Abstract
This study was designed to investigate the possible effects of 24-Epibrassinolide (BR), arbuscular mycorrhizal (AM) fungus, Glomus mosseae, singularly and collectively under salt stress in wheat (Triticum aestivum L.) plants. After foliar spraying of mycorrhizal and non-mycorrhizal plants by 5 µM epibrassinolide (24-Epi), they were treated with 0 and 150 mM NaCl for 2 weeks and then harvested. The results showed interactions of G. mosseae and 24-Epi could alleviate the adverse effects of salinity by improving relative water content (RWC) of leaves (62%), relative growth rate (40.74%), shoot fresh weights (39.83%) and shoot phosphorous content (63.93%), stimulating leaf enzymatic antioxidant activities including catalase (2.24 fold) and ascorbate peroxidase (2.18 fold) as well as malondialdehyde (36.17%) and H2O2 concentrations (49.74%) as compared to those of NaCl treatments. Moreover, mycorrhizal dependency of root dry weight (2%) and phosphorus concentration (0.4%) increased with AM infection and 24-Epi application under saline condition. Leaf RWC, also, negatively correlated with membrane electrolyte leakage. Furthermore, the greatest mitigating effects were observed in mycorrhizal plants subjected to NaCl and 24-Epi. This study indicated that 24-Epi application and AM fungi may synergistically mitigate harmful impacts of salinity in wheat plants.
Keywords: Arbuscular mycorrhizal symbiosis, Brassinosteroids, NaCl, Wheat
Introduction
An important aspect of agriculture is the cultivation of plants for food, fiber, biofuel, medicine and other products used to sustain and enhance human life. Agriculture is the key development in the rise of sedentary human civilization, whereby farming of domesticated species created food surpluses that nurtured the development of civilization (Mishra et al. 2015; Nemli et al. 2015; Ipek et al. 2016; Tsou et al. 2016). Salt stress depresses wheat growth and yield in addition to other main physiological and metabolic processes (Ashraf and Harris 2004; Evelin et al. 2009). Salinity induces ion toxicity, osmotic and oxidative stress as well as a reduction in growth, protein synthesis and photosynthetic capacity (Campanelli et al. 2013). Furthermore, high level of NaCl leads to an increase in lipid peroxidation which disrupts cell membrane integrity (Beltrano et al. 1999). Although, wheat is a moderately sensitive plant to salinity, there are differences in salt tolerance according to plant growth stage or cultivar genetics. Plants employ a number of strategies to cope with salt stress including biochemical and molecular alterations and may respond to salinity by enhancement of antioxidant defense systems. Many studies have indicated an increase in activities of peroxidase (POD), catalase (CAT) or superoxide dismutase (SOD) to scavenge detrimental reactive oxygen species (ROS) produced in saline condition (Jahnke and White 2003). In addition, synthesis of some protector components such as osmoprotectants are common responses of plants to salt stress (Ashraf and Harris 2004). Enhancing the salt tolerance of wheat plants could improve their growth and productivity in saline conditions. It has been demonstrated that arbuscular mycorrhizal (AM) fungi symbiosis contributes to plant tolerance by increased access to water and nutrients under salt stress (Evelin et al. 2009). Besides, AM fungi application, as a biological method, may alleviate adverse influences of salinity by enhancing the activity of antioxidant enzymes and accumulation of compatible solutes in plants (Feng et al. 2002; Evelin et al. 2009). On the other hand, brassinosteroids (BR), isolated first from rape (Brassica napus L.) pollen, as a group of steroidal plant growth regulators play an essential role in a wide range of physiological and developmental processes and stress responses (Grove et al. 1979; Krishna 2003). BRs have been shown to improve plant tolerance to salt stress by modifying the activity of antioxidant enzymes, enhancing relative water content (RWC), protein accumulation and photosynthesis efficiency (Özdemir et al. 2004). The present work was carried out to investigate whether independent and possible synergistic effects of AM fungus (Glomus mosseae) and foliar 24-Epi applications may have any role in improving plant tolerance to salinity.
Materials and methods
Wheat (Triticum aestivum L. cv Pishtaz) seeds (procured from Seed and Plant Improvement Institute, Karaj, Iran) were surface sterilized with 5% (v/v) sodium hyopchlorite for 3 min and washed with distilled water thrice. Then, the washed seeds were sown in plastic pots containing 2 kg of sterilized soil (peat/soil mixed with perlite at 2:1 (v/v) ratio, Table 1) with or without mycorrhizal fungi. For mycorrhization, 2 g of G. mosseae containing root fragments spores of mycorrhizal fungus (obtained from Zist Fanavar Touran Company, Shahrood, Iran) was placed 2 cm below the seed before sowing. Control treatments remained without mycorrhizal fungus. After examining 15-day old plants for AM colonization, each inoculated and non-inoculated plants were sprayed with 0 and 5 µM 24-Epi solution (0.01% Tween 20 used as surfactant). The 24-Epi (Sigma Chemicals) was foliar sprayed three times (once every 2 days). Then, each mentioned group was sub-divided into other two groups, and irrigated with 0 or 150 mM NaCl solutions once every 3 days for 2 weeks. Plants were grown under a light density of approximately 100 µmol m−2 s−1, day/night temperatures of 26 ± 1/17 ± 1 °C under a 16 h photoperiod in a greenhouse. Eight treatment groups were defined as follows: Control: (0 mM NaCl + 0 µM 24-Epi + without AM); NaCl: (150 mM NaCl + 0 µM 24-Epi + without AM); BR: (0 mM NaCl + 5 µM 24-Epi + without AM); AM: (0 mM NaCl + 0 µM 24-Epi + with AM); NaCl × BR: (150 mM NaCl + 5 µM 24-Epi + without AM); NaCl × AM: (150 mM NaCl + 0 µM 24-Epi + with AM); BR × AM: (0 mM NaCl + 5 µM 24-Epi + with AM); NaCl × BR × AM: (150 mM NaCl + 5 µM 24-Epi + with AM) 36-day old plants were harvested to analyze certain physiological and biochemical parameters before stem elongation occurs.
Table 1.
The physico-chemical properties of soil used in this research
| pH | EC (dS m−1) | FC (%) | Sand (%) | Clay (%) | Silt (%) | Soil texture | Organic carbon (%) | N (%) | P (mg kg−1) | K (mg kg−1) | Zn | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 1.54 | 23 | 67 | 12 | 21 | S.L | 1.8 | 0.04 | 12.5 | 159 | 1.3 | 2.9 |
Growth parameters
After harvesting, fresh weights (FWs) of total plants (shoots and roots) were recorded. To determine the dry weights (DWs), the freshly plants were oven-dried at 70 °C for 72 h for each plant and weighed again. Relative growth rate (RGR) was calculated as:
where; W1 and W2 are plant dry weights at times t1 and t2.
Plant tolerance (Pt) was calculated according to the following formula:
where DWSP and DWNSP are dry weights of stressed and non-stressed plants, respectively (Campanelli et al. 2013).
Mycorrhizal dependency (DM)
Mycorrhizal dependency was estimated as a percentage increase in each parameter of mycorrhizal (AM) plants over that of non-mycorrhizal (non-AM) ones (Graham and Syvertsen 1985) (determination of root colonization not shown).
Estimation of phosphorus (P) concentration
Oven-dried shoots were grounded and used to analyze P level which was extracted by nitric-perchloric acid digestion (Jackson 1973).
Determination of antioxidant enzyme activities
Extraction
0.5 g of fresh leaf material was ground in 2 ml of 50 mM potassium phosphate buffer (pH 7) under ice cold condition. Then, the homogenate mixture was centrifuged at 12,000 × g for 20 min at 4 °C. The supernatant was used to determine ascorbate peroxidase (APX) and catalase (CAT) activities.
Ascorbate peroxidase (APX) activity assay
The activity of APX was estimated as described by Rao et al. (1996) using reaction solution consisting of (3 ml) of 50 mM potassium phosphate buffer (pH 7), 0.15 mM H2O2, 0.5 mM ascorbic acid, 0.1 mM (EDTA) and 100 µL enzyme extract. APX activity was assayed by following the decrease in absorbance at 290 nm as the amount of ascorbic acid oxidation and expressed as ΔOD mg−1 protein min−1.
Catalase (CAT) activity assay
CAT activity was assayed according to Aebi (1983). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 30% (w/v) H2O2 and 100 µL enzyme extract. One unit of enzyme activity was measured as a decrease in absorbance at 420 nm for 1 min. Enzyme activity was expressed as ΔOD mg−1 protein min−1.
Lipid peroxidation assay
Lipid peroxidation level was measured as the content of malondialdehyde (MDA) according to Hu et al. (2006). MDA content was estimated using thiobarbituric acid (TBA) reaction. 0.5 g fresh leaf tissue samples were homogenized in 5 ml of 5% trichloroacetic acid (TCA). The homogenate was centrifuged at 3600 × g for 10 min. 2 ml of 0.67% TBA was added to 2 ml of supernatant and mixture was heated at 95 °C for 20 min and then cooled immediately. The absorbance of supernatant was evaluated at 450 and 600 nm. Then, MDA content was calculated using the following formula:
Leaf H2O2 content assay
The content of H2O2 was determined according to Loreto and Velikova (2001). 0.2 g of leaf tissue was homogenised in cold with 5 ml of cold 0.1% (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 12,000 × g for 15 min and 500 µL of supernatant was then added to 500 µL of 10 mM potassium phosphate buffer (pH 7.0) and 1 ml of 1 M KI. The absorbance was read at 390 nm and expressed as µg g−1 FW.
Electrolyte leakage determination
Electrolyte leakage was used to calculate the relative membrane permeability (Beltrano and Ronco 2008). The uppermost fully expanded leaves per treatment were collected, immediately cut out into 1 cm diameter. Samples were washed and placed into closed test tubes containing 10 ml of deionized water. The leaf samples were incubated at room temperature for 4 h and subsequently the electrical conductivity (EC) of the solution (EC1) was measured using a conductivity meter. Then, the same leaf segments were boiled in a water bath for 15 min. After cooling at room temperature, the EC of solution was measured (EC2). Electroyte leakage was determined as EC1/EC2 and expressed as percentage.
Relative water content (RWC)
Four mature leaves were collected and their leaf fresh weights (FW) were measured immediately. Thereafter, the leaves were kept in distilled water in close petri dishes for 4 h at room temperature and weighed again (as turgid weight or TW). Then, samples were oven-dried at 80 °C for 24 h to determine the dry weight (DW). RWC was calculated as follows (Beltrano and Ronco 2008):
Statistical analysis
All data were statistically analyzed by both SAS and SPSS Softwares version no. 18 and the treatment means were compared by using duncan test at P ≤ 0.05 level of significant.
Results
Growth parameters, Pt and leaf RWC
Results showed a significantly greater reduction (P ≤ 0.05) in RGR (6.89%), shoot (37.6%) and root (45.45%) fresh weights of plants under NaCl condition as compared to controls (Table 2). Mycorrhizal inoculation of plants by G. mosseae, significantly increased RGR, fresh shoot and root weights by 22.22, 26.20 and 53.33%, respectively relative to those grown under saline condition. On the other hand, 24-Epi application at 5 µM, significantly improved RGR and shoot fresh weights by 1.11 and 12.83% under NaCl stress. Moreover, plants grown in saline soil and treated with both BR and AM fungi showed the highest RGR and shoot fresh weights improvement in comparison with NaCl-treated plants (40.74% in RGR and 39.83% in shoot fresh weights). Also, these increases in NaCl × AM × BR-treated plants were significant in comparison with those of NaCl × BR- (26.66 in RGR and 23.93 in shoot fresh weights) and NaCl × AM-treated ones (15.15% in RGR and 10.8 in shoot fresh weights). On the other hand, root fresh weights in NaCl × BR-, NaCl × AM- and dual -treated plants increased as compared to those of NaCl-treated ones by 41.66, 53.33 and 63.33% respectively. AM × BR-treated wheat plants showed higher RGR and shoot fresh weights than those of controls. Besides, enhanced Pt was observed in NaCl × AM- (70.27%), NaCl × AM × BR-treated (75.7%) plants, respectively (Table 2) whereas an increase in NaCl × BR-treated plants (65/3%) was not significant at P ≤ 0.05. Furthermore, salt stress significantly reduced leaf RWC b 39.14% in comparison with that under non-stressed condition (P ≤ 0.05, Table 2). G. mosseae and BR application improved leaf water status by 18.32 and 33.84%, respectively as compared to those of NaCl-treated plants (P ≤ 0.05). Leaf RWC in mycorrhizal plants subjected to both NaCl and BR treatments were 62., 36.91 and 21.03% higher than those of NaCl-, NaCl × AM- and NaCl × BR-treated plants, respectively which were all significant at P ≤ 0.05.
Table 2.
Influence of 150 mM NaCl, 5 µM epibrassinolide (BR) and G. mosseae (AM) on total fresh weights, RGR, RWC, membrane leakage and plant tolerance (Pt) in T. aestivum L. plants
| Shoot fresh weights (g) | Root fresh weights (g) | RGR (g kg−1 d−1) | RWC (%) | Membrane electrolyte leakage (%) | Pt (%) | |
|---|---|---|---|---|---|---|
| Control | 0.6 ± 0.01c | 0.11 ± 0.004b | 0.029 ± 0.000e | 77.5 ± 0.714c | 23.4 ± 1.186c | |
| NaCl | 0.374 ± 0.01 g | 0.06 ± 0.000d | 0.027 ± 0.000f | 47.16 ± 1.722f | 32.4 ± 0.334a | 64.02 |
| BR | 0.682 ± 0.004b | 0.114 ± 0.004b | 0.04 ± 0.000c | 89.7 ± 0.893a | 17.5 ± 0.237f | |
| AM | 0.697 ± 0.022b | 0.13 ± 0.000a | 0.05 ± 0.001b | 85.3 ± 1.578b | 20.4 ± 0.017e | |
| NaCl × BR | 0.422 ± 0.001f | 0.066 ± 0.001d | 0.03 ± 0.000de | 63.12 ± 1.353d | 22.35 ± 0.187 cd | 65.3 |
| NaCl × AM | 0.472 ± 0.002e | 0.092 ± 0.002c | 0.033 ± 0.000d | 55.8 ± 1.067e | 28.5 ± 0.081b | 71.02 |
| AM × BR | 0.743 ± 0.006a | 0.137 ± 0.001a | 0.59 ± 0.001a | 93.3 ± 0.988a | 15.05 ± 0.032 g | |
| NaCl × BR × AM | 0.523 ± 0.013d | 0.097 ± 0.001c | 0.038 ± 0.000c | 76.4 ± 1.560c | 21.65 ± 0.049de | 75.7 |
Values represent mean ± SE of four replicates. Different letters indicate significant differences at P ≤ 0.05
ns Not significant
DM for DW and P concentration
Plants showed 40 and 37.77% dependency on G. mosseae for shoot and root DW as well as 28.05 and 2.81% dependency for shoot and root P content under saline condition (Table 3). However, parameters mentioned above remained unchanged in the AM inoculated plants using both BR and NaCl.
Table 3.
The mycorrhizal dependency (MD) or shoot and root dry weight (DW) and phosphorus (P) content of mycorrhizal wheat plants treated with NaCl and BR
| Treatments | MD (%) | |||
|---|---|---|---|---|
| Plant shoot DW | Plant root DW | Shoot P content | Root P content | |
| 0 NaCl | 29.31b | 17.14c | 25.51b | 28b |
| 150 m NaCl | 40a | 37.77a | 28.05a | 28.81a |
| 150 mM NaCl × 5 µM BR | 39.2a | 36.17b | 28.26a | 28.94c |
Values represent mean ± SE of four replicates. Different letters indicate significant differences at P ≤ 0.05
ns Not significant
P concentration
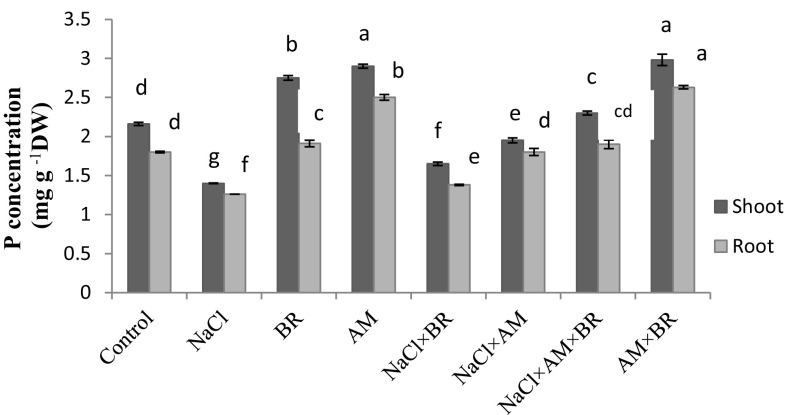
There was a significant decrease in shoot and root under salt stress condition (35 and 30%) at P ≤ 0.05 (Fig. 1). AM-inoculated, BR-sprayed and dual treated wheat shoots showed higher content of P in shoots in salinity 38.98, 17.6 and 63.93% respectively. The increment in P concentration of NaCl × AM × BR-treated shoots was also significant when compared to singular NaCl × AM-treated (39.39%) and NaCl × BR-treated (17.94%) ones (Fig. 1).
Fig. 1.
Influence of 150 mM NaCl, 5 µM epibrassinolide (BR) and G. mosseae (AM) on Phosphorus (P) concentrations in shoots and roots of T. aestivum L. plants. Different letters indicate significant differences at P ≤ 0.05
Moreover, there was an increase in P content in NaCl × AM (42.85%) and NaCl × AM × BR-treated (50.79%) plant roots only in comparison with that of NaCl-treated ones.
Leaf membrane leakage
NaCl significantly increased membrane injury (P ≤ 0.05, Table 2). Both AM fungal inoculation and BR application as well as their interactions improved membrane stability by 31.01, 12.03 and 33.17% in comparison with NaCl-treated plants, respectively (P ≤ 0.05, Table 2). In addition, NaCl × AM × BR-treated plants showed 24.03 and 3.13% decreases in membrane leakage compared to NaCl × AM- and NaCl × BR-treated ones. However, the latter reduction was not significant at P ≤ 0.05.
Lipid peroxidation
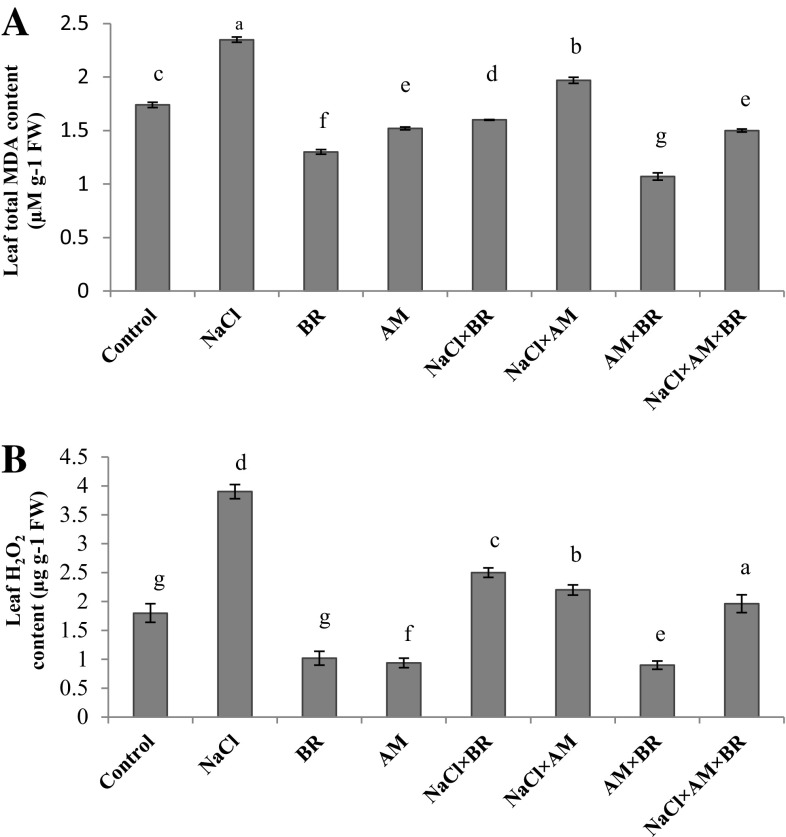
Lipid peroxidation level increased significantly by 35% under salt stress in comparison with controls (P ≤ 0.05; Fig. 2a). Foliar BR spraying, mycorrhizal inoculation and their interactions reduced MDA level by 31.91, 16.17 and 36.17% as compared to those of salt stress condition, respectively (significant at P ≤ 0.05). Besides, declined amounts of MDA in NaCl × AM × BR-treated plants were significant by 6.25 and 23.85% when compared with NaCl × BR- and NaCl × AM-treated plants, respectively.
Fig. 2.
Influence of 150 mM NaCl, 5 µM epibrassinolide (BR) and G. mosseae (AM) on Leaf MDA (a) and H2O2 (b) content, in T. aestivum L. plants. Different letters indicate significant differences at P ≤ 0.05
Leaf H2O2 concentration
In NaCl salt-stressed plants, H2O2 content increased considerably in comparison with controls (significant at P ≤ 0.05, Fig. 2b). AM fungi and BR applications reduced H2O2 content by 43.58 and 35.89% respectively in relation to salt-stressed plants. Likewise, NaCl × AM × BR-treated plants showed a significant decrease in H2O2 content by 49.74, 21.6 and 10.9% compared to NaCl- and NaCl × BR- and NaCl × AM-treated plants.
Changes in antioxidant enzymes activities
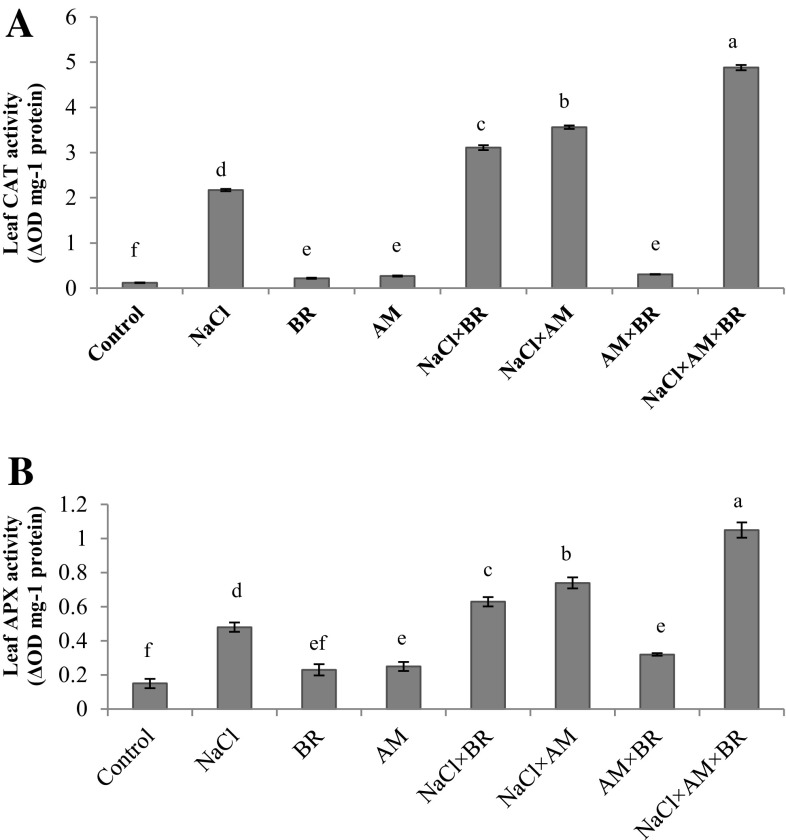
Leaf CAT and APX activities remarkably increased under salinity (P ≤ 0.05; Fig. 3a and b). AM fungi (64.05, 54.16%) and BR application (43.31, 31.25%) and their interactions (2.24 fold, 2.18 fold) significantly enhanced CAT and APX activities, respectively than those recorded for plants grown under saline condition (P ≤ 0.05).
Fig. 3.
Influence of 150 mM NaCl, 5 µM epibrassinolide (BR) and G. mosseae (AM) on Leaf CAT (a) and APX (b) activity, in T. aestivum L. plants. Different letters indicate significant differences at P ≤ 0.05
Discussion
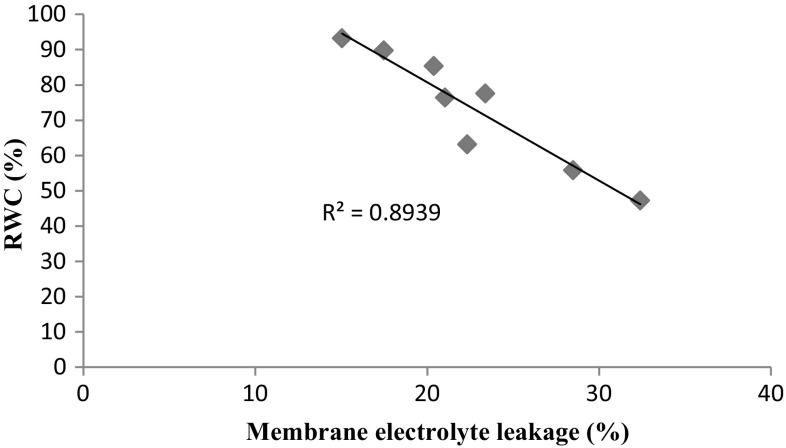
Salinity (150 mM NaCl) inhibited wheat growth including RGR, shoot and root fresh weights. This inhibition may be due to unbalanced nutrient and water uptake caused by salinity which inhibits cell elongation and photosynthesis capacity in plant (Grattan and Grieve 1999; Hasegawa et al. 2000). Both AM fungus and BR treatments independently as well as their interactions remarkably alleviated the harmful impacts of NaCl on plant growth. G. mosseae inoculated wheat roots (data not shown) which may improve growth and tolerance by enhancing nutrient availability, water uptake and hydraulic conductivity which leads to metabolic changes including increased protein biosynthesis that decreases growth inhibition under salt stress (Mathur and Vyas 2000; Evelin et al. 2009). The results of the present work support the findings of previous studies reporting better growth of mycorrhizal plants in saline condition (Evelin et al. 2009; Campanelli et al. 2013). Moreover, BRs serve an important function in promoting growth such as increased cell division or elongation, activation of proton pumps and protein synthesis under salinity (Krishna 2003; Cao et al. 2005). However, BR application had no effects on improving root fresh weights under salinity condition. Interestingly, AM symbiosis in combination with foliar BR spray were the most efficient treatments in improving shoot fresh weight and plant tolerance under salt stress which have not been reported yet. These synergistic effects may, in part, result from their singular improving effects on promoting plant growth or stimulation of other metabolic pathways or hormones such as auxins which lead to better plant growth. MD was considerably increased with AM infection under saline condition. However, salinity reduced mycorrhizal colonization (data not shown), but AM fungi inoculation of wheat plants was strengthened in NaCl treatment by increasing MD. Rabie and Almadini (2005) observed increased MD and P in mycorrhizal Vicia faba plants inoculated by Glomus clarum under salinity. Similarly, Campanelli et al. (2013) concluded that this may show the ecological importance of AM fungi for plant survival in saline condition once the symbiosis is established. However, it seems that BR application at 5 µM was not effective enough to enhance root P absorption for increasing MD. Soil salinity reduced the uptake of P due to its precipitation with ions including calcium and magnesium in soil (Azcón-Aguilar et al. 1979). Mycorrizal plants showed increased P content which supports previous findings (Roychoudhury et al. 2010). The higher P levels in mycorrhizal wheat plants may be due to enhanced P uptake by hyphae of fungus which improve growth and salt tolerance (Evelin et al. 2009). Foliar BR application increased P concentration in shoot and root under saline condition. This increase seems to be greater in shoots than in roots which may explain greater shoot improvement in comparison to root growth. Vardhini et al. (2012) observed an increase in Raphanus sativus roots under 28-Homobrassinolide and 24-Epibrassiolide at 3 µM. Kuno (1987) reported increased translocation of P after BR foliar spraying in mulberry. BR may improve P content by enhancing P absorption due to positive effects of BR on phosphate transporters. The results of the present work revealed a remarkable decline in leaf water content under salinity condition, which may be due to reduced cell osmotic potential. AM fungi helped improving leaf RWC in salt-stressed wheat plants by increasing water availability. These results are in accordance with previous findings (Campanelli et al. 2013). Furthermore, leaf RWC was considerably improved by foliar BR application in NaCl-treated plants. This increase can be explained by decreased water loss or improved membrane stability. The results revealed an inverse relationship (R2 = 0.893) between leaf RWC and membrane electrolyte leakage (Fig. 4). NaCl-stressed wheat plants which were treated with both G. mosseae and BR showed the most distinct increase in leaf water content in comparison with salt-stressed plants. In addition to maintenance in cell water potential, improved membrane integrity and root water uptake, this may have been caused by modifying some plant hormones such as decreasing ABA which consequently leads to high leaf water status. Excessive salt in soil induced production of H2O2 as one of the most important free radicals which could enhance oxidative damage by reacting with cellular components (Jones and Dangl 2006). However, H2O2 may have a role as a mediator in signaling pathways (Noctor et al. 2014). AM fungi together with BR application reduced H2O2 content in NaCl-treated plants. Moreover, enhanced CAT and APX activities were along with this fact that BR and mycorrhizal applications singularly reduce free radicals such as H2O2, OH− (hydroxyl) or O2 − (superoxide) by enhancing antioxidant defense system comprising antioxidant enzymes and metabolites (Evelin et al. 2009). Moreover, elevated activities of CAT and APX may be explained by an increase in nutrient availability which modifies the gene expression and biosynthesis of these metalloenzymes (Alguacil et al. 2003). Membrane stability is an indicator of plant salt and drought tolerance which is measured by electrolyte leakage of leaves. Salinity stress disrupts cell membrane integrity and causes an increase in leakage resulting from oxidative injury. This damage is due to ROS produced under stress condition which could change membrane lipid composition as demonstrated in several studies (Beltrano et al. 1999). Lower membrane leakage observed in mycorrhizal plants may be attributed to increased water and mineral (such as phosphorus) uptake or antioxidant defense system (Evelin et al. 2009). This result is in line with those reported by Beltrano and Ronco (2008) in wheat plants under water deficiency and Feng et al. (2002) in NaCl-treated maize plants. Similarly, BR improved membrane integrity in plants grown in saline condition which agrees with earlier reports (Houimli et al. 2010; Bartwal et al. 2012). Highest membrane stability tended to occur in NaCl × AM × BR-treated plants which may be due to singular or collective strategies of each treatment in reducing cell membrane structure damage. These include increased membrane unsaturated acids, enhanced antioxidant system activity in scavenging ROS including H2O2 produced during salinity and reduced lipid peroxidation. Both AM fungi and BR treatments inhibited lipid peroxidation by inducing antioxidant system which reduce free radicals under stress condition. These findings are in accordance with similar studies (Evelin et al. 2009; Feng et al.2002; Özdemir et al. 2004). Besides, improved effects of G. mosseae and BR were observed in non-stressed plants compared with controls. Thus, an increase in growth and cell membrane stability was most distinct for AM × BR-treated plants. However, little is reported on any significant difference between BR-treated plant and controls (Özdemir et al. 2004) although BR seemed to be in part more efficient in maintaining cell membrane integrity than AM fungi. On the whole, there are few reports regarding interactive influences of AM fungi and BR applications on salt tolerance and any other biotic or abiotic stresses.
Fig. 4.
Relationship between leaf RWC and membrane electrolyte leakage of wheat plant leaves under NaCl, AM fungi and BR treatments singularly and collectively
Conclusion
In conclusion, these findings provide evidence that plant tolerance to salinity could be improved by AM fungi in combination with BR application. Promotion in growth, cell membrane integrity, RWC and activity of antioxidant enzymes were observed in mycorrhizal wheat plants subjected to BR under salinity in comparison with NaCl-treated plants. Similarly, when compared to controls, AM × BR-treated plants showed improved growth and physiological parameters. However, plant responses may vary not only among different wheat cultivars but also depending other factors involved including the mode and type of BR applications, hormone concentration, the AM fungi species inoculated and plant growth stage. However, BR seemed to be in part more effective in maintaining cell integrity than AM fungi. This may results from probable crosstalks with other hormones such as jasmonic acid which seems to be higher in AM-inoculated plants (Nair et al. 2015). Thus, AM fungi together with BR could induce multiple pathways involved in the regulation of biochemical and molecular responses, thereby leading to mitigate adverse effects of salinity in plants.
Footnotes
Cobra Tofighi: Principal investigator.
References
- Aebi HE. Catalase. In: Bergemeyer HU, editor. Methods of enzymatic analysis. Weinheim: Verlag Chemie; 1983. pp. 273–285. [Google Scholar]
- Alguacil MM, Hernandez JA, Caravaca F, Portillo B, Roldan A. Antioxidant enzyme activities in shoots from three mycorrhizal shrub species afforested in a degraded semi-arid soil. Physiol Plant. 2003;118:562–570. doi: 10.1034/j.1399-3054.2003.00149.x. [DOI] [Google Scholar]
- Ashraf M, Harris PJC. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004;166:3–6. doi: 10.1016/j.plantsci.2003.10.024. [DOI] [Google Scholar]
- Azcón-Aguilar C, Azcón R, Barea JM. Endomyorrhizal fungi and rhizobium as biological fertilizers for Medicago sativa in normal cultivation. Nature. 1979;279:325–327. doi: 10.1038/279325a0. [DOI] [Google Scholar]
- Bartwal A, Mall R, Lohani P, Guru SK, Arora S. Role of secondary metabolites and brassinosteroids in plant defense against environmental stress. J Plant Growth Regul. 2012;32:216–232. doi: 10.1007/s00344-012-9272-x. [DOI] [Google Scholar]
- Beltrano J, Ronco MG. Improved tolerance of wheat plants (Triticum aestivum L.) to drought stress and rewatering by the arbuscular mycorrhizal fungus Glomus clariodeum effects on growth and cell membrane stability. Braz J Plant Physiol. 2008;20:29–37. doi: 10.1590/S1677-04202008000100004. [DOI] [Google Scholar]
- Beltrano J, Ronco MG, Montaki ER. Drought stress syndrome in wheat is provoked by ethylene evolution imbalance and reversed by rewatering, aminoethoxy vinylglycine or sodium benzoate. J Plant Growth Regul. 1999;18:54–64. doi: 10.1007/PL00007049. [DOI] [PubMed] [Google Scholar]
- Campanelli A, Ruta C, Mastro GD, Morone-Fortunato I. The role of arbuscular mycorrhizal fungi in alleviating salt stress in Medicago sativa L. var icon. Symbiosis. 2013;59:65–76. doi: 10.1007/s13199-012-0191-1. [DOI] [Google Scholar]
- Cao S, Xu Q, Cao Y, Qian K, An K, Zhu Y, Binzeng H, Zhao H, Kuai B. Loss of function mutation in DET2 gene lead to an enhanced resistance to oxidative stress in Arabidopsis. Physiol Plant. 2005;123:57–66. doi: 10.1111/j.1399-3054.2004.00432.x. [DOI] [Google Scholar]
- Evelin H, Kapoor R, Giri B. Arbuscular mycorrhizal fungi in alleviation of salt stress: a review. Ann Bot. 2009;104:1263–1280. doi: 10.1093/aob/mcp251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G, Zhang FS, Li XI, Tian CY, Tang C, Rengel Z. Improved tolerance of maize plants to salt stress by arbuscular mycorrhizal is related to higher accumulation of soluble sugars in roots. Mycorrhiza. 2002;12:185–190. doi: 10.1007/s00572-002-0170-0. [DOI] [PubMed] [Google Scholar]
- Graham HJ, Syvertsen JP. Do mycorrhizae influence the drought tolerance of citrus? J Environ Hortic. 1985;5:37–39. [Google Scholar]
- Grattan SR, Grieve CM. Salinity-mineral nutrient relations in horticultural crops. Sci Hortic. 1999;78:127–157. doi: 10.1016/S0304-4238(98)00192-7. [DOI] [Google Scholar]
- Grove DM, Spencer GF, Rohwedder WK, Mandava N, Worly JF, Warthen JD, Stefens JL, Flippen-Anderson GL, Cook JC. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature (London) 1979;281:216–217. doi: 10.1038/281216a0. [DOI] [Google Scholar]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular resposes to high salinity. Ann Rev Plant Physiol Plant Mol Biol. 2000;51:463–499. doi: 10.1146/annurev.arplant.51.1.463. [DOI] [PubMed] [Google Scholar]
- Houimli SIM, Denden M, Mouhandes BD. Effects of 24-epibrassinlide on growth, chlorophyll, electrolyte leakage and proline by pepper plants under NaCl-stress. Eurasia J Biosci. 2010;4:96–104. doi: 10.5053/ejobios.2010.4.0.12. [DOI] [Google Scholar]
- Hu J, Xie XJ, Wang ZF, Song WJ. Sand priming improves alfalfa germination under high-salt concentration stress. Seed Sci Technol. 2006;34:199–204. doi: 10.15258/sst.2006.34.1.22. [DOI] [Google Scholar]
- Ipek A, Yilmaz K, Sıkıcı P, Tangu NA, Oz AT, Bayraktar M, Ipek M, Gulen H. SNP discovery by GBS in olive and the construction of a high-density genetic linkage map. Biochemical Genet. 2016;54:313–325. doi: 10.1007/s10528-016-9721-5. [DOI] [PubMed] [Google Scholar]
- Jackson ML. Soil chemical analysis. New Delhi: Prentice Hall of India Pvt. Ltd.; 1973. pp. 53–81. [Google Scholar]
- Jahnke LS, White A. Long-term hyposaline and hypersaline stresses produce distinct antioxidant responses in the marine algae Dunaliella tertiolecta. J Plant Physiol. 2003;160:1193–1202. doi: 10.1078/0176-1617-01068. [DOI] [PubMed] [Google Scholar]
- Jones JDG, Dangl JL. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- Krishna P. Brassinosteroid-mediated stress responses. J Plant Growth Regul. 2003;22:353–364. doi: 10.1007/s00344-003-0058-z. [DOI] [PubMed] [Google Scholar]
- Kuno K. Effects of plant steroid brassinolide on dry weight, growth and nutrient translocation in mulberry shoots. J Seric Sci Jpn. 1987;66:57–58. [Google Scholar]
- Loreto F, Velikova V. Isoprene produced by leaves protects the photosynthetic apparatus agents zone damage, quenches ozone products and reduces lipid peroxidation of cellular membranes. Plant Phyiol. 2001;127:1781–1787. doi: 10.1104/pp.010497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur N, Vyas A. Influence of arbuscular mycorrhiza on biomass production, nutrient uptake and mycorrhizal changes in Ziziphus mauritiana Lan. Under water stress. J Arid Environ. 2000;45:191–195. doi: 10.1006/jare.2000.0644. [DOI] [Google Scholar]
- Mishra PK, Ram RB, Kumar N. Genetic variability, heritability, and genetic advance in strawberry (Fragaria × ananassa Duch.) Turk J Agric For. 2015;39:451–458. doi: 10.3906/tar-1408-99. [DOI] [Google Scholar]
- Nair A, Kolet SP, Thulairam HV, Bhargava S. Systematic jasmonic acid modulation in mycorrhizal tomato plants and its role in induced resistance against Alternaria alternate. Plant Biol. 2015;17:625–631. doi: 10.1111/plb.12277. [DOI] [PubMed] [Google Scholar]
- Nemli S, Kianoosh T, Tanyolac MB. Genetic diversity and population structure of common bean (Phaseolus vulgaris L.) accessions through retrotransposon-based interprimer binding sites (iPBSs) markers. Turk J Agric For. 2015;39:940–948. doi: 10.3906/tar-1505-59. [DOI] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. The roles of reactive oxygen metabolism in drought: not so cut and dried. Plant Physiol. 2014;164:1636–1648. doi: 10.1104/pp.113.233478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özdemir F, Bor M, Demiral T, Turkan I. Effects of 24-epibrassinolide on seed germination, seedling growth, lipid peroxidation, proline content and antioxidative system of rice (Oryza sativa L.) under salinity stress. Plant Growth Regul. 2004;42:203–211. doi: 10.1023/B:GROW.0000026509.25995.13. [DOI] [Google Scholar]
- Rabie GH, Almadini AM. Role of bioinoculants in development to salt-tolerance of Vicia faba plants under salinity stress. Afr J Biotechnol. 2005;4:210–222. [Google Scholar]
- Rao MV, Paliyath G, Ormod DP. Ultraviolet-B radiation and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996;110:125–136. doi: 10.1104/pp.110.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhury A, Basu S, Sengupta DN. Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J Plant Physiol. 2010;18:12–17. doi: 10.1016/j.jplph.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Tsou C, Li L, Vijayan K. The intra-familial relationships of Pentaphylacaceae as revealed by DNA sequence analysis. Biochem Genet. 2016;54:270–282. doi: 10.1007/s10528-016-9717-1. [DOI] [PubMed] [Google Scholar]
- Vardhini BV, Sujatha E, Ramrao SS. Studies on the effects of brassinosteroids on the qualitative changes in the storage roots of radish. Bulg J Agric Sci. 2012;18:63–69. [Google Scholar]