Abstract
This study was aimed to identify plant growth-promoting bacterial isolates from soil samples and to investigate their ability to improve plant growth and salt tolerance by analysing phytohormones production and phosphate solubilisation. Among the four tested bacterial isolates (I-2-1, H-1-4, H-2-3, and H-2-5), H-2-5 was able to enhance the growth of Chinese cabbage, radish, tomato, and mustard plants. The isolated bacterium H-2-5 was identified as Bacillus amyloliquefaciens H-2-5 based on 16S rDNA sequence and phylogenetic analysis. The secretion of gibberellins (GA4, GA8, GA9, GA19, and GA20) from B. amyloliquefaciens H-2-5 and their phosphate solubilisation ability may contribute to enhance plant growth. In addition, the H-2-5-mediated mitigation of short term salt stress was tested on soybean plants that were affected by sodium chloride. Abscisic acid (ABA) produced by the H-2-5 bacterium suppressed the NaCl-induced stress effects in soybean by enhancing plant growth and GA4 content, and by lowering the concentration of ABA, salicylic acid, jasmonic acid, and proline. These results suggest that GAs, ABA production, and the phosphate solubilisation capacity of B. amyloliquefaciens H-2-5 are important stimulators that promote plant growth through their interaction and also to improve plant growth by physiological changes in soybean at saline soil.
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0449-4) contains supplementary material, which is available to authorized users.
Keywords: Abscisic acid, Bacillus amyloliquefaciens H-2-5, Gibberellins, Phosphate solubilisation, Plant growth, Salt stress
Introduction
The human population is increasing every year, and food insecurity and malnutrition is a major challenge in developing countries. Therefore, environment-friendly, low-cost technology is needed to insure food for poor people (Schreiner et al. 2013). As an alternative to chemical fertilisers, the use of bio-stimulants, such as microbial inoculants, humic acids, fulvic acids, protein hydrolysates, and amino acid and seaweed extracts, has greatly increased in agriculture during the twentyfirst century, and has increased the safety of agricultural products (Calvo et al. 2014). Beneficial free-living or endophytic bacteria and fungi in the rhizosphere of plants can cause yield increases by interacting with plant roots and stimulating plant metabolism throughout the crop life-cycle. Such organisms have been isolated and identified from different sources and utilised as biofertilisers. An effective bacterial biofertiliser can stimulate plant growth and nutrient uptake by fixing atmospheric nitrogen, solubilising complex substances to simple nutrients, sequestering iron through siderophore production, and producing volatile organic compounds and phytohormones (Kang et al. 2014a; Ryu et al. 2003; Wilson et al. 2006).
Seed germination and development of crop plants is significantly regulated by several endogenous hormone concentrations, and specifically, auxin plays a major role in root growth in terms of initiation, elongation, division, and apical dominance of root cells, and differentiation of vascular tissues. Gibberellins (GAs) are also involved in a number of cellular processes that regulate seed germination and growth of aerial plant parts, including floral induction and fruit development (Spaepen et al. 2009; Yamaguchi, 2008). The phytohormone-producing bacteria can enhance the growth of crop plants, even under unfavourable environmental stress conditions (Kang et al. 2014a). The exact mechanism underlying the interaction between bacterial GA and improved plant growth remains unclear (Calvo et al. 2014). A few GA-producing bacteria have been reported to be plant growth-promoting bacteria, such as Acetobacter diazotropicus, Acinetobacter calcoaceticus, Azospirillum brasilense, Bacillus cereus, Bacillus licheniformis, Bacillus macroides, Bacillus pumilus, Herbaspirillum seropedicae, Leifsonia soli, Promicromonospora sp., and Pseudomonas putida (Kang et al. 2014a; Bastian et al. 1998; Janzen et al. 1992; Joo et al. 2004; Kang et al. 2009, 2012, 2014b). These reports revealed that the application of GA-secreting bacteria to soil or the rhizosphere in croplands is an effective technique that can be used in sustainable agriculture.
Several reports have shown that beneficial rhizosphere bacteria interact with host plants to increase stress tolerance against biotic and abiotic factors (Grover et al. 2014; Solanki et al. 2015). Bacterial inoculation can regulate phytohormones and other defence metabolites in plants, which help to mitigate the stress induced by environmental factors (Kang et al. 2014a). The identification of bacterial isolates with phytohormone production capacities that can be used to enhance horticultural crop growth has also received attention because the quality and quantity of commercial horticultural fruits, vegetables, and flowers is affected by adverse changes in climate and by pollution. Although, the bio-inoculants that could be used to increase horticultural crop yields, but very few bacteria have been commercialised (Kang et al. 2015a). An efficient microbial fertiliser should have positive effects on a number of different crop plants and be able to survive in various selected formulations (Calvo et al. 2014). Therefore, this study was aimed to identify a suitable GA-secreting and plant growth-promoting bacterium that would enhance the growth of different horticultural plants, such as Chinese cabbage, radish, tomato, and mustard, and to investigate whether the bacterium could mitigate the detrimental effects of short term soil salinity during soybean growth.
Materials and methods
Isolation and screening of plant growth-promoting bacteria
The soil samples used to isolate the plant growth-promoting bacteria were collected from agricultural fields that were located in different areas in the Republic of Korea; these included Danyang, Jecheon, Jeongseon, and Yeongwol. Ten grams of soil were suspended in sterile 0.85% saline solution, and the soil suspensions were serially diluted with the same solution. Subsequently, 0.1 ml aliquots were spread on solid tryptic soy and agar (TSA) medium and incubated for 48 h at 30 °C. Each individual colony was separated, based on bacterial colony morphology and pigmentation, and cultured on fresh TSA medium. The selected colonies were pure cultured and stored in 50% glycerol at −80 °C for further use. Tryptic soy broth was inoculated with 10 bacterial isolates: H-1-3, H-1-4, H-2-3, H-2-4, H-2-5, H-3-2, I-1-5, I-2-1, I-2-3, and I-2-4, so that their plant growth-promoting ability in Chinese cabbage could be investigated. Three-day-old bacterial cultures were diluted 10 times with sterile distilled water and applied to surface-sterilised Chinese cabbage seeds in Petri-dishes. The seedling fresh weight and length were measured after 5 days.
Bacterial isolates (I-2-1, H-1-4, H-2-3 and H-2-5) inoculation and plant growth
Radish (Raphanus sativus L.), tomato (Lycopersicon esculentum L.), and mustard (Brassica juncea L.) seeds were surface-sterilised with 5% sodium hypochloride and rinsed with sterile distilled water. The sterilised seeds were sown in autoclaved plastic pots containing an artificial horticultural soil mixture [peat moss (13–18% (w/v)], perlite [7–11% (w/v)], coco-peat [63–68% (w/v)], zeolite [6–8% (w/v)] and other nutrients [~90 mg/kg NH4+, ~205 mg/kg NO3 −, ~350 mg/kg P2O5 and ~100 mg/kg K2O). They were then cultivated in a green house at 30 ± 2 °C. Five millilitres of the 3-day-old selected bacterial cultures (I-2-1, H-1-4, H-2-3, and H-2-5) were applied to 2-week-old radish, and 3-week-old tomato and mustard plants. The plant growth attributes, i.e., length and biomass of shoots and roots, leaf numbers and chlorophyll content (Chlorophyll Meter, Minolta Co. Ltd, Japan) were measured at 10 days after inoculation.
Genomic DNA extraction and B. amyloliquefaciens H-2-5 bacterial identification
The H-2-5 bacterial strain was cultured on NB medium for 3 days and then the culture medium was separated from the bacterial pellet. The genomic DNA was extracted from the H-2-5 strain, and 16S rDNA sequence was amplified using the 27F primer (5′-AGAGTTTGATC(AC)TGGCTCAG-3′) and the 1492R primer (5′-CGG (CT) TACCTTGTTACGACTT-3′), which were complementary to the 5′ end and 3′ end of the prokaryotic 16S rDNA, respectively. The BLAST search program (http://www.ncbi.nlm.nih.gov/BLAST/) was used to compare the nucleotide sequence homology of the H-2-5 strain. The obtained relatively similar sequences were aligned by ClustalW using MEGA version 5.0 software and the phylogenetic tree was constructed by the maximum parsimony method using the same software. Bootstrap replication (1000 replications) was used to statistically support the nodes in the phylogenetic tree.
Gibberellin secretions from H-2-5 in the culture medium
Bacillus amyloliquefaciens H-2-5 culture (3 days-old) was used for GA quantification. The culture medium was separated from the culture and the GAs were extracted and quantified using the method described by Kang et al. (2014a). The retention time was determined using GA standards and each GA was calculated from their peak area ratios.
Phosphate solubilisation ability of H-2-5
To determine the phosphate solubilisation ability of B. amyloliquefaciens H-2-5, the bacterial isolate was inoculated on 0.5% National Botanical Research Institute’s Phosphate media [glucose, 10 g; Ca3(PO4)2, 5 g; (NH4)2SO4, 0.5 g; NaCl, 0.2 g; MgSO4·7H2O, 0.1 g; KCl, 0.2 g; MnSO4·H2O, 0.002 g and FeSO4·7H2O, 0.002 g/L] containing plates and incubated for 3 days at 30 °C. The clear zone formations on the Petri-plates caused by the bacterial isolate were noted.
Abscisic acid quantification in H-2-5
Bacillus amyloliquefaciens H-2-5 was cultured on LB medium for 3 days and the culture filtrate was then portioned with an isopropanol:glacial acetic acid (95:5) solution mixture. The ABA in the culture medium was extracted and quantified according to the method followed by Kang et al. (2014a).
Soybean growth under salt stress and H-2-5 treatment
Soybean (cv. Taekwang) seeds were surface-sterilised with Tween 80 solution and perchloric acid, and then rinsed with sterile distilled water. Surface-sterilised seeds were sown in a horticultural soil mixture. The H-2-5 isolate was inoculated into nutrient broth and then cultured at 200 rpm and 30 °C for 3 days. Soybeans at the two-leaf emergence stage were treated with 10 ml of 10 times diluted bacterial culture and irrigated at regular intervals in a greenhouse (30 ± 2 °C). To induce salt stress, 120 mM NaCl was applied to the plants after 7 days post bacterial inoculation. The length and weight of the shoots were measured after 10 days. The collected plant samples were freeze-dried and used for plant hormone and proline analyses.
Phytohormonal analysis of soybean plants
The H-2-5 induced endogenous phytohormones, such as GAs, ABA, SA and JA, in salt-affected soybean plants were quantified from lyophilised leaf samples. GAs (GA4) and ABA were extracted and measured in plant samples as described above. The freeze-dried leaf tissues of all treated samples were ground to powder for SA analysis (Kang et al. 2014a). The endogenous JA from the soybean plant leaf samples was extracted according to the method used by Kang et al. (2014a). The amount of endogenous JA was calculated by comparing the peak areas for endogenous JA to the corresponding standards.
Proline analysis
The leaf samples (50 mg) were homogenised with liquid nitrogen and hydrolysed using 0.02 N HCl at 110 °C for 24 h (Kang et al. 2015b). The crude extract was filtered and injected into an amino acids analyser (L-8900, Hitachi, Japan). The peak values of the proline standard were used to calculate the amount of proline in the soybean leaf samples.
Statistical analysis
A randomised block design was used in the plant growth experiment. The data obtained from the results were statistically analysed for mean ± standard deviation using Sigma Plot software. The analysis of variance (ANOVA) results between mean values were compared by Duncan’s multiple range test (DMRT) at p ≤ 0.05 with the use of statistical analysis software (SAS).
Results and discussion
Effect of bacterial isolates on the growth of horticultural plants
The detection of plant beneficial micro-organisms is important to develop the effective bio-fertilisers and sustainable agriculture (Sturz et al. 2000). Several isolates in the genera including Acinetobacter, Alcaligenes, Arthrobacter, Azospirillium, Azotobacter, Bacillus, Beijerinckia, Burkholderia, Enterobacter, Erwinia, Flavobacterium, Rhizobium, and Serratia have been reported to be plant growth-promoting bacteria (Rodriguez and Fraga 1999; Sahin et al. 2004; Shoebitz et al. 2009). These bacteria not only improve plant growth, but also effectively reduce the toxic effects of salt, drought, heavy metals, insects, and phytopathogen infection (Kang et al. 2014a; Burges 1982; Correa et al. 2009; Zribi et al. 2012). Although more than 100 bacterial species have been recognised as bio-insecticides, only a few bacteria, including Bacillus spp., have been commercially successful in agriculture (Starnes et al. 1993). In this study, we focused on detecting efficient plant growth-promoting bacteria in soil collected from different places in the Republic of Korea.
Two hundred and twenty one bacterial colonies were detected from soil samples inoculated on TSA medium. Among these, ten bacterial isolates: H-1-3, H-1-4, H-2-3, H-2-4, H-2-5, H-3-2, I-1-5, I-2-1, I-2-3 and I-2-4, were tested on Chinese cabbage to determine their effect on seedling growth (Supplementary figure 1). The bacterial treatments, excluding H-3-2, increased seedling height compared to their control, but the H-3-2 interaction did not cause any significant changes to seedling height. The effects of the bacterial medium (NB) components on plant growth were investigated and the results showed that the NB had a negative effect on plant development because it inhibited seedling growth. The significant increases in seedling height were in the following order: H-1-3 > I-1-5 > I-2-4 > I-2-3 > H-2-4 > H-2-3 > H-1-4 > I-2-1 > H-2-5 compared to non-inoculated control plants. The fresh weight of the seedlings was also increased after inoculation with most of the bacterial isolates (Supplementary figure 1). However, no significant changes to fresh weight were observed when the seedlings were inoculated with the H-2-4 isolate and culture medium. The H-2-5 and H-2-3 isolates effectively enhanced the seedling biomass and similar significant plant growth-promoting effects were recorded than other bacterial isolate treated seedlings when compared to their controls.
Bacterial isolates, such as I-2-1, H-1-4, H-2-3 and H-2-5, were separately applied to soil containing radish plants and their individual plant growth stimulating effect was compared to untreated controls. The positive interaction between the bacterial inoculation and radish plant growth is shown in Supplementary figure 2. The root length of the plants was significantly increased by H-2-5 treatment, followed by H-2-3 and H-1-4, compared to their controls (Table 1). The increased shoot length growth was found in plants associated with H-2-3, whereas H-1-4 isolates enhanced the shoot and root fresh weights, and the number of leaves when compared to the untreated plants. Moreover, all the bacterial isolates enhanced chlorophyll content in the treated plants.
Table 1.
Effect of I-2-1, H-1-4, H-2-3 and H-2-5 isolates on R. sativus plant growth
| Treatment | RL (cm) | SL (cm) | SFW (g) | RFW (g) | LN | Chl (SPAD) |
|---|---|---|---|---|---|---|
| Control | 9.3 ± 1.4a | 11.30 ± 0.8b | 0.90 ± 0.2c | 0.60 ± 0.2c | 4.40 ± 1.1c | 32.1 ± 0.9b |
| I-2-1 | 9.24 ± 1.5a | 13.26 ± 1.0a | 1.57 ± 0.3b | 0.68 ± 0.2b | 5.00 ± 1.0bc | 35.5 ± 2.5a |
| H-1-4 | 9.60 ± 1.1a | 13.80 ± 0.8a | 2.03 ± 0.2a | 1.65 ± 0.2a | 6.20 ± 0.5a | 34.5 ± 1.5a |
| H-2-3 | 9.56 ± 2.6a | 14.04 ± 0.8a | 1.41 ± 0.3b | 0.17 ± 0.1b | 5.80 ± 0.5ab | 36.4 ± 2.1a |
| H-2-5 | 11.34 ± 2.2a | 12.96 ± 1.2a | 1.59 ± 0.3b | 0.53 ± 0.2b | 6.00 ± 0.7ab | 35.9 ± 2.7a |
RL root length, SL shoot length, SFW shoot fresh weight, RFW root fresh weight, LN leaf numbers, Chl chlorophyll
The means followed by the same letter in each column are not significantly different (p ≤ 0.05) as determined by DMRT
In tomato plants, the four bacterial cultures effectively promoted root, shoot and leaf growth compared to the untreated control plants (Table 2). H-2-5 stimulated plant growth by enhancing root length, leaf numbers and chlorophyll content. Longer shoot lengths, and higher shoot and root fresh weights were also recorded for plants treated with the I-2-1 bacterium. In addition, mustard plant growth after I-2-1, H-1-4, H-2-3 and H-2-5 bacterial treatment was measured. However, the I-2-1 and H-2-3 isolates inhibited root length growth and there were no significant changes in root length between the control and the H-1-4 and H-2-5 treatments (Table 3). All bacterial treatments increased the shoot length and the longest shoot lengths were recorded for the H-2-3 treated plants. Differences in biomass were detected in plants treated with bacterial isolates. The four bacterial treatments enhanced the shoot fresh weights and reduced the root fresh weights compared to the untreated control plants. Although there was no significant increase in leaf numbers after mustard plants had been treated with the bacteria, the chlorophyll content was significantly enhanced after all treatments, and H-2-3 and H-2-5 effectively stimulated chlorophyll synthesis in mustard leaves.
Table 2.
Influence of bacterial treatment (I-2-1, H-1-4, H-2-3 and H-2-5) on L. esculentum plant growth
| Treatment | RL (cm) | SL (cm) | SFW (g) | RFW (g) | LN | Chl (SPAD) |
|---|---|---|---|---|---|---|
| Control | 9.26 ± 1.2a | 20.52 ± 1.1b | 1.47 ± 0.6a | 0.37 ± 0.1a | 16.40 ± 1.1b | 30.1 ± 0.9c |
| I-2-1 | 8.46 ± 0.6a | 25.38 ± 0.8a | 1.93 ± 0.5a | 0.52 ± 0.1a | 17.60 ± 1.1b | 33.1 ± 2.2bc |
| H-1-4 | 9.34 ± 1.9a | 24.36 ± 1.4a | 1.81 ± 0.2a | 0.40 ± 0.1a | 16.60 ± 1.3b | 32.1 ± 1.8b |
| H-2-3 | 10.18 ± 2.2a | 25.32 ± 1.0a | 1.86 ± 0.2a | 0.42 ± 0.1a | 18.60 ± 3.8b | 34.4 ± 1.1ab |
| H-2-5 | 12.08 ± 5.7a | 23.28 ± 5.3ab | 1.70 ± 0.6a | 0.45 ± 0.1a | 22.60 ± 2.4a | 36.2 ± 2.1a |
RL root length, SL shoot length, SFW shoot fresh weight, RFW root fresh weight, LN leaf numbers, Chl chlorophyll
The means followed by the same letter in each column are not significantly different (p ≤ 0.05) as determined by DMRT
Table 3.
Role of I-2-1, H-1-4, H-2-3 and H-2-5 bacterial isolates on growth of B. juncea
| Treatment | RL (cm) | SL (cm) | SFW (g) | RFW (g) | LN | Chl (SPAD) |
|---|---|---|---|---|---|---|
| Control | 14.54 ± 3.1a | 12.92 ± 0.9e | 1.19 ± 0.3d | 0.46 ± 0.1a | 4.2 ± 0.6a | 29.1 ± 1.9b |
| I-2-1 | 12.30 ± 3.0b | 16.62 ± 1.5b | 2.29 ± 0.1a | 0.48 ± 0.1a | 4.4 ± 0.6a | 33.5 ± 1.5a |
| H-1-4 | 14.92 ± 4.1a | 13.22 ± 1.0d | 1.31 ± 0.2d | 0.27 ± 0.1c | 4.4 ± 0.6a | 31.5 ± 3.5ab |
| H-2-3 | 12.56 ± 3.0b | 17.12 ± 1.0a | 1.80 ± 0.2b | 0.30 ± 0.1b | 4.6 ± 0.6a | 35.7 ± 3.1a |
| H-2-5 | 14.16 ± 1.5a | 15.66 ± 1.2c | 1.65 ± 0.3c | 0.28 ± 0.1c | 4.2 ± 0.5a | 34.4 ± 1.7a |
RL root length, SL shoot length, SFW shoot fresh weight, RFW root fresh weight, LN leaf numbers, Chl chlorophyll
The means followed by the same letter in each column are not significantly different (p ≤ 0.05) as determined by DMRT
The significant enhancement of plant growth by beneficial microbes is achieved by improvements in photosynthesis (Weston et al. 2012). The availability of soil nutrients and the supply of water and nutrients to roots may increase in crops cultivated on agricultural land where biofertilisers have been applied (Vessey, 2003). Leaves use water and nutrients to synthesise photosynthetic pigments and in other metabolic processes that enhance growth. Increased chlorophyll content in plants during fungal and bacterial interactions has been widely reported (Kang et al. 2014c; Radhakrishnan et al. 2014). A similar chlorophyll content response to B. amyloliquefaciens H-2-5 inoculation was found in radish, tomato and mustard plants. These results revealed that the bacterial isolates I-2-1, H-1-4, H-2-3 and H-2-5 significantly promoted plant growth in Chinese cabbage, radish, tomato and mustard plants. The external application of H-2-5 improved plant growth in terms of increases in root, shoot and leaf growth, and this may be due to the GA production and phosphate solubilisation ability of H-2-5. Furthermore, the H-2-5 isolate was also identified by phylogenetic analysis.
Molecular identification of isolate H-2-5
The genomic DNA of H-2-5 was used to sequence the 16S rDNA in order to identify the bacterial isolate. A BLAST search showed that isolate H-2-5 had the highest sequence homology proportion and query coverage, and the lowest E values with B. amyloliquefaciens. Sequences of other genera were also used to determine the actual relationship among participating candidates in the group. In the phylogenetic dendrogram, the H-2-5 isolate formed a sub-clade with different B. amyloliquefaciens (Supplementary figure 4). The obtained 16S rDNA sequence for H-2-5 was submitted to the NCBI GenBank and was assigned Accession No. KF731679. On the basis of sequence homology and phylogenetic analysis, bacterial isolate H-2-5 was identified as B. amyloliquefaciens and named B. amyloliquefaciens H-2-5.
Secretion of phytohormones and the phosphate solubilisation characteristics of the H-2-5 isolate
The synthesis and secretion of bioactive substances by bacteria can help to enhance the growth and yield of many crop plants. The first commercial biofertiliser, Alinit, was based on a Bacillus species and is used to raise the yield of crop plants up to 40% (Kilian et al. 2000). Although Pseudomonas species can promote plant growth, Bacillus species are preferred because their spore forming nature helps to increase their viability in commercial products (Haas and Defago 2005). However, more extensive uses in crop cultivation are still limited because of a lack of knowledge of the mechanisms underlying the interactions between the plant and Bacillus species (Borriss 2011). In this study, we propose that the GA production capacity of B. amyloliquefaciens can improve the growth of horticultural plants. This is the first report to show that GAs, such as GA4, GA8, GA9, GA19 and GA20, in a culture filtrate of H-2-5 are produced in varying concentrations. We used HPLC coupled with GC–MS-SIM to detect and quantify several GAs because it measures available GAs in bacterial culture more reliably than other TLC and HPLC-UV methods (Kang et al. 2014b). Several GAs have been identified previously, and their quantities were measured in bacterial and fungal cultures by HPLC coupled with GC–MS-SIM (Kang et al. 2014b; Radhakrishnan et al. 2013), which suggests that this method gives unbiased values.
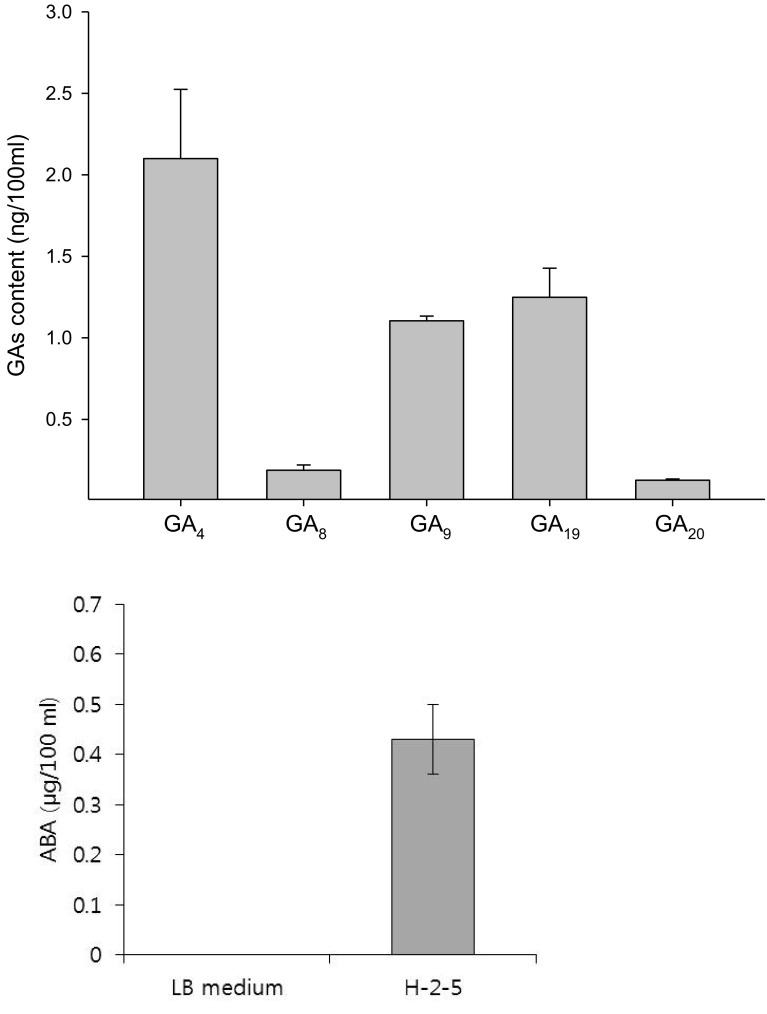
Bacillus amyloliquefaciens H-2-5 produced phytohormones, such as GAs (GA4, GA8, GA9, GA19, and GA20) and ABA (0.43 µg/100 ml) in their culture medium. Figure 1 shows that GA4 levels in the bacterial culture were highest (2.1 ng/100 ml), followed by GA19 (1.25 ng/100 ml) and GA9 (1.1 ng/100 ml), whereas GA8 (0.19 ng/100 ml) and GA20 (0.13 ng/100 ml) levels were lowest in the culture medium. Some reports have shown that the GA producing ability of Bacillus species plays a prominent role in plant growth promotion (Joo et al. 2004). In a previous study, the beneficial role of GA producing bacteria was tested on a GA deficient mutant dwarf rice cultivar (Waito C), which had a blocked C13-hydroxylation pathway for GA biosynthesis. The results showed that increased rice plant growth when the plants were associated with the bacteria (Kang et al. 2014a). This study has revealed that B. amyloliquefaciens H-2-5 application can trigger plant growth through their production of GAs.
Fig. 1.
Gibberellin and ABA production in the Bacillus amyloliquefaciens H-2-5 culture medium
The phosphate-solubilising ability of H-2-5 was confirmed in Petri-plates containing phosphate medium by the formation of clear zones around the bacterial growth (Supplementary figure 3). Recently, Kang et al. (2014c) reported that the phosphate-solubilising capacity of B. megaterium mj1212 helped to increase chlorophyll synthesis and other subsequent metabolic processes, i.e., sugars and amino acids, which led to enhanced mustard plant growth. This study showed that B. amyloliquefaciens H-2-5 solubilised phosphate. The conversion of insoluble phosphate into soluble phosphate by the bacteria helps to increase phosphate transport through the roots (Relwani et al. 2008). Phosphorus is an important constituent of cellular membranes, nucleic acids, and ATP, and it plays a major role in cellular functions (Theodorou and Plaxton, 1993). Phosphate-solubilising microbes also help plants to tolerate unfavourable environmental conditions as well as solubilising phosphates, which leads to increased plant growth (Kang et al. 2014a; Radhakrishnan et al. 2015).
Effect of B. amyloliquefaciens H-2-5 on the growth of soybean, phytohormones and proline content under salt stress
Soil salinity reduces soybean growth (Fig. 2; Table 4). The B. amyloliquefaciens H-2-5 mitigated salt stress effects in soybean. The results showed that H-2-5 association with soybean significantly improved the growth of host plants (Table 4). The shoot length and shoot fresh weight were 17 and 8% higher in plants associated with the bacteria, respectively. Under salt stress, soybean plants had reduced shoot lengths (10%) and fresh weights (9%) compared to their non-stressed controls. However, the plant growth parameters were significantly higher in salt affected plants when the plants were inoculated with H-2-5 bacteria, which enhanced shoot length by 10% compared to salt stressed plants.
Fig. 2.

Effect of B. amyloliquefaciens H-2-5 on soybean plant growth when under salt stress. The soybean plants under salt stress either had no association with B. amyloliquefaciens (control) or were inoculated with B. amyloliquefaciens H-2-5
Table 4.
Effect of B. amyloliquefaciens H-2-5 on soybean plant growth performance at NaCl stressed and non-stressed conditions
| Treatments | Shoot length (cm) | Shoot weight (g, FW) |
|---|---|---|
| Con | 31.34 ± 0.4b | 16.38 ± 3.2b |
| NaCl | 28.42 ± 1.3c | 14.92 ± 3.6c |
| H-2-5 | 36.60 ± 2.2a | 17.70 ± 1.5a |
| H-2-5 + NaCl | 31.21 ± 1.8b | 14.88 ± 1.5c |
The means denoted by the same letter are not significantly different (p ≤ 0.05)
Applying B. amyloliquefaciens H-2-5 to the soil mitigated salt stress effects in soybean by regulating gibberellin, abscisic acid, jasmonic acid and salicylic acid. The gibberellin (GA4) content in the soybean plants significantly increased (more than threefold) in the presence of B. amyloliquefaciens H-2-5 compared to the control plants under no-stress conditions (Fig. 3). The soybean plant growth reduction caused by the soil salinity was probably due to the lower GA content. The positive effect against salt stress of bacterial interactions in the host plants was strongly supported by the increased synthesis of GA in the inoculated soybean compared to the control plants. Several reports have suggested that salt stress inhibits soybean growth (Kang et al. 2014a; Radhakrishnan and Lee 2014). This study also showed a similar soybean response to salt stress. Soil salinity disturbs the normal metabolism of plant growth regulating hormones. This study found that GA4 levels decreased, but ABA and SA synthesis increased in salt stressed soybean. The inhibition of bioactive GA production in plants stunted growth, which was also reported in previous rice-mutant studies (Kang et al. 2014a). Radhakrishnan and Lee (2013a) demonstrated that plants respond to salt stress by increasing stress hormone levels, including ABA and SA. The plant beneficial rhizosphere-microorganisms help plants tolerate saline soil induced stress effects by secreting phytohormones and through phosphate solubilisation (Kang et al. 2014a; Radhakrishnan et al. 2013, 2015). In this study, applying B. amyloliquefaciens H-2-5 to the soil mitigated salt stress effects on soybean by secreting GAs and ABA into soil and by solubilising phosphate. The increased GA levels in bacterium-treated plants suggested that the exogenous secretion of phytohormone (GAs) from B. amyloliquefaciens H-2-5 was involved in stress tolerance. It has also been reported that GA producing rhizobacteria are able to increase plant tolerance against abiotic stress (Kang et al. 2014a, d).
Fig. 3.
Influence of B. amyloliquefaciens H-2-5 on GA4 content in plants under salt stress. The bar represents mean ± standard deviation (n = 3) and means followed by the same letter are not significantly different (p ≤ 0.05) according to DMRT
However, the roots associated with B. amyloliquefaciens H-2-5 had higher concentrations of ABA in soybean plants than the bacterium-free plants in a non-stress environment (Fig. 4). A significant increase in ABA content was found in salt stressed plants, but co-treatment with H-2-5 effectively decreased the ABA accumulation in stressed plants. Similarly, Cohen et al. (2015) demonstrated that ABA producing A. brasilense enhanced endogenous ABA content in both drought stressed and non-stressed Arabidopsis thaliana plants, which improved plant performance. Under stress conditions, the ABA content indirectly reduces water loss in plants by regulating the activity of stomata, and increases stress tolerance by triggering stress-responsive genes (Herrera-Medina et al. 2007; Leung and Giraudat 1998). Treating soils with Burkholderia cepacia SE4, Promicromonospora sp. SE188, A. calcoaceticus SE370 or P. putida H-2-3 has been shown to promote plant growth and decrease stress induced ABA accumulation in salt or drought affected plants (Kang et al. 2014a, d). Therefore, the tolerance of soybean to salt stress when it is associated with B. amyloliquefaciens H-2-5 might be due to the regulation of endogenous GA and ABA synthesis. The levels of another stress related hormone, JA, also decreased in H-2-5 treated plants and this occurred in salt stressed and non-salt stressed plants (Fig. 4). The combined effect of bacterial inoculation and salinity significantly reduced JA synthesis in soybean plants. The JA pathway in plants induces systemic resistance and is activated after plant interaction with rhizosphere bacterial communities (Carvalhais et al. 2013). Soil osmotic stress inhibits JA synthesis in soybean plants (Radhakrishnan and Lee, 2013b). This study revealed that B. amyloliquefaciens H-2-5 association suppressed endogenous JA production in soybean, which helped mitigate the salt stress effects.
Fig. 4.
Changes to ABA, JA and SA in soybean plants treated with B. amyloliquefaciens H-2-5 and NaCl. The bar represents mean ± standard deviation (n = 3) and means followed by the same letter are not significantly different (p ≤ 0.05) according to DMRT
In addition, SA content was higher in plants subjected to a salt stress environment (Fig. 4), and the bacterial association decreased the stress effects by reducing SA synthesis. However, the expression of SA increased in plants grown under saline conditions and reduced when there was an association with B. amyloliquefaciens H-2-5. A similar response by soybean was reported in a previous study (Kang et al. 2014a), which suggested that P. putida H-2-5 was also able to protect salt affected plants by inhibiting SA accumulation.
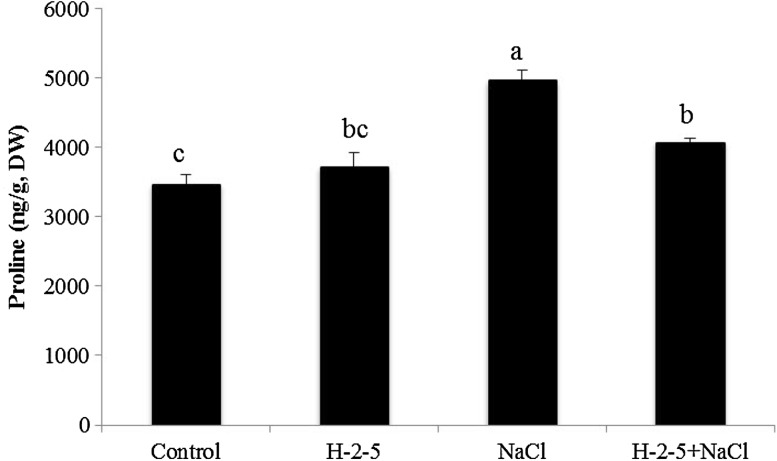
Proline levels increased in soybean plants when subjected to NaCl treatment (Fig. 5), but there was no significant change in proline synthesis between the control and the H-2-5 bacterial treatment under non-stress conditions. Under salt stress, the B. amyloliquefaciens H-2-5 association reduced proline levels in salt stressed plants compared to the plants that had not been inoculated with bacteria. In addition, proline, a stress responsive amino acid, has a well-known role in plant tolerance to abiotic stresses. It acts as a compatible solute during osmotic adjustment and stabilises cellular structures and membranes. The accumulation of proline during abiotic stress condition is an adaptive response by plants (Bartels and Sunkar, 2006; Hong et al. 2000). Ruiz-Lozano et al. (2008) reported that rhizosphere microflora effectively alleviate the effects of abiotic stress by altering proline accumulation in plant tissues. This study showed that when soybean was inoculated with B. amyloliquefaciens H-2-5, proline content was enhanced compared to the controls, but it was decreased under stress conditions, which would help reduce stress effects and increase plant tolerance to stress.
Fig. 5.
Effect of B. amyloliquefaciens H-2-5 on the proline content in soybean plants subjected to salt stress. The bar represents mean ± standard deviation (n = 3) and means followed by the same letter are not significantly different (p ≤ 0.05) according to DMRT
In conclusion, these in vitro and greenhouse studies have revealed that B. amyloliquefaciens H-2-5 promote Chinese cabbage, radish, tomato and mustard plant growth due to its GA production and phosphate solubilisation characters. This significant growth-promoting effect by B. amyloliquefaciens H-2-5 on several crop plants strongly suggests that this bacterium could be used as a biofertiliser. The regulation of phytohormones and proline content in salt tolerant soybean plants when they interact with B. amyloliquefaciens suggests that inoculation with H-2-5 bacterium improves physiological condition of soybean in saline agricultural soil. Further work should focus on developing a suitable B. amyloliquefaciens H-2-5 formulation that can be used in the field.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This research was supported by Agenda Program (Project No. PJ01228603) through the Rural Development Administration, Republic of Korea.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s12298-017-0449-4) contains supplementary material, which is available to authorized users.
Min-Ji Kim and Ramalingam Radhakrishnan have contributed equally to this work.
References
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit Rev Plant Sci. 2006;24:23–28. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- Bastian F, Cohen A, Piccoli P, Luna V, Baraldi R, Bottini R. Production of indole-3-acetic acid and gibberellins A1 and A3 by Acetobacter diazotrophicus and Herbaspirillum seropedicae in chemically defined media. Plant Growth Regul. 1998;24:7–11. doi: 10.1023/A:1005964031159. [DOI] [Google Scholar]
- Borriss R. Use of plant-associated bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari DK, editor. Bacteria in agrobiology: plant growth responses. Heidelberg: Springer; 2011. pp. 41–76. [Google Scholar]
- Burges HD. Control of insects by bacteria. Parasitology. 1982;84:79–117. doi: 10.1017/S0031182000053610. [DOI] [Google Scholar]
- Calvo P, Nelson L, Kloepper JW. Agricultural uses of plant biostimulants. Plant Soil. 2014;383:3–41. doi: 10.1007/s11104-014-2131-8. [DOI] [Google Scholar]
- Carvalhais LC, Dennis PG, Badri DV, Tyson GW, Vivanco JM, Schenk PM. Activation of the jasmonic acid plant defence pathway alters the composition of rhizosphere bacterial communities. PLoS ONE. 2013;8(2):e56457. doi: 10.1371/journal.pone.0056457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AC, Bottini R, Pontin M, Berli FJ, Moreno D, Boccanlandro H, Travaglia CN, Piccoli PN. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol Plant. 2015;153:79–90. doi: 10.1111/ppl.12221. [DOI] [PubMed] [Google Scholar]
- Correa OS, Montecchia MS, Berti MF, Ferrari MCF, Pucheu NL, Kerber BL, Garcia AF. Bacillus amyloliquefaciens BNM122, a potential microbial biocontrol agent applied on soybean seeds, causes a minor impact on rhizosphere and soil microbial communities. Appl Soil Ecol. 2009;41:185–194. doi: 10.1016/j.apsoil.2008.10.007. [DOI] [Google Scholar]
- Grover M, Madhubala R, Ali SZ, Yadav SK, Venkateswarlu B. Influence of Bacillus spp. strains on seedling growth and physiological parameters of sorghum under moisture stress conditions. J Basic Microbiol. 2014;54:951–961. doi: 10.1002/jobm.201300250. [DOI] [PubMed] [Google Scholar]
- Haas D, Defago G. Biological control of soil-borne pathogens by fluorescent Pseudomonas. Nat Rev Microbiol. 2005;3:307–319. doi: 10.1038/nrmicro1129. [DOI] [PubMed] [Google Scholar]
- Herrera-Medina MJ, Steinkellner S, Vierheilig H, Bote JAO, Garrido JMG. Abscisic acid determines arbuscule development and functionality in the tomato arbuscular mycorrhiza. New Phytol. 2007;175:554–564. doi: 10.1111/j.1469-8137.2007.02107.x. [DOI] [PubMed] [Google Scholar]
- Hong ZL, Lakkineni K, Zhang ZM, Verma DPS. Removal of feed back inhibition of delta-1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol. 2000;122:1129–1136. doi: 10.1104/pp.122.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen R, Rood S, Dormar J, McGill W. Azospirillum brasilense produces gibberellins in pure culture and chemically defined medium and in co-culture on straw. Soil Biol Biochem. 1992;24:1061–1064. doi: 10.1016/0038-0717(92)90036-W. [DOI] [Google Scholar]
- Joo GJ, Kim YM, Lee IJ, Song KS, Rhee IK. Growth promotion of red pepper plug seedlings and the production of gibberellins by Bacillus cereus, Bacillus macrolides and Bacillus pumilus. Biotechnol Lett. 2004;26:487–491. doi: 10.1023/B:BILE.0000019555.87121.34. [DOI] [PubMed] [Google Scholar]
- Kang SM, Joo GJ, Hamayun M, Na CI, Shin DH, Kim HY, Hong JK, Lee IJ. Gibberellin production and phosphate solubilization by newly isolated strain of Acinetobacter calcoaceticus and its effect on plant growth. Biotechnol Lett. 2009;31:277–281. doi: 10.1007/s10529-008-9867-2. [DOI] [PubMed] [Google Scholar]
- Kang SM, Khan AL, Hamayun M, Hussain J, Joo GJ, You Kim JG, Lee IJ. Gibberellin-producing Promicromonospora sp. SE188 improves Solanum lycopersicum plant growth and influences endogenous plant hormones. J Microbiol. 2012;50:902–909. doi: 10.1007/s12275-012-2273-4. [DOI] [PubMed] [Google Scholar]
- Kang SM, Radhakrishnan R, You YH, Joo GJ, Lee IJ, Lee KE, Kim JH. Phosphate solubilizing Bacillus Megaterium mj1212 regulates endogenous plant carbohydrates and amino acids contents to promote mustard plant growth. Indian J Microbiol. 2014;54:427–433. doi: 10.1007/s12088-014-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SM, Khan AL, Waqas M, You YH, Kim JH, Kim JG, Hamayun M, Lee IJ. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J Plant Interact. 2014;9:673–682. doi: 10.1080/17429145.2014.894587. [DOI] [Google Scholar]
- Kang SM, Khan AL, You YH, Kim JG, Kamran M, Lee IJ. Gibberellin production by newly isolated strain Leifsonia soli SE134 and its potential to promote plant growth. J Microbiol Biotechnol. 2014;24:106–112. doi: 10.4014/jmb.1304.04015. [DOI] [PubMed] [Google Scholar]
- Kang SM, Radhakrishnan R, Khan AL, Kim MJ, Park JM, Kim BR, Shin DH, Lee IJ. Gibberellin secreting rhizobacterium, Pseudomonas putida H-2-3 modulates the hormonal and stress physiology of soybean to improve the plant growth under saline and drought conditions. Plant Physiol Biochem. 2014;84:115–124. doi: 10.1016/j.plaphy.2014.09.001. [DOI] [PubMed] [Google Scholar]
- Kang SM, Radhakrishnan R, Lee KE, You YH, Ko JH, Kim JH, Lee IJ. Mechanism of plant growth promotion elicited by Bacillus sp. LKE15 in oriental melon. Acta Agric Scand B Soil Plant Sci. 2015;65(7):637–647. [Google Scholar]
- Kang SM, Radhakrishnan R, You YH, Khan AL, Park JM, Lee SM, Lee IJ. Cucumber performance is improved by inoculation with plant growth-promoting microorganisms. Acta Agric Scand B Soil Plant Sci. 2015;65(1):36–44. [Google Scholar]
- Kilian M, Steiner U, Krebs B, Junge H, Schmiedeknecht G, Hain R. FZB24®Bacillus subtilis-mode of action of a microbial agent enhancing plant vitality. Pflanzenschutz Nachr Bayer. 2000;1:72–93. [Google Scholar]
- Leung J, Giraudat J. Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:199–222. doi: 10.1146/annurev.arplant.49.1.199. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Lee IJ. Regulation of salicylic acid, jasmonic acid and fatty acids in cucumber (Cucumis sativus L.) by spermidine promotes plant growth against salt stress. Acta Physiol Plant. 2013;35:3315–3322. doi: 10.1007/s11738-013-1364-0. [DOI] [Google Scholar]
- Radhakrishnan R, Lee IJ. Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, jasmonic acid signals in soybean. J Plant Growth Regul. 2013;32:22–30. doi: 10.1007/s00344-012-9274-8. [DOI] [Google Scholar]
- Radhakrishnan R, Lee IJ. Effect of low dose of spermidine on physiological changes in salt-stressed cucumber plants. Russ J Plant Physiol. 2014;61:90–96. doi: 10.1134/S1021443714010129. [DOI] [Google Scholar]
- Radhakrishnan R, Khan AL, Lee IJ. Endophytic fungal pre-treatments of seeds alleviates salinity stress effects in soybean plants. J Microbiol. 2013;51:850–857. doi: 10.1007/s12275-013-3168-8. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Kang SM, Baek IY, Lee IJ. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J Plant Interact. 2014;9:754–762. doi: 10.1080/17429145.2014.930524. [DOI] [Google Scholar]
- Radhakrishnan R, Khan AL, Kang SM, Lee IJ. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann Microbiol. 2015;65:585–593. doi: 10.1007/s13213-014-0894-z. [DOI] [Google Scholar]
- Relwani L, Krishna P, Reddy MS. Effect of carbon and nitrogen sources on phosphate solubilization by a wild-type strain and UV-induced mutants of Aspergillus tubingensis. Curr Microbiol. 2008;57:401–406. doi: 10.1007/s00284-008-9212-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez H, Fraga R. Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv. 1999;17:319–339. doi: 10.1016/S0734-9750(99)00014-2. [DOI] [PubMed] [Google Scholar]
- Ruiz-Lozano JM, Porcel R, Aroca R. Evaluation of the possible participation of drought-induced genes in the enhanced tolerance of arbuscular mycorrhizal plants to water deficit. In: Varma A, editor. Mycorrhiza: state of the art, genetics and molecular biology, eco-function, biotechnology, eco-physiology, structure and systematics. Berlin: Springer; 2008. pp. 185–207. [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW. Bacterial volatiles promote growth in arabidopsis. Proc Natl Acad Sci USA. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin F, Cakmakci R, Kantar F. Sugar beet and barley yields in relation to inoculation with N2-fixing and phosphate solubilizing bacteria. Plant Soil. 2004;265:123–129. doi: 10.1007/s11104-005-0334-8. [DOI] [Google Scholar]
- Schreiner M, Korn M, Stenger M, Holzgreve L, Altmann M. Current understanding and use of quality characteristics of horticulture products. Sci Hortic. 2013;163:63–69. doi: 10.1016/j.scienta.2013.09.027. [DOI] [Google Scholar]
- Shoebitz M, Ribaudo CM, Pardo MA, Cantore ML, Ciampi L, Cura JA. Plant growth promoting properties of a strain of Enterobacter ludwigii isolated from Lolium perenne rhizosphere. Soil Biol Biochem. 2009;41:1768–1774. doi: 10.1016/j.soilbio.2007.12.031. [DOI] [Google Scholar]
- Solanki MK, Singh RK, Srivastava S, Kumar S, Kashyap PL, Srivastava AK. Characterization of antagonistic-potential of two Bacillus strains and their biocontrol activity against Rhizoctonia solani in tomato. J Basic Microbiol. 2015;55:82–90. doi: 10.1002/jobm.201300528. [DOI] [PubMed] [Google Scholar]
- Spaepen S, Vanderleyden J, Okon Y. Plant growth promoting actions of rhizobacteria. Adv Bot Res. 2009;51:283–320. doi: 10.1016/S0065-2296(09)51007-5. [DOI] [Google Scholar]
- Starnes RL, Liu CL, Marrone PG. History, use, and future of microbial insecticides. Am Entomol. 1993;39:83–91. doi: 10.1093/ae/39.2.83. [DOI] [Google Scholar]
- Sturz AV, Christie BR, Novak J. Bacterial endophytes: potential role in developing sustainable system of crop production. Crit Rev Plant Sci. 2000;19:1–30. doi: 10.1016/S0735-2689(01)80001-0. [DOI] [Google Scholar]
- Theodorou ME, Plaxton WC. Metabolic adaptations of plant respiration to nutritional phosphate deprivation. Plant Physiol. 1993;101:339–344. doi: 10.1104/pp.101.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil. 2003;255:571–586. doi: 10.1023/A:1026037216893. [DOI] [Google Scholar]
- Weston DJ, Pelletier DA, Morrell-Falvey JL, Tschaplinski TJ, Jawdy SS, Lu TY, Allen SM, Melton SJ, Martin MZ, Schadt CW, Karve AA, Chen JG, Yang X, Doktycz MJ, Tuskan GA. Pseudomonas fluorescens induces strain-dependent and strain-independent host plant responses in defense networks, primary metabolism, photosynthesis, and fitness. Mol Plant Microbe Interact. 2012;25(6):765–778. doi: 10.1094/MPMI-09-11-0253. [DOI] [PubMed] [Google Scholar]
- Wilson MK, Abergel RJ, Raymond KN, Arceneaux JEL, Byers BR. Siderophores of Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis. Biochem Biophys Res Commun. 2006;348:320–325. doi: 10.1016/j.bbrc.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S. Gibberellin metabolism and its regulation. Annu Rev Plant Biol. 2008;59:225–251. doi: 10.1146/annurev.arplant.59.032607.092804. [DOI] [PubMed] [Google Scholar]
- Zribi K, Djebali N, Mrabet M, Khayat N, Smaoui A, Mlayah A, Aouani ME. Physiological responses to cadmium, copper, lead, and zinc of Sinorhizobium sp. strains nodulating Medicago sativa grown in Tunisian mining soils. Ann Microbiol. 2012;62:1181–1188. doi: 10.1007/s13213-011-0358-7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.