Abstract
Foxtail millet [Setaria italica (L.) P. Beauv.] is an important small millet, grown as a short duration, drought tolerant crop across the world. This crop can be grown on wide ranges of soil conditions and has an immense potential for food and fodder in rainfed and arid regions of the India. In the present study, 31 primer pairs (27 SSR and 4 EST-SSR) were used to analyse the genetic diversity in 223 core collection accessions. Analysis resulted in detection of a total of 136 alleles with an average of 4.38 alleles per locus. Among these 136 alleles, 22 were rare, 70 were common and 44 were frequent. The PIC value ranged from 0.01 to 0.86 with an average of 0.31. The average number of observed alleles ranged from 2.0 (northern hills of India accessions) to 4.06 (exotic) with an average of 2.72. The mean Shannon’s Information Index ranged from 0.44 (northern hills of India) to 0.69 (exotic) with an average of 0.52. Pair-wise Fst values indicated little to moderate genetic differentiation among the group of accessions. UPGMA clustering grouped the accessions into two major groups while analysis for population substructure indicated presence of four subpopulations. However there was no statistically well supported grouping of the accessions based on eco-geographic specificities. The core collection designated here represented substantial genetic diversity at molecular level, hence may be a good source of diversity for use in foxtail improvement programs in the region.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0448-5) contains supplementary material, which is available to authorized users.
Keywords: Core collection, Polymorphic loci, Setaria italica, Shannon’s information index
Introduction
Foxtail millet [Setaria italica (L.) P. Beauv.] is a short duration, drought tolerant crop, which can withstand severe moisture stress. It can be grown on wide range of soil conditions, thus has an immense potential as food and fodder in the arid regions. In India it is grown mostly in drier regions of Rajasthan, Gujarat, Himachal Pradesh, Uttarakhand and Jammu & Kashmir and limited areas in the states of Andhra Pradesh, Tamil Nadu and Karnataka, extending from the Himalayan region to coastal areas of Southern India (Kumari et al. 2011). It belongs to family Poaceae and subfamily Panicoideae and has its origin in Eastern Asia particularly in the China region. Foxtail millet is considered as one of the oldest crops in the world probably domesticated in China approximately 8700 years ago. It ranks second in total world millet production, accounting for six million tons of grain from southern Europe and Asia (Li and Wu 1996; Yang et al. 2012). Recent excavations at Sisak, Croatia led to discovery of archeobotanical evidence of foxtail millet and its contribution to the diet of early Iron Age Population (Reed and Drnic 2016). It is a diploid (2n = 2x = 18), self-pollinating, C4 panicoid crop with high photosynthetic efficiency. It has a small genome with reports varying from ~400 Mb (Bennetzen et al. 2012), ~490 Mb (Doust et al. 2009) and 515 Mb (Pandey et al. 2013). Due to its smaller genome size and inbreeding nature, foxtail millet can be used as a model plant to study functional genomics and also for physiological and evolutionary studies in grasses (Doust et al. 2009).
Taxonomists have classified foxtail millet into two subspecies, S. italica subsp. italica and subsp. viridis. Cytological studies suggest that S. italica subsp. viridis is the wild ancestor of foxtail millet (Kihara and Kishimoto 1942; Li et al. 1945). It is a good source of protein (12.3 g/100 g), dietary fiber (14 g/100 g), minerals (3 g/100 g) and phytochemicals but has low carbohydrate content (60.9 g/100 g). It is rich in ß carotene i.e. 126–191 μg/100 g (Goudar et al. 2011). The foxtail millet bran constitutes 9.39% crude oil, 12.48% crude protein and 51.69% crude fiber (Liang et al. 2010). Coulibaly and Chen (2011) reported presence of vitamins B1, B2, B6, C and E to the extent of 0.393, 0.142, 0.724, 0.120 and 0.176 mg/100 g respectively. It has medicinal importance too as it helps in reducing blood glucose level. It has been reported that a local recipe named ‘jaula’ made out of foxtail millet works as medicine for people suffering from chicken pox (Pathak et al. 2000). Jali et al. (2012) reported that consumption of foxtail millet can result in good glycemic control, inhibit hyperinsulinemia and decrease plasma lipid concentrations, and is good for people suffering with type-2 diabetes (Anju and Sarita 2010; Thathola et al. 2010).
Simple sequence repeat (SSR) markers are used in the diversity analysis of germplasm and plant breeding studies. They offer several advantages compared to other molecular markers as they are reproducible, highly polymorphic, detect high level of variation, reveal abundant information, are readily portable within a species; and are thus commonly used in many crop species (Kumar et al. 2009; Shahroodian et al. 2011). SSRs have been used in genetic diversity study in foxtail millet by some workers (Liu et al. 2011; Kim et al. 2012; Lin et al. 2012; Vetriventhan et al. 2012; Gupta et al. 2014). Microsatellite markers were used for the development of core collection in Peanut (Kottapalli et al. 2007), Japanese Rice landrace (Ebana et al. 2008), Persimmon (Zhang et al. 2009), Garlic (Zhao et al. 2011) Olive (Diez et al. 2012) and wild rice (Liu et al. 2015). SSR markers were also used for validation of core collection using various genetic diversity parameters (Zong et al. 2009; Tiwari et al. 2013; Vetriventhan et al. 2014; Wu et al. 2014; Subudhi et al. 2016).
Pritchard et al. (2000) suggested that population structure is difficult to detect using visible traits however; it may be easily detectable with molecular characters. Recently, population structure has been studied by many researchers in foxtail millet (Gupta et al. 2014; Vetriventhan et al. 2014; Ali et al. 2016), broomcorn millet (Liu et al. 2016), finger millet (Ramakrishnan et al. 2016), sorghum (Kondombo et al. 2016), Solanum pimpinellifolium (Rao et al. 2012) and soybean (Kumar et al. 2014).
A total of 933 and 1403 accessions of foxtail millet (Indian origin) are maintained at International Crops Research Institute for the Semi-Arid Tropics (ICRISAT), Hyderabad and All India Coordinated Research Project (AICRP) on small millet (Bengaluru) respectively. Upadhyaya et al. (2008) developed core collection of foxtail millet germplasm conserved at ICRISAT using traditional hierarchical cluster analysis method (Ward 1963). In this study we used the approach in Power Core software to develop the core collection of foxtail millet with 223 accessions. The present study was conducted with the objectives of analysing the molecular diversity and its partitioning in the newly identified core collection and thus validating the representation of the genetic diversity in core collection identified using morpho-agronomic traits vis-a-vis total collection.
Materials and methods
Plant material and DNA extraction
A set of 223 foxtail core accessions identified earlier (Singhariya et al. 2017) including three checks varieties, SiA 326, SiA 3085 and PS-4 (Table S1) were grown in augmented design (Federer 1956) at ICAR-National Bureau of Plant Genetic Resources (NBPGR) Regional Station, Akola (Maharashtra) and University of Agricultural Sciences, Bengaluru (Karnataka) during July, 2012. This core collection comprises accessions from 20 states of India and 20 different countries.
Fresh leaf samples from the core accessions and released varieties were collected at 4–5 leaf stage and used for DNA isolation. DNA extraction was carried out using modified CTAB method (Saghai-Maroof et al. 1984). About 2–5 g leaf material was crushed in liquid nitrogen and DNA was extracted by lysing cells in 10 ml of preheated (60 °C) CTAB Buffer (100 mM Tris, pH-8.0, 1.4 M NaCl, 20 mM EDTA pH-8.0, 0.2% β-mercaptoethanol, 2.0% CTAB). The supernatant was extracted twice with 24:1 mixture of Chloroform-Isoamyl alcohol and thereafter DNA was precipitated with 0.7 X volume of isopropanol. The DNA pellet was washed twice with 70% ethanol and air dried. After drying, the DNA pellet was dissolved in limited volume of TE (Tris–EDTA, 10:1 mM pH 8.0). For purification, the DNA was treated with RNase A (10 mg/ml) and incubated at 37 °C for one hour followed by extraction once with Tris saturated phenol: chloroform: iso-amyl alcohol (25:24:1) and twice with chloroform: iso-amyl alcohol. DNA was thereafter precipitated by adding 1/10th volume of 3 M sodium acetate and 2.5X volume chilled ethanol. The precipitated DNA was washed twice with 70% ethanol, air dried and dissolved in limited volume of TE (10:1). The purified DNA was quantified using a low volume spectrophotometer (ND1000 Spectrophotometer, NanoDrop Technologies, Montchanin, DE). After quantification, a working DNA solution of concentration 20 ng/μl was prepared and stock was stored at −20 °C until further use.
PCR amplification
The microsatellite primers were designed from the survey of genome and EST sequences of NCBI (National Center for Biotechnology Information). A total of 203 primer pairs [104 Simple Sequence Repeats (SSR) and 99 Expressed Sequence Tag—Simple Sequence Repeats (EST-SSR)] were selected and used for this study. PCR amplification was optimized and conducted in a reaction volume of 25 μl, comprising 1X PCR buffer; 1U Taq DNA polymerase; 0.2 mM each of dATP, dCTP, dGTP and dTTP; 2 mM MgCl2 (all the reagents from GeNei™ Bangalore Genei, Bangalore, India), 0.1 µM of respective forward and reverse primer and 40 ng of genomic DNA. PCR reactions were carried out in a Bioer XP cycler (Bioer Technology Co. Ltd, Hangzhou, P.R. China) using PCR microplates (Axygen Inc., California, USA). The PCR amplification products were resolved by electrophoresis on 3.5% agarose gel, stained with ethidium bromide and photographed under UV light in a gel documentation system. A 100 bp DNA size ladder (GeNei™ Bangalore Genei, Bangalore, India) was used as standard for scoring.
Data analysis
The amplification products (bands) were scored by comparing their respective molecular weights with the DNA size standard across the lanes. The bands were scored as present (1) or absent (0) in all samples for each of the primer. Only clear and consistent bands were scored and no amplification or very faint band was scored as ‘9’. Each band was treated as one STMS (Sequence Tagged Microsatellite Site) allele. Scoring data was then used for further analyses.
Polymorphism information content (PIC) was calculated online from the website (http://www.liv.ac.uk/~kempsj/pic.html). Occurrence of the rare, common and most frequent allele was calculated manually. Alleles with frequencies of ≤1, 1–20 and >20% were considered rare, common and most frequent respectively (Upadhyaya et al. 2008). Clustering was done on the basis of Jaccard’s similarity matrix (Jaccard 1902) using NTSYSpc version 2.2 (Exeter Corporation, USA).
The 223 core collection accessions were grouped into eight populations on the basis of their source viz. Central India (24), Eastern India (9), North Eastern India (7), Northern Hills of India (8), Northern India (41), Southern India (66) Western India (9) and Exotic (59). Genetic variation among these eight populations was analyzed on the basis of the allelic profile using various genetic diversity parameters such as number of allele per locus (N), observed number of alleles (Na), effective number of alleles (Ne) (Kimura and Crow 1964), Shannon’s Information index (I) (Lewontin 1972), number of polymorphic loci (P), expected heterozygosity (HE), observed heterozygosity (HO), Nei’s (1973) gene diversity, F statistic (FST) and gene flow (Nm) using POPGEN 1.32 software (Yeh et al. 2000). Line diagram were used to illustrate the genetic diversity in the 8 populations (Liu et al. 2015).
Population sub-structure was assessed using 31 polymorphic SSR markers data following admixture model implemented in STRUCTURE (Pritchard et al. 2000) with a burn-in of 5000 and 50,000 iterations for 2–15 K populations, each having five independent runs. The value of k was calculated on the basis of the rate of change in LnP(D) between successive k, stability of grouping pattern across five runs. The optimum number of subpopulations was determined using Evanno’s ad-hoc statistic ΔK (Evanno et al. 2005).
Results
Primer selection and allelic diversity
A total of 31 primers pairs (27 SSR and 4 EST-SSR) were selected based on the polymorphism (Table S2). The number of alleles per locus ranged from 2 (Fox gssr-67, 72, 76, 101, FOX EST- 71, 96, 97, 98) to 10 (Fox gssr-7, 63) with an average number of allele per locus at 4.38 (Fig. 1s). Among 136 alleles detected, 22 were rare alleles (16.18%), 70 were common (51.47%) and 44 were most frequent (32.35%). A total of 11 unique alleles (8.09%) were detected which were present in single accession and not present in rest of the accessions. PIC value is the reflection of the allele diversity and the informativeness of each marker. The PIC value ranged from 0.01 (FOX EST- 97) to 0.86 (Fox gssr-62) with an average of 0.31 (Table 1). Out of 31 markers, 12 showed PIC value >0.30.
Fig. 1.
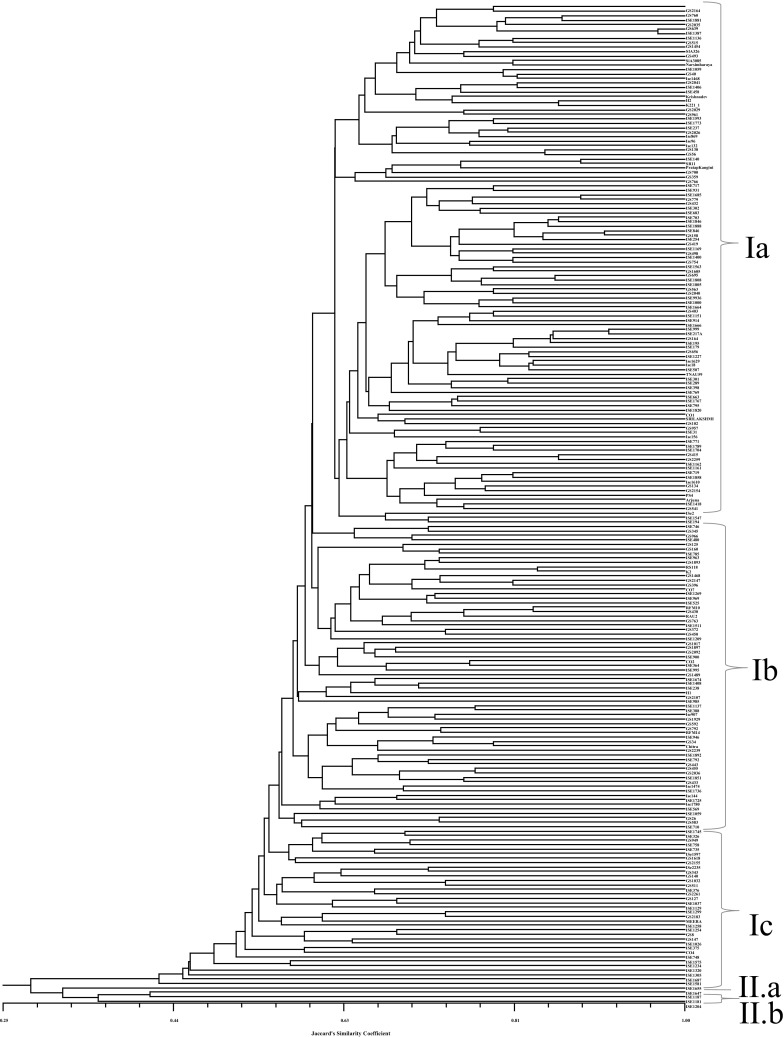
UPGMA dendrogram based on Jaccard's similarity coefficient among 223 accessions of foxtail millet core collection
Table 1.
Number of alleles per locus (N), polymorphism information content (PIC) and allele size range for the 31 SSRs used in analysis of foxtail millet core collection
| SSR Markers | PIC | N | Allele Size (bp) | |
|---|---|---|---|---|
| Range | Difference | |||
| Fox g ssr-2 | 0.25 | 3 | 380–400 | 20 |
| Fox g ssr-7 | 0.60 | 10 | 200–310 | 110 |
| Fox g ssr-9 | 0.14 | 3 | 380–420 | 40 |
| Fox g ssr-22 | 0.07 | 3 | 320–380 | 60 |
| Fox g ssr-24 | 0.35 | 5 | 260–300 | 40 |
| Fox g ssr-25 | 0.28 | 3 | 340–400 | 60 |
| Fox g ssr-27 | 0.76 | 7 | 250–310 | 60 |
| Fox g ssr-36 | 0.63 | 6 | 240–290 | 50 |
| Fox g ssr-46 | 0.51 | 6 | 300–370 | 70 |
| Fox g ssr-47 | 0.11 | 5 | 350–420 | 70 |
| Fox g ssr-51 | 0.76 | 7 | 300–370 | 70 |
| Fox g ssr-53 | 0.12 | 4 | 160–210 | 50 |
| Fox g ssr-54 | 0.54 | 6 | 210–270 | 60 |
| Fox g ssr-59 | 0.74 | 5 | 230–290 | 60 |
| Fox g ssr-61 | 0.19 | 3 | 340–360 | 20 |
| Fox g ssr-62 | 0.86 | 9 | 170–340 | 170 |
| Fox g ssr-63 | 0.80 | 10 | 290–390 | 100 |
| Fox g ssr-66 | 0.04 | 2 | 210–250 | 40 |
| Fox g ssr-67 | 0.07 | 2 | 210–240 | 30 |
| Fox g ssr-69 | 0.10 | 3 | 290–360 | 70 |
| Fox g ssr-70 | 0.13 | 5 | 420–520 | 100 |
| Fox g ssr-72 | 0.02 | 2 | 240–270 | 30 |
| Fox g ssr-74 | 0.51 | 7 | 170–250 | 80 |
| Fox g ssr-75 | 0.26 | 4 | 370–400 | 30 |
| Fox g ssr-76 | 0.05 | 2 | 390–400 | 10 |
| Fox g ssr-100 | 0.16 | 4 | 450–700 | 250 |
| Fox g ssr-101 | 0.37 | 2 | 200–240 | 40 |
| Fox EST- 71 | 0.01 | 2 | 380–390 | 10 |
| Fox EST- 96 | 0.20 | 2 | 130–140 | 10 |
| Fox EST- 97 | 0.009 | 2 | 290–300 | 10 |
| Fox EST- 98 | 0.02 | 2 | 170–180 | 10 |
| Total | 136 | |||
| Mean | 0.31 | 4.38 | ||
PIC polymorphic information content, N number of alleles
Mean of observed number of alleles per locus Na (4.06), Ne (1.95), Shannon’s information index (0.69), Ne’s gene diversity (0.35), percent polymorphism (100%), HE (0.35) and Ho (0.12) were high for the Exotic accessions. However, accessions of Indian origin particularly from Northern, Eastern and Southern populations of India also showed high genetic variability (Table 2). Line diagram of Na, I and Ne’s genetic diversity parameter showed ten conserved position or loci in foxtail millet populations (Fig. 4). These ten loci, include Fox gssr 22, Fox gssr 47, Fox gssr 53, Fox gssr 61, Fox gssr 66, Fox gssr 72, Fox gssr 76, Fox estssr 71, Fox estssr 97 and Fox estssr 98.
Table 2.
Summary of genetic diversity parameters for different sub-populations of foxtail millet core collection
| Population | Sample size | P % | Na | Ne | I | Nei’s h | HE | HO |
|---|---|---|---|---|---|---|---|---|
| CI | 24 | 64.52 | 2.64 ± 1.94 | 1.67 ± 1.04 | 0.47 ± 0.54 | 0.23 ± 0.28 | 0.25 ± 0.25 | 0.07 ± 0.17 |
| EI | 9 | 67.74 | 2.51 ± 1.59 | 1.79 ± 1.09 | 0.54 ± 0.55 | 0.29 ± 0.28 | 0.31 ± 0.25 | 0.11 ± 0.19 |
| EXO | 59 | 100 | 4.06 ± 2.20 | 1.95 ± 1.22 | 0.69 ± 0.53 | 0.35 ± 0.25 | 0.35 ± 0.25 | 0.12 ± 0.14 |
| NEI | 7 | 67.74 | 2.12 ± 1.12 | 1.65 ± 0.77 | 0.48 ± 0.45 | 0.28 ± 0.25 | 0.31 ± 0.25 | 0.09 ± 0.15 |
| NHI | 8 | 58.06 | 2.00 ± 1.12 | 1.61 ± 0.78 | 0.44 ± 0.45 | 0.26 ± 0.26 | 0.28 ± 0.25 | 0.09 ± 0.19 |
| NI | 41 | 70.97 | 3.06 ± 1.93 | 1.76 ± 1.10 | 0.54 ± 0.55 | 0.28 ± 0.28 | 0.28 ± 0.25 | 0.08 ± 0.15 |
| SI | 66 | 80.65 | 3.19 ± 1.97 | 1.76 ± 1.17 | 0.52 ± 0.57 | 0.26 ± 0.28 | 0.26 ± 0.25 | 0.09 ± 0.17 |
| WI | 9 | 64.52 | 2.19 ± 1.32 | 1.54 ± 0.71 | 0.43 ± 0.46 | 0.24 ± 0.25 | 0.26 ± 0.25 | 0.07 ± 0.14 |
| Average | 71.77 | 2.72 | 1.72 | 0.51 | 0.28 | 0.29 | 0.09 |
CI Central India, EI Eastern India, EXO Exotic population, NEI North Eastern India, NHI Northern Hills of India, SI Southern India, WI Western India
P%– % polymorphic loci; Na-mean number of observed allele; Ne-mean number of effective allele; I-mean of Shannon’s information index; Ne’s He-Ne’s gene diversity; HE-expected heterozygosity; Ho-observed heterozygosity
Fig. 4.
Line diagram of the genetic diversity parameters (Na- Number of observed alleles; I- Shannon's information index; and Nei’s gene diversity) at the 31 SSR loci in foxtail millet core collection populations (See Table 2 for details of populations; *indicate the conserved loci)
Fst values for group 1 (northern, northern hill and western region of India), group 2 (north-eastern and eastern region of India) and group 4 populations (central and southern regions of India) were 0.0607, 0.0461 and 0.0117 respectively and gene flow in group 1, Group 2 and Group 4 was 3.87, 5.17 and 21.19 respectively (Table 3) contributing to low population sub-division observed in foxtail millets.
Table 3.
Summary of percentage of polymorphism, Fst and geneflow in group of populations of foxtail millet core collection
| Group (Population) | Sample size | P% | Fst | Nm |
|---|---|---|---|---|
| 1 (NHI, NI, WI) | 58 | 74.19% | 0.0607 | 3.87 |
| 2 (EI, NEI) | 16 | 74.19% | 0.0461 | 5.17 |
| 3 (EXO) | 59 | 100.00% | – | – |
| 4 (CI, SI) | 90 | 80.65% | 0.0117 | 21.19 |
CI Central India, EI Eastern India, EXO Exotic population, NEI North Eastern India, NHI Northern Hills of India, SI Southern India, WI Western India P%-percent polymorphic loci, Nm-Gene flow
Clustering and population structure
Based on pair-wise Jaccard’s similarity coefficients, a dendrogram was constructed using UPGMA method. The average Jaccard’s similarity coefficient was (0.55) and 223 accessions grouped into two major groups I and II (Fig. 1). Group I further divided into 3 subgroups i.e. Ia, Ib and Ic. Subgroup Ia includes 121 accessions [Uttarakhand (15); Tamil Nadu and Andhra Pradesh (14 each); Karnataka (12); Madhya Pradesh (8); Maharashtra (6); Uttar Pradesh, Syria and China (5 each); Bihar, NE Region, Rajasthan, West Bengal and Korea (3 each); Himachal Pradesh, Kerala, Punjab, Kenya and Pakistan (2 each); Gujarat, Jammu & Kashmir, Orissa, Ethiopia, Nepal, Russia, Srilanka, Switzerland, Turkey and USA (1 each)]. Subgroup Ib included 63 accessions [Tamil Nadu (9); Karnataka and Andhra Pradesh (8); Uttarakhand (7); Madhya Pradesh (5); Uttar Pradesh (4); Maharashtra (3); Bihar and Orissa (2 each); Gujarat, Himachal Pradesh, NE Region, West Bengal, Bangladesh, China, Myanmar, Nepal, Russia, South Africa, Syria, United Kingdom and USA (1 each)]. Subgroup Ic included 35 accessions [Uttarakhand (6); Andhra Pradesh, NE Region and Russia (3 each); Jammu & Kashmir, Orissa, Korea, Lebanon and USA (2 each); Bihar, Gujarat, Karnataka, Rajasthan, Tamil Nadu, Iran, Spain, Syria, Taiwan, and Pakistan (1 each)]. Subgroup IIa included only one accession from Taiwan and IIb included three accessions, 2 from China and 1 from Russia.
The population structure of 223 foxtail millet core accessions was assessed using software STRUCTURE 2.3.3 (Pritchard et al. 2000) and subpopulations (k) were determined using Evanno’s ad-hoc statistic ΔK (Evanno et al. 2005). The LnP(D) values for subpopulations displayed a peak value at k = 4 (Fig. 2). The LnP(D) values after k = 4, were decreasing to −ve value up to k = 11 and further sharp increase up to k = 13. The results of structure analysis pointed out that the k values peaked at k = 4 and then decreased before another peak at k = 6. Hence, the number of sub-populations was inferred to be 4 (Fig. 3). SP1 to SP4 subpopulations included 23, 61, 70 and 69 accessions respectively. The genotypes of various eco-regions were intermixed in all the sub-populations (Table 4).
Fig. 2.
Plot of K value against delta LnPd showing optimim K = 4 (k averaged over the five run)
Fig. 3.
Population structure of foxtail core collection based on 31 SSR markers showing four sub-populations with admixtures indicated in different colours
Table 4.
Distribution of 223 foxtail millet core accessions based on ‘STRUCTURE’ analysis
| Regions | States in group | SP1 | SP2 | SP3 | SP4 |
|---|---|---|---|---|---|
| Central India (C) | Madhya Pradesh, Maharashtra | 0 | 8 | 8 | 7 |
| Eastern India (E) | Bihar, Orissa, West Bengal | 0 | 3 | 6 | 5 |
| Eastern Plains of India (EP) | Uttar Pradesh | 0 | 3 | 2 | 5 |
| North East India (NE) | Tripura, Assam, Manipur, Meghalaya | 2 | 0 | 3 | 2 |
| North West India (NW) | Himachal Pradesh, Jammu & Kashmir, Uttarakhand | 3 | 12 | 17 | 12 |
| Southern India (S) | Andhra Pradesh, Karnataka, Kerala, Tamil Nadu | 4 | 21 | 24 | 24 |
| Western India (W) | Gujarat, Punjab, Rajasthan | 0 | 1 | 0 | 2 |
| Africa (AF) | Africa, Kenya, South Africa | 0 | 3 | 1 | 1 |
| America (AM) | USA | 1 | 2 | 1 | 2 |
| East Asia (EA) | China, Korea, Taiwan | 6 | 5 | 2 | 4 |
| Eastern Region (ER) | Turkey; Spain, UK, Switzerland | 1 | 0 | 1 | 1 |
| North East Asia (NEA) | Iran, Pakistan | 3 | 1 | 1 | 1 |
| South Asia (SA) | Bangladesh, Nepal, | 0 | 1 | 0 | 0 |
| South East Asia (SEA) | Myanmar | 0 | 1 | 0 | 0 |
| West Asia (WA) | Syria, Lebanon | 3 | 0 | 4 | 3 |
| Total | 23 | 61 | 70 | 69 |
SP sub-population
Discussion
Large collections of an array of crops are present in the different gene banks all over the world, but their effective utilization is far from satisfactory. It is of utmost importance to identify manageable size of collections to represent the genetic diversity contained in whole collection using the core collection concept in order to utilize the genetic diversity assembled in gene banks for crop improvement. Further understanding the nature of genetic variation in the identified core collection is a key factor for germplasm conservation, management and effective utilization of genetic resources (Subudhi et al. 2016). The average number of alleles/locus observed in this study (4.38) is comparable to earlier studies in foxtail millet by Pandey et al. 2013 (2.1) and Gupta et al. 2014 (4.3). However, in a previous study in foxtail millet core collection, the average number of alleles per locus observed was 16.69 (Vetriventhan et al. 2014). Similarly, higher number of alleles/locus was observed in core collections of wheat (Hao et al. 2006); rice (Li et al. 2010) and common bean (Gill-Langarica et al. 2011). In the present study, lower number of alleles/locus may be due to the genome-wide, unlinked SSR markers selected for the analyses in comparison with earlier studies (Jia et al. 2013).
Presence of substantial number of rare alleles in the core accessions could be useful to identify specific trait associations of interest using allele mining procedures (Upadhyaya et al. 2010) since these may be due to presence of adaptive signatures in specific accessions. Unique alleles (8.08%) were higher than the number observed by Vetriventhan et al. (2012) and may be an important resource for marker-assisted breeding and in cultivar identification. Greater number of rare and unique alleles in the population may be due to sampling of unlinked SSR loci selected in this study unlike earlier reports (Senior et al. 1998) and also due to the effect of different selection pressures on landraces cultivated under diverse agronomic conditions. PIC value indicates the allele diversity and the informativeness of each marker. Average PIC value in the present research was comparable to earlier studies (Gupta et al. 2011; Kim et al. 2012; Lin et al. 2012; Wu et al. 2014). Vetriventhan et al. (2012) used 75 markers with PIC value >0.30 for validation of ICRSAT core collection, showed the high informativeness and polymorphic nature of the markers. EST-SSR markers used in this study had lowest PIC values which corroborate the findings of Obidiegwu et al. 2013. Present study is in general agreement with the Kumari et al. (2011) that higher value of Ne, I and Na was for the northern and southern foxtail millet accessions indicating presence of higher diversity in the core collection.
Pairwise Fst is an indication of the degree of the genetic differentiation between the populations. Present study showed little to moderate genetic differentiation among the sub-populations, which is contrary to the results obtained (Fst value >0.25) by Kumari et al. (2011) in different Indian foxtail millet populations. The reasons for lower estimates of genetic differentiation might be due to greater representation of accessions from India of overlapping nature resulting in lower estimates of pairwise Fst. Gene flow in Group 1 accessions (north, north hill and western region of India) was lower than in other groups because of lack of physical contiguity on account of their cultivation in small disjoined patches well separated from one another. The accessions under group 4 from central and southern regions of India had highest estimates of gene flow, since in these regions foxtail millet is cultivated on a larger scale and the regions are in closer proximity compared to the northern part of India thereby resulting in higher exchange of genetic material (Fig. 4).
The core collection of foxtail designated here included accessions of diverse origin both within and outside the country with 75.78% from India. The UPGMA clustering and STRUCTURE analysis showed, no clear-cut segregation of the accessions based on eco-geographic regions. Previous study on foxtail millet core collection by Vetriventhan et al. (2014) had identified four sub-populations which were also not correlated to the countries or regions of origin. Such similarities in pattern of distribution of diversity might be due to frequent exchange of germplasm from one region to other and their use in crop improvement programs across the countries (Ali et al. 2016). The exceptions reported were for the 250 foxtail millet Chinese landraces wherein structure was in accordance with eco-geographical distribution (Wang et al. 2012).
In the present study, the accessions from Gujarat (Western India) was represented in a single group in population structure based analysis which may be due to confined cultivation in contiguous dry area of Gujarat and limited exchange with other parts of the country. The clustering pattern supports the results obtained by Kumari et al. 2011 and Kim et al. 2012, that there is no discrete relationship observable with the origin of accessions. Further it is contrary to the result obtained by Fukunaga et al. (2002), that there is geographic differentiation observed among the five region of origin. Accessions from China and East Asia were distributed in the two major groups which indicate that accessions from these regions are highly diverse and support the hypothesis that China and East Asia are the probable centres of diversity for foxtail millet (Fukunaga et al. 2002). The Group I in the present study included accessions from exotic sources along with Indian accessions which indicated the their genetic affinities are probably due to exchange of landraces across the regions of cultivation (Li et al. 1998). However, the Group II included only the accessions from Taiwan, China and Russia exhibiting higher diversity which may be of much use in the foxtail breeding programme. The core collection identified here included the accessions from diverse regions of origin (Subudhi et al. 2016). The patterns of grouping of accessions indicated presence of high genetic diversity among the accessions designated as the Indian foxtail millet core collection. The accessions thus designated here are likely to be useful in large scale evaluation and screening programs to facilitate their use in cross-breeding programs in the country and elsewhere.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are thankful to the Director, National Bureau of Plant Genetic Resources and Indian Agricultural Research Institute, New Delhi, India, for providing the opportunity for the study. The scholarship provided to the senior author (Subhash Chander) by the Rajeev Gandhi National Fellowship-University Grant Commission for PhD degree is gratefully acknowledged.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0448-5) contains supplementary material, which is available to authorized users.
References
- Ali A, Choi YM, Yoon-Hyun D, Lee S, Sejong O, Hong-Jae P, Yang-Hee C, Myung CL. EST-SSR based genetic diversity and population structure among Korean landraces of foxtail millet (Setaria italica L.) Korean J Plant Res. 2016;29:322–330. doi: 10.7732/kjpr.2016.29.3.322. [DOI] [Google Scholar]
- Anju T, Sarita S. Suitability of foxtail millet (Setaria italica) and barnyard millet (Echinochloa frumentacea) for development of low glycemic index biscuits. Malays J Nutr. 2010;16:361–368. [PubMed] [Google Scholar]
- Bennetzen JL, Schmutz J, Wang H, Percifield R, Hawkins J, Pontaroli AC, Estep M, Feng L, Vaughn JN, Grimwood J. Reference genome sequence of the model plant Setaria. Nat Biotech. 2012;30:555–561. doi: 10.1038/nbt.2196. [DOI] [PubMed] [Google Scholar]
- Coulibaly A, Chen J. Evolution of energetic compounds, antioxidant capacity, some vitamins and minerals, phytase and amylase activity during the germination of foxtail millet. Am J Food Technol. 2011;6:40–51. doi: 10.3923/ajft.2011.40.51. [DOI] [Google Scholar]
- Diez CM, Imperato A, Rallo L, Barranco D, Trujillo I. Worldwide core collection of olive cultivars based on simple sequence repeat and morphological markers. Crop Sci. 2012;52:211–221. doi: 10.2135/cropsci2011.02.0110. [DOI] [Google Scholar]
- Doust AN, Elizabeth AK, Katrien MD, Jeffery LB. Foxtail Millet: a sequence-driven grass model system. Plant Physiol. 2009;149:137–141. doi: 10.1104/pp.108.129627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebana K, Kojima Y, Fukuoka S, Nagamine T, Kawase M. Development of mini core collection of Japanese rice landrace. Breed Sci. 2008;58:281–291. doi: 10.1270/jsbbs.58.281. [DOI] [Google Scholar]
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Federer WT. Augmented (or hoonuiaku) designs. Hawaii Plant Rec. 1956;2:191–208. [Google Scholar]
- Fukunaga K, Wang Z, Kato K, Kawase M. Geographical variation of nuclear genome RFLPs and genetic differentiation of foxtail millet, Setaria italica (L.) P. Beauv. Genet Resour Crop Evol. 2002;49:95–101. doi: 10.1023/A:1013852007770. [DOI] [Google Scholar]
- Gill-Langarica HR, Muruaga-Martinez JS, Vargas-Vazquez MLP, Rosales-Serna R, Mayek-Perez N. Genetic diversity analysis of common beans based on molecular markers. Genet Mol Biol. 2011;34:595–605. doi: 10.1590/S1415-47572011005000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudar G, Hemalatha S, Naik RK, Kamatar MY (2011) Evaluation of nutritional composition of foxtail millet (Setaria italica) grains cultivated in agro climatic zones of Karnataka by NIR. In: National symposium on recapturing nutritious millets for health and management of diseases, UAS, Dharwad, pp 37
- Gupta S, Kumari K, Sahu PP, Vidapu S, Prasad M. Sequence-based novel genomic microsatellite markers for robust genotyping purposes in foxtail millet [Setaria italica (L.) P. Beauv] Plant Cell. 2011;31:323–337. doi: 10.1007/s00299-011-1168-x. [DOI] [PubMed] [Google Scholar]
- Gupta S, Kumari K, Muthamilarasan M, Parida SK, Prasad M. Population structure and association mapping of yield contributing agronomic traits in foxtail millet. Plant Cell Rep. 2014 doi: 10.1007/s00299-014-1564-0. [DOI] [PubMed] [Google Scholar]
- Hao CY, Zhang XY, Wang LF, Dong YS, Shang XW, Jia JZ. Genetic diversity and core collection evaluations in common wheat germplasm from the North Western spring wheat region in China. Mol Breed. 2006;17:69–77. doi: 10.1007/s11032-005-2453-6. [DOI] [Google Scholar]
- Jaccard P. Lois de distribution florale. Bulletin de la Socíeté Vaudoise des Sciences Naturelles. 1902;38:67–130. [Google Scholar]
- Jali MV, Kamatar MY, Jali SM, Hiremath MB, Naik RK. Efficacy of value added foxtail millet therapeutic food in the management of diabetes and dyslipidamea in type 2 diabetic patients. Recent Res Sci Technol. 2012;4:03–04. [Google Scholar]
- Jia G, Shi S, Wang C, Niu Z, Chai Y, Zhi H, Diao X. Molecular diversity and population structure of Chinese green foxtail [Setaria viridis (L.) Beauv.] revealed by microsatellite analysis. J Exp Bot. 2013;64:3645–3655. doi: 10.1093/jxb/ert198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara H, Kishimoto E. Bastradezwischen Setaria italica and S. viridis. (in Japanese with German summary) Bot Mag Tokyo. 1942;56:62–67. doi: 10.15281/jplantres1887.56.62. [DOI] [Google Scholar]
- Kim EJ, Sa KJ, Park KC, Lee JK. Study of genetic diversity and relationship among accessions of foxtail millet [Setaria italica (L.) P. Beauv.] in Korea, China and Pakistan using SSR markers. Genes Genomics. 2012;34:529–538. doi: 10.1007/s13258-012-0074-0. [DOI] [Google Scholar]
- Kimura M, Crow JF. The number of alleles that can be maintained in a finite population. Genetics. 1964;49:725–738. doi: 10.1093/genetics/49.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondombo CP, Deu M, Chantereau J, Martin G, Chapuis E, Letourmy P, Zongo JD, Sagnard F, Brocke KV. Patterns of genetic structure and phenotypic diversity in sorghum landraces in relation to farmers’ management in Burkina Faso. Int J Biol Chem Sci. 2016;10:1747–1764. doi: 10.4314/ijbcs.v10i4.24. [DOI] [Google Scholar]
- Kottapalli KR, Burow MD, Burow G, Burke J, Puppala N. Molecular characterization of the U.S. peanut mini core collection using microsatellite markers. Crop Sci. 2007;47:1718–1727. doi: 10.2135/cropsci2006.06.0407. [DOI] [Google Scholar]
- Kumar P, Gupta VK, Misra AK, Modi DR, Pandey BK. Potential of molecular markers in plant biotechnology. Plant OMIC. 2009;2:141–162. [Google Scholar]
- Kumar B, Talukdar A, Bala I, Verma K, Lal SK, Sapra RL, Namita B, Chander S, Tiwari R. Population structure and association mapping studies for important agronomic traits in soybean. J Genet. 2014;93:775–784. doi: 10.1007/s12041-014-0454-0. [DOI] [PubMed] [Google Scholar]
- Kumari R, Dikshit N, Sharma D, Bhat KV. Analysis of molecular genetic diversity in a representative collection of foxtail millet [Setaria italica (L.) P. Beauv.] from different agro-ecological regions of India. Physiol Mol Biol Plants. 2011;17:363–374. doi: 10.1007/s12298-011-0085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewontin RC. The apportionment of human diversity. Evol Biol. 1972;6:381–398. [Google Scholar]
- Li Y, Wu SZ. Traditional maintenance and multiplication of foxtail millet [Setaria italica (L) P. Beauv.] landraces in China. Euphytica. 1996;87:33–38. doi: 10.1007/BF00022961. [DOI] [Google Scholar]
- Li HW, Li CH, Pao WK. Cytological and genetical studies of the interspecific cross of the cultivated foxtail millet, Setaria italica (L.) P. Beauv. and the green foxtail millet, S. viridis L. J Am Soc Agron. 1945;37:32–54. doi: 10.2134/agronj1945.00021962003700010004x. [DOI] [Google Scholar]
- Li Y, Jia J, Wang Y, Wu S. Intraspecific and interspecific variation in Setaria revealed by RAPD analysis. Genet Res Crop Evol. 1998;45:279–285. doi: 10.1023/A:1008600123509. [DOI] [Google Scholar]
- Li X, Yan W, Agrama H, Hu B, Jia L, Jia M, Jackson A, Moldenhauer K, McClung A, Wu D. Genotypic and phenotypic characterization of genetic differentiation and diversity in the USDA rice mini-core collection. Genetica. 2010;138:1221–1230. doi: 10.1007/s10709-010-9521-5. [DOI] [PubMed] [Google Scholar]
- Liang S, Yang G, Ma Y. Chemical characteristics and fatty acid profile of foxtail millet bran oil. J Am Oil Chem Soc. 2010;87:63–67. doi: 10.1007/s11746-009-1475-3. [DOI] [Google Scholar]
- Lin HS, Liao GI, Chiang CY, Kuoh CS, Chang SB. Genetic diversity in the foxtail millet (Setaria italica) germplasm as determined by agronomic traits and microsatellite markers. AJCS. 2012;6:342–349. [Google Scholar]
- Liu Z, Bai G, Zhang D, Znu C, Xia X, Cheng Z, Shi Z. Genetic diversity and population structure of elite foxtail millet (Setaria italica (L.) P. Beauv.) germplasm in China. Crop Sci. 2011;51:1655–1663. doi: 10.2135/cropsci2010.11.0643. [DOI] [Google Scholar]
- Liu W, Shahid MQ, Bai L, Lu Z, Chen Y, Jiang L, Diao M, Liu X, Lu Y. Evaluation of genetic diversity and development of a core collection of wild rice (Oryza rufipogon Griff.) populations in china. PLOS ONE. 2015 doi: 10.1145/2818302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Xu Y, He J, Zhang S, Wang Y, Lu P. Genetic diversity and population structure of broomcorn millet (Panicum miliaceum L.) cultivars and landraces in china based on microsatellite markers. Int J Mol Sci. 2016;17:370. doi: 10.3390/ijms17030370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Analysis of gene diversity in subdivided populations. Proc Natl Acad Sci USA. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obidiegwu OS, Obidiegwu JE, Parzies H. Development of SSR for foxtail millet [Setaria italica (L.) P. Beauv] and its utility in genetic discrimination of a core set. Genes Genom. 2013 [Google Scholar]
- Pandey G, Mishra G, Kumari K, Gupta S, Parda SK, Chattopadhyay Prasad M. Genome-wide development and use of microsatellite markers for large-scale genotyping applications in foxtail millet (Setaria italica) DNA Res. 2013;20:197–207. doi: 10.1093/dnares/dst002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Srivastava S, Grover S. Development of food products based on millets, legumes and fenugreek seeds and their suitability in the diabetic diet. Int J Food Sci Nutr. 2000;51:409–414. doi: 10.1080/096374800427019. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan M, Antony CS, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S (2016) Assessment of genetic diversity, population structure and relationships in Indian and non-Indian genotypes of finger millet [Eleusine coracana (L.) Gaertn] using genomic SSR markers. SpringerPlus 5:120 [DOI] [PMC free article] [PubMed]
- Rao ES, Kadirve P, Symonds RC, Geethanjali S, Ebert AW. Using SSR markers to map genetic diversity and population structure of Solanum pimpinellifolium for development of a core collection. PGR Charact Util. 2012;10:38–48. doi: 10.1017/S1479262111000955. [DOI] [Google Scholar]
- Reed K, Drnic I. Iron age diet at Sisak, Croatia: archaeobotanical evidence of foxtail millet [Setaria italica P. Beauv.] Oxf J Archaeol. 2016;35:359–368. [Google Scholar]
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior ML, Murphy JP, Goodman MM, Stuber CW. Utility of SSRs for determining genetic similarities and relationships in maize using an agarose gel system. Crop Sci. 1998;38:1088–1098. doi: 10.2135/cropsci1998.0011183X003800040034x. [DOI] [Google Scholar]
- Shahroodian SH, Azadfar D, Soltanloo H, Ramezanpour SS. Genetic variability in natural Iranian populations of Cupressus sempervirens var. horizontalis in Caspian Sea coastward assessed by SSR markers. Plant OMIC. 2011;4:19–24. [Google Scholar]
- Singhariya SC, Bhat KV, Gowda MVC, Dikshit N (2017) Identification, characterization and validation of core collection of foxtail millet [Setaria italica (L.) P.Beauv.] Indian J Agr Sci (in press)
- Subudhi E, Das A, Joshi RK, Mohanty S, Nayak S. Genetic diversity analysis and redundant identification in 48 core collections of Zingiber officinale Rosc. (Zingiberaceae) Braz J Bot. 2016 [Google Scholar]
- Thathola A, Srivastava S, Singh G. Effect of foxtail millet (Setaria italica) supplementation on serum glucose, serum lipids and glycosylated hemoglobin in type 2 diabetics. Diabetol Croat. 2010;40:23–28. [Google Scholar]
- Tiwari JK, Singh BP, Gopal J, Poonam Patil VU. Molecular characterization of the Indian Andigena potato core collection using microsatellite markers. Afr J Biotechnol. 2013;12:1025–1033. [Google Scholar]
- Upadhyaya HD, Dwivedi SL, Baum M, Varshney RK, Udupa SM, Gowda CLL, Hoisinton D, Singh S. Genetic structure, diversity and allelic richness in composite collection and reference set in chick pea (Cicer arietinum L.) BMC Plant Biol. 2008;8:106. doi: 10.1186/1471-2229-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyaya HD, Yadav D, Dronavalli N, Gowda CLL, Singh S. Mini core germplasm collections for infusing genetic diversity in plant breeding programmes. Electron J Plant Breed. 2010;1:1294–1309. [Google Scholar]
- Vetriventhan M, Upadhyaya HD, Anandakumar CR, Senthilvel S, Parzies HK, Bharathi A, Varshney RK, Gowda CLL. Assessing genetic diversity, allelic richness and genetic relationship among races in ICRISAT foxtail millet core collection. PGR Charact Util. 2012;10:214–223. doi: 10.1017/S1479262112000287. [DOI] [Google Scholar]
- Vetriventhan M, Upadhyaya HD, Anandakumar CR, Senthilvel S, Varshney RK, Parzies HK. Population structure and linkage disequilibrium of ICRISAT foxtail millet [Setaria italica (L.) P. Beauv.] core collection. Euphytica. 2014;196:423–435. doi: 10.1007/s10681-013-1044-6. [DOI] [Google Scholar]
- Wang C, Jia G, Zhi H, Niu Z, Chai Y, Li W, Wang Y, Li H, Lu P, Zhao B, Diao X. Genetic diversity and population structure of Chinese foxtail millet [Setaria italica (l.) beauv.] landraces. Genes Genomes Genet. 2012;2:769–777. doi: 10.1534/g3.112.002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. Hierarchical grouping to optimize an objective function. JASA. 1963;38:236–244. doi: 10.1080/01621459.1963.10500845. [DOI] [Google Scholar]
- Wu K, Yang M, Liu H, Tao Y, Mei Ju, Zhao Y. Genetic analysis and molecular characterization of Chinese sesame (Sesamum indicum L.) cultivars using Insertion-Deletion (InDel) and Simple Sequence Repeat (SSR) markers. BMC Genet. 2014;15:35. doi: 10.1186/1471-2156-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wan Z, Perry L. Early millet use in northern China. Proc Natl Acad Sci USA. 2012;109:3726–3730. doi: 10.1073/pnas.1115430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Yang R, Boyle T (2000) Popgene. http://ualberta.ca/~fyeh/F. Accessed 25 Feb 2004
- Zhang YF, Zhang QL, Yang Y, Luo ZR. Development of Japanese persimmon core collection by genetic distance sampling based on SSR markers. Biotechnol Biotechnol Equip. 2009 [Google Scholar]
- Zhao WG, Chung JW, Lee GA, Ma KH, Kim HH, Kim KT, Chung IM, Lee JK, Kim NS, KIM SM, Park YJ. Molecular genetic diversity and population structure of a selected core set in garlic and its relatives using novel SSR markers. Plant Breed. 2011;130:46–54. doi: 10.1111/j.1439-0523.2010.01805.x. [DOI] [Google Scholar]
- Zong X, Redden RJ, Liu Q, Wang S, Guan J, Liu J, Xu Y, Liu X, Gu J, Yan L, Ades P, Ford R. Analysis of a diverse global Pisum sp. collection and comparison to a Chinese local P. sativum collection with microsatellite markers. Theor Appl Genet. 2009;118:193–204. doi: 10.1007/s00122-008-0887-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





