly49H+ NK cells when infected with the WT MCMV strain, whereas enhanced NKG2D signaling amplifies proliferation of Ly49H+ NK cells after infection with MCMV strains capable of inducing expression of NKG2D ligands in the infected cells.
Figure 1. NKG2D enhances expansion of Ly49H+ NK cells after MCMV infection.
(A) One hundred thousand CD45.1+ WT Ly49H+ NK cells and CD45.2+ NKG2D-deficient Ly49H+ NK cells were co-transferred into DAP10- and DAP12-double deficient recipient mice and infected with 1 x 105 PFU tissue culture-propagated WT MCMV. The absolute numbers of donor Ly49H+ NK cells in the blood are shown. Data were pooled from 2 experiments (n = 7 mice). (B) Expression of RAE-1 proteins on C57BL/6 3T3 cells at 60 hours after infection with tissue culture-propagated MCMV strains at 1 PFU/cell. Thin and bold lines represent staining with an isotype-matched control and anti-RAE-1 pan-specific mAb. Data are representative of 2 experiments. (C) NK cells were purified from CD45.1+ WT and CD45.2+ Klrk1−/− mixed BM chimeric mice. One hundred thousand WT and Klrk1−/− Ly49H+ NK cells were transferred into DAP10- and DAP12-double deficient mice and infected with 1 x 105 PFU tissue culture-propagated WT MCMV, Δm152 MCMV, and RAE-1γ-MCMV. The kinetics of the absolute number of donor Ly49H+ NK cells in the blood are represented as the ratio relative to the number of donor Ly49H+ NK cells in the blood on day 0 (before infection). Data are representative of 2 independent experiments (n = 4 mice per experiment and MCMV strain). *p <0.05.
NKG2D signaling alone is insufficient for expansion of NK cells after MCMV infection
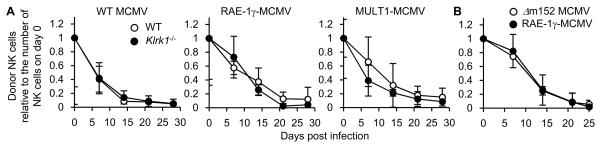
In mice activated NK cells can express a NKG2D isoform, NKG2D-S, that pairs and signals through both DAP12 and DAP10, similar to Ly49H, which also associates with both DAP10 and DAP12 (13) Therefore, we addressed the possibility that NKG2D signaling through NKG2D-S alone, in the absence of Ly49H signaling, might support activation and proliferation of NK cells after infection with MCMV strains engineered to allow expression of NKG2D ligands in infected cells. Purified CD45.1+Ly49H− WT NK cells and CD45.2+Ly49H− Klrk1−/− NK cells were adoptively co-transferred into DAP10- and DAP12-double deficient recipient mice, and then infected with WT MCMV, RAE-1γ-MCMV, and a MCMV strain expressing the high-affinity mouse NKG2D ligand MULT1 (MULT1-MCMV). Surprisingly, donor WT Ly49H− NK cells did not robustly proliferate in the blood after infection with either WT or mutant MCMV strains allowing expression of RAE-1γ or MULT1 on infected cells (Figure 2A). To confirm these results, CD45.1+ Ly49H-deficient (Klra8−/−) NK cells were transferred into DAP10- and DAP12-double deficient recipient mice and infected with Δm152 MCMV and RAE-1γ-MCMV. These donor Ly49H-deficient NK cells failed to expand after mutant MCMV infection (Figure 2B). These results indicate that NKG2D signaling alone is inadequate for clonal expansion of NK cells, in contrast to Ly49H.
Figure 2. NKG2D signaling is insufficient for expansion of NK cells after MCMV infection.
(A) Purified 5 x 104 CD45.1+Ly49H− WT NK cells and CD45.2+Ly49H− Klrk1−/− NK cells were co-transferred into DAP10- and DAP12-double deficient mice and infected with 1 x 105 PFU tissue culture-propagated WT MCMV, RAE-1γ-MCMV, and MULT1-MCMV. Donor NK cells were detected by expression of CD45 congenic markers. The kinetics of the absolute number of donor Ly49H− NK cells in the blood are represented as the ratio relative to the number of donor Ly49H− NK cells in the blood on day 0 (before infection). Data are representative of 2 independent experiments (n = 3 mice per experiment and MCMV strain). (B) Two hundred thousand CD45.1+ Ly49H-deficient NK cells were transferred into DAP10- and DAP12-double deficient mice and infected with 1 x 104 PFU tissue culture-propagated Δm152 MCMV and RAE-1γ-MCMV. The kinetics of the absolute number of donor NK cells in the blood are represented as the ratio relative to the number of donor NK cells in the blood on day 0 (before infection). Data are representative of 2 independent experiments (n = 3 mice per experiment and MCMV strain).
NK cells downregulate NKG2D by binding with its ligand after MCMV infection
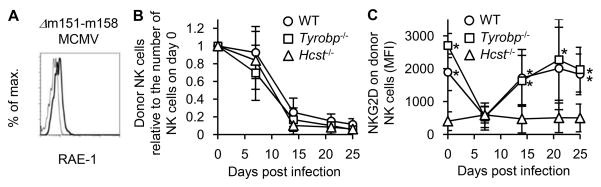
To investigate the roles of DAP10 and DAP12 in NKG2D signaling in the absence of Ly49H during MCMV infection, we used a mutant MCMV strain Δm151-m158 MCMV, which lacks the m151-m158 MCMV gene loci. This strain lacks the m157 ligand of Ly49H, thereby preventing the immunodominant NK cell response to m157, and deletion of m152 allows the infected cells to express RAE-1 ligands (Figure 3A). WT NK cells, DAP12-deficient (Tyrobp−/−) NK cells, and DAP10-deficient (Hcst−/−) NK cells were co-transferred into DAP10- and DAP12-double deficient mice and infected with Δm151-m158 MCMV. In the infected mice, the activated WT NK cells would be capable of expressing both the NKG2D-DAP12 and NKG2D-DAP10 receptor complexes, the Tyrobp−/− NK cells would exclusively express NKG2D-DAP10 receptor complexes, and the Hcst−/− NK cells would express only NKG2D-DAP12 receptor complexes. After infection with Δm151-m158 MCMV none of the NK cells expanded and all declined in frequency over the course of infection (Figure 3B), unlike the robust expansion of Ly49H+ NK cells that is observed after infection with a MCMV strain expressing m157 (4). Although naïve WT and Tyrobp−/− NK cells expressed comparable amounts of NKG2D on their cell surface, consistent with the lack of NKG2D-S in resting NK cells, naïve Hcst−/− NK cells lacked expression of NKG2D. After Δm151-m158 MCMV infection, the cell surface density of NKG2D on WT and Tyrobp−/− NK cells significantly decreased, indicating that the NK cells had likely interacted with NKG2D ligand-bearing cells, which would cause internalization of NKG2D (35) (Figure 3C). Little NKG2D was observed on the cell surface of Hcst−/− NK cells at any time point during the infection (Figure 3C).
Figure 3. NK cells downregulate NKG2D after MCMV infection.
(A) Expression of RAE-1 proteins on C57BL/6 3T3 cells at 60 hours after infection with tissue culture-propagated Δm151-m158 MCMV at 1 PFU/cell. Thin and bold lines represent staining with an isotype-matched control and anti-RAE-1 pan-specific mAb. Data are representative of 2 experiments. (B–C) Two hundred thousand CD45.1+CD45.2+ WT NK cells, CD45.1+ Tyrobp−/− NK cells, and CD45.2+ Hcst−/− NK cells were co-transferred into DAP10- and DAP12-double deficient mice and infected with 1 x 105 PFU tissue culture-propagated Δm151-m158 MCMV. Donor WT NK cells and Tyrobp−/− NK cells were detected by expression of CD45 congenic markers and donor Hcst−/− NK cells were detected by expression of Ly49H and CD45 congenic markers. (B) The kinetics of the absolute number of donor NK cells in the blood are represented as the ratio relative to the number of donor NK cells in the blood on day 0 (before infection). (C) Expression of NKG2D on donor NK cells in the blood is represented as mean fluorescent intensity (MFI). Data were pooled from 2 experiments (n = 8 mice). *p <0.05.
NK cells only transiently express NKG2D-DAP12 receptor complexes during MCMV infection
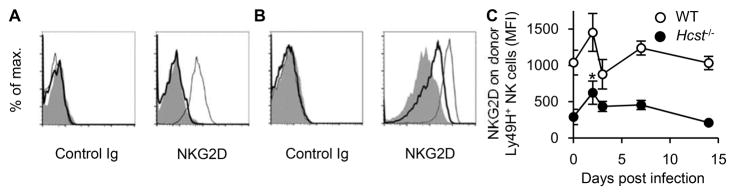
Naïve Hcst−/− NK cells do not express appreciable NKG2D on their cells surface (Figure 4A), whereas naïve WT NK cells express NKG2D. Consistent with prior studies (19, 20), we observed that in vitro culture of Hcst−/− NK cells in IL-2 induces expression of NKG2D-DAP12 receptor complexes on the cell surface (Figure 4B). Although we did not detect expression of NKG2D on the cell surface of Hcst−/− NK cells after infection with Δm151-m158 MCMV, it is possible that NKG2D-S was induced during the infection, but the NKG2D-DAP12 receptor complex was down modulated by interaction with RAE-1 on the infected cell, as in the absence of m152 RAE-1 is expressed on the surface of infected cells. Therefore, to determine whether NKG2D-DAP12 complexes are expressed on NK cells after MCMV infection, WT and Hcst−/− mice were infected with WT MCMV, as WT MCMV efficiently blocks expression of NKG2D ligands on infected cells, therefore, avoiding ligand-induced down modulation of NKG2D. Hcst−/− Ly49H+ NK cells indeed expressed low amounts of DAP12-associated NKG2D on day 2 p.i.; however, this was transient and no NKG2D was detected on Ly49H+ NK cells by day 14 (Figure 4C). These findings imply that expression of NKG2D-DAP12 complexes are only transiently expressed during infection and once induced, expression apparently is not stable.
Figure 4. NK cells only transiently express DAP12-associated NKG2D after MCMV infection.
(A–B) WT, Hcst−/−, and DAP10- and DAP12-double deficient NK cells were stained with control Ig or anti-NKG2D mAb. (A) Expression of NKG2D on naïve WT NK cells (thin line), Hcst−/− NK cells (bold line), and DAP10- and DAP12-double deficient NK cells (shaded histogram) is shown. (B) WT, Hcst−/−, and DAP10- and DAP12-double deficient NK cells were cultured in the presence of 1000 U/ml human IL-2 for 3 days. Expression of NKG2D on IL-2-cultured WT NK cells (thin line), Hcst−/− NK cells (bold line), and DAP10- and DAP12-double deficient NK cells (shaded histogram) is shown. (C) WT and Hcst−/− mice were infected with 1 x 104 PFU in vivo salivary gland-passaged WT MCMV. Expression of NKG2D on Ly49H+ NK cells in the blood is represented as MFI. Data were pooled from 2 experiments (n = 2–10 mice). *p <0.05 vs. day 0.
A prior study demonstrated that the deficiency of NKG2D causes faster homeostatic cell division of NK cells and augments their sensitivity to apoptosis in the naïve state (36). Although Klrk1−/− NK cells were reported to demonstrate a faster maturation and enhanced IFN-γ production when infected with a MCMV strain lacking m157 (36), our results demonstrate that expansion of MCMV-specific Ly49H+ NK cells lacking NKG2D is intact when infected with WT MCMV. The activating Ly49H and Ly49D receptors associate with DAP12 and DAP10 and both receptors are capable of driving NK cell expansion when stimulated by their cognate ligands, MCMV m157 (4, 6) and H-2Dd alloantigens (5), respectively. As NKG2D can also pair with DAP10 and DAP12, this prompted us to determine whether activation through NKG2D could also drive clonal expansion of NK cells. As NKG2D ligands are express by many tumors and by cells infected with numerous pathogens (37), if NKG2D receptor signaling were capable of driving NK cell expansion this would be beneficial in host defense and provide a therapeutic opportunity for cancer. However, in contrast to Ly49H, NKG2D alone is unable to promote robust expansion of NK cells during MCMV infection, even when the MCMV genes that prevent RAE-1 expression in infected cells were removed from the viral genome or in MCMV stains engineered to overexpress mouse NKG2D ligands in infected cells. We also failed to induce NKG2D-dependent NK cell expansion when mice were challenged with NKG2D ligand-bearing tumors in the context of a viral infection to provide pro-inflammatory cytokines to augment the response (unpublished observations). In the case of Ly49H, Ly49H-DAP12 receptor complexes are essential to drive extensive proliferation during MCMV infection (4, 38). Although Ly49H-DAP10 receptors can be detected in low amounts on the surface of Tyrobp−/− NK cells, NK cells lacking DAP12 had a significantly impaired proliferative response after MCMV infection compared with WT NK cells (13). The inability of NKG2D-DAP12 receptors to induce a robust expansion is likely due to the transient expression of NKG2D-DAP12 complexes during MCMV infection. These findings support the concept that the strength of DAP10 signaling modulates the magnitude of expansion of NK cells, whereas DAP12 is the essential driver for expansion of antigen-specific NK cells. The action of DAP10 in NK cells is reminiscent of costimulatory signals for T cell receptors in T cells. In accordance with this, prior studies have reported that NKG2D acts as a co-stimulatory receptor on CD8+ T cells (39–42). Reciprocally, DAP12 serves as the dominant driver of activation by its recruitment of Syk and ZAP-70 tyrosine kinases, which are also responsible for downstream signal transduction of the CD3 subunits of the T cell receptor (43–45). Conceptually, DAP12 signaling through activating Ly49 receptors on NK cells is functionally analogous to T cell receptor signaling in T cells with DAP10 functioning in a costimulatory role. Intriguingly, mice immunized with a MCMV strain engineered to express a mouse NKG2D ligand exhibit an amplified CD8+ T cell response and improved survival against the secondary challenge with virulent MCMV as compared with mice initially immunized with WT MCMV (30). Similarly, our current studies revealed an enhanced Ly49H+ NK cell response in mice infected with the MCMV strain expressing mouse NKG2D ligands, suggesting a new strategy to perhaps augment NK cell responses in the context of vaccination.
Supplementary Material
Acknowledgments
The work was supported by NIH grant AI068129 and European Research Council Advanced Grant (no. 322693). L.L.L. is an American Cancer Society Professor. The Uehara Memorial Foundation, the Natio Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, the Japan Society for the Promotion of Science, the Friends of Leukemia Research Fund, and the Nakajima Foundation support T.N.
We thank the Lanier laboratory members for helpful comments and discussions. We are grateful to Dr. Maelig Morvan (University of California, San Francisco) for generation of Klrk1−/− mice. We thank Prometheus Laboratories, Inc. for providing the recombinant human IL-2.
Abbreviations in this article
- BM
bone marrow
- MCMV
mouse cytomegalovirus
- p.i
post-infection
- WT
wild-type
Footnotes
Disclosures
The authors have no conflicting financial interests.
References
- 1.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 2.Lanier LL. Natural killer cell receptor signaling. Curr Opin Immunol. 2003;15:308–314. doi: 10.1016/s0952-7915(03)00039-6. [DOI] [PubMed] [Google Scholar]
- 3.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabekura T, Lanier LL. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J Exp Med. 2014;211:2455–2465. doi: 10.1084/jem.20140798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nabekura T, Lanier LL. Tracking the fate of antigen-specific versus cytokine-activated natural killer cells after cytomegalovirus infection. J Exp Med. 2016;213:2745–2758. doi: 10.1084/jem.20160726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 8.Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier LL. Costimulatory Molecule DNAM-1 Is Essential for Optimal Differentiation of Memory Natural Killer Cells during Mouse Cytomegalovirus Infection. Immunity. 2014 doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madera S, Sun JC. Cutting Edge: Stage-Specific Requirement of IL-18 for Antiviral NK Cell Expansion. J Immunol. 2015 doi: 10.4049/jimmunol.1402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabekura T, Girard JP, Lanier LL. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. J Immunol. 2015;194:5948–5952. doi: 10.4049/jimmunol.1500424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orr MT, Sun JC, Hesslein DG, Arase H, Phillips JH, Takai T, Lanier LL. Ly49H signaling through DAP10 is essential for optimal natural killer cell responses to mouse cytomegalovirus infection. J Exp Med. 2009;206:807–817. doi: 10.1084/jem.20090168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 16.Champsaur M, Lanier LL. Effect of NKG2D ligand expression on host immune responses. Immunol Rev. 2010;235:267–285. doi: 10.1111/j.0105-2896.2010.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 18.Houchins JP, Yabe T, McSherry C, Bach FH. DNA sequence analysis of NKG2, a family of related cDNA clones encoding type II integral membrane proteins on human natural killer cells. J Exp Med. 1991;173:1017–1020. doi: 10.1084/jem.173.4.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diefenbach A, Tomasello E, Lucas M, Jamieson AM, Hsia JK, Vivier E, Raulet DH. Selective associations with signaling proteins determine stimulatory versus costimulatory activity of NKG2D. Nat Immunol. 2002;3:1142–1149. doi: 10.1038/ni858. [DOI] [PubMed] [Google Scholar]
- 20.Gilfillan S, Ho EL, Cella M, Yokoyama WM, Colonna M. NKG2D recruits two distinct adapters to trigger NK cell activation and costimulation. Nat Immunol. 2002;3:1150–1155. doi: 10.1038/ni857. [DOI] [PubMed] [Google Scholar]
- 21.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 22.Billadeau DD, Upshaw JL, Schoon RA, Dick CJ, Leibson PJ. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4:557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 23.Zompi S, Hamerman JA, Ogasawara K, Schweighoffer E, Tybulewicz VL, Di Santo JP, Lanier LL, Colucci F. NKG2D triggers cytotoxicity in mouse NK cells lacking DAP12 or Syk family kinases. Nat Immunol. 2003;4:565–572. doi: 10.1038/ni930. [DOI] [PubMed] [Google Scholar]
- 24.Fodil-Cornu N, Lee SH, Belanger S, Makrigiannis AP, Biron CA, Buller RM, Vidal SM. Ly49h-deficient C57BL/6 mice: a new mouse cytomegalovirus-susceptible model remains resistant to unrelated pathogens controlled by the NK gene complex. J Immunol. 2008;181:6394–6405. doi: 10.4049/jimmunol.181.9.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, Colonna M, Takai T. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc Natl Acad Sci U S A. 2009;106:4816–4821. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hyka-Nouspikel N, Phillips JH. Physiological roles of murine DAP10 adapter protein in tumor immunity and autoimmunity. Immunol Rev. 2006;214:106–117. doi: 10.1111/j.1600-065X.2006.00456.x. [DOI] [PubMed] [Google Scholar]
- 27.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 28.Wagner M, Jonjic S, Koszinowski UH, Messerle M. Systematic excision of vector sequences from the BAC-cloned herpesvirus genome during virus reconstitution. J Virol. 1999;73:7056–7060. doi: 10.1128/jvi.73.8.7056-7060.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krmpotić A, Busch DH, Bubić I, Gebhardt F, Hengel H, Hasan M, Scalzo AA, Koszinowski UH, Jonjić S. MCMV glycoprotein gp40 confers virus resistance to CD8+ T cells and NK cells in vivo. Nat Immunol. 2002;3:529–535. doi: 10.1038/ni799. [DOI] [PubMed] [Google Scholar]
- 30.Slavuljica I, Busche A, Babić M, Mitrović M, Gašparović I, Cekinović D, Markova Car E, Pernjak Pugel E, Ciković A, Lisnić VJ, Britt WJ, Koszinowski U, Messerle M, Krmpotić A, Jonjić S. Recombinant mouse cytomegalovirus expressing a ligand for the NKG2D receptor is attenuated and has improved vaccine properties. J Clin Invest. 2010;120:4532–4545. doi: 10.1172/JCI43961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubić I, Wagner M, Krmpotić A, Saulig T, Kim S, Yokoyama WM, Jonjić S, Koszinowski UH. Gain of virulence caused by loss of a gene in murine cytomegalovirus. J Virol. 2004;78:7536–7544. doi: 10.1128/JVI.78.14.7536-7544.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brune W, Hengel H, Koszinowski UH. A mouse model for cytomegalovirus infection. Curr Protoc Immunol Chapter. 2001;19(Unit 19.17) doi: 10.1002/0471142735.im1907s43. [DOI] [PubMed] [Google Scholar]
- 33.Lodoen M, Ogasawara K, Hamerman JA, Arase H, Houchins JP, Mocarski ES, Lanier LL. NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules. J Exp Med. 2003;197:1245–1253. doi: 10.1084/jem.20021973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arapovic J, Lenac T, Antulov R, Polic B, Ruzsics Z, Carayannopoulos LN, Koszinowski UH, Krmpotic A, Jonjic S. Differential susceptibility of RAE-1 isoforms to mouse cytomegalovirus. J Virol. 2009;83:8198–8207. doi: 10.1128/JVI.02549-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara K, Hamerman JA, Hsin H, Chikuma S, Bour-Jordan H, Chen T, Pertel T, Carnaud C, Bluestone JA, Lanier LL. Impairment of NK cell function by NKG2D modulation in NOD mice. Immunity. 2003;18:41–51. doi: 10.1016/s1074-7613(02)00505-8. [DOI] [PubMed] [Google Scholar]
- 36.Zafirova B, Mandarić S, Antulov R, Krmpotić A, Jonsson H, Yokoyama WM, Jonjić S, Polić B. Altered NK cell development and enhanced NK cell-mediated resistance to mouse cytomegalovirus in NKG2D-deficient mice. Immunity. 2009;31:270–282. doi: 10.1016/j.immuni.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3:575–582. doi: 10.1158/2326-6066.CIR-15-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sjölin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Kärre K, Vivier E, Cerboni C. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J Exp Med. 2002;195:825–834. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, Spies T. Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol. 2001;2:255–260. doi: 10.1038/85321. [DOI] [PubMed] [Google Scholar]
- 40.Maasho K, Opoku-Anane J, Marusina AI, Coligan JE, Borrego F. NKG2D is a costimulatory receptor for human naive CD8+ T cells. J Immunol. 2005;174:4480–4484. doi: 10.4049/jimmunol.174.8.4480. [DOI] [PubMed] [Google Scholar]
- 41.Kavazović I, Lenartić M, Jelenčić V, Jurković S, Lemmermann NAW, Jonjić S, Polić B, Wensveen FM. NKG2D stimulation of CD8(+) T cells during priming promotes their capacity to produce cytokines in response to viral infection in mice. Eur J Immunol. 2017 doi: 10.1002/eji.201646805. [DOI] [PubMed] [Google Scholar]
- 42.Tomić A, Varanasi PR, Golemac M, Malić S, Riese P, Borst EM, Mischak-Weissinger E, Guzmán CA, Krmpotić A, Jonjić S, Messerle M. Activation of Innate and Adaptive Immunity by a Recombinant Human Cytomegalovirus Strain Expressing an NKG2D Ligand. PLoS Pathog. 2016;12:e1006015. doi: 10.1371/journal.ppat.1006015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier LL, Bakker AB. The ITAM-bearing transmembrane adaptor DAP12 in lymphoid and myeloid cell function. Immunol Today. 2000;21:611–614. doi: 10.1016/s0167-5699(00)01745-x. [DOI] [PubMed] [Google Scholar]
- 44.Lanier LL, Corliss BC, Wu J, Leong C, Phillips JH. Immunoreceptor DAP12 bearing a tyrosine-based activation motif is involved in activating NK cells. Nature. 1998;391:703–707. doi: 10.1038/35642. [DOI] [PubMed] [Google Scholar]
- 45.Straus DB, Weiss A. The CD3 chains of the T cell antigen receptor associate with the ZAP-70 tyrosine kinase and are tyrosine phosphorylated after receptor stimulation. J Exp Med. 1993;178:1523–1530. doi: 10.1084/jem.178.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.