Abstract
The objective of this study was to investigate the effectiveness of calcium chloride (CaCl2), as potential elicitor, on tomato plants against Fusarium oxysporum f. sp. lycopersici. Foliar application of CaCl2 showed significant reduction of wilt incidence after challenge inoculation. Increased production of defense and antioxidant enzymes was observed in elicitor treated sets over control. Simultaneously, altered amount of phenolic acids were analyzed spectrophotometrically and by using high performance liquid chromatography. Significant induction of defense-related genes expressions was measured by semi-quantitative RT-PCR. Greater lignifications by microscopic analysis were also recorded in elicitor treated plants. Simultaneously, generation of nitric oxide (NO) in elicitor treated plants was confirmed by spectrophotometrically and microscopically by using membrane permeable fluorescent dye. Furthermore, plants treated with potential NO donor and NO modulators showed significant alteration of all those aforesaid defense molecules. Transcript analysis of nitrate reductase and calmodulin gene showed positive correlation with elicitor treatment. Furthermore, CaCl2 treatment showed greater seedling vigor index, mean trichome density etc. The result suggests that CaCl2 have tremendous potential to elicit defense responses as well as plant growth in co-relation with NO, which ultimately leads to resistance against the wilt pathogen.
Keywords: Defense enzymes, Gene expression, High performance liquid chromatography, Seedling vigor index, Wilt
Introduction
Tomato (Lycopersicon esculentum Mill.) is the second most economically important vegetable crop cultivated throughout the world. It has immense nutritional value and antioxidant properties (Nahar and Ullah 2012). The crop is susceptible to over 200 plant diseases of which mostly are of fungal in nature. In field condition, yield of tomato is severely hampered by wilt disease caused by Fusarium oxysporum f. sp. lycopersici (Sacc) Snyder et Hansen (Medeiros et al. 2009; Agrios 2005; Srivastava et al. 2010). This pathogen occurs throughout most tomato-growing areas and infects the secondary root hairs, wherein it penetrates the epidermis and finally it progresses the xylem vessels through the pits and clogs the path for water transport (Zvirin et al. 2010). It is very difficult to eradicate the pathogen from soil because they produce resting spore (chlamydospores) that can survive for many years (Amini 2009). Although, application of fungicides as well as soil fumigators are the regular practice but fruitful results may not come since the disease occurs in the later stages of crop growth when persistence and effectiveness of the applied fungicides are doubtful. Furthermore, biological control by using of plant growth promoting rhizobacterial (PGPR) strains represent a potential alternative disease management approach since PGPR are recognized for growth promotion and disease reduction in crops (Jetiyanon and Kloepper 2002; Acharya et al. 2011a). However, use of a single biocontrol agent often showed conflicting results when applied to the field as it is less liable to be active in diverse soil surroundings and agricultural ecosystems (Raupach and Kloepper 1998; Shanmugam and Kanoujia 2011). So, in the present scenario of global food demand and environmental consciousness, significant research efforts have been shifted to search the alternative non-hazardous compounds, which are capable of triggering plant immune responses for longer period of time (Abdel-Kader et al. 2012; Biswas et al. 2012; Chandra et al. 2014a). According to earlier reports both primary elicitors derived from pathogen and secondary endogenous signals may activate a varied array of plant defense related genes, encoding antioxidant enzymes like peroxidases (PO), glutathione S-transferases (GST), cell wall components, pathogenesis-related proteins, proteinase inhibitors, hydrolytic enzymes, phytoalexin biosynthetic enzymes etc. (Wang et al. 2010). Over expression of defense related genes like thaumatin from rice has been demonstrated to reduce infection of rice by Rhizoctonia solani (Grover and Gowthaman 2003) and of carrot by Alternaria dauci, A. petroselini, A. radicina, Botrytis cinerea, R. solani, and Sclerotinia sclerotiorum (Punja 2005).
Calcium plays an essential function in plant growth and also acts as a universal second messenger involved in various aspects of biotic and abiotic stress responses in plants (Chandra et al. 2014a; Peng et al. 2014). Earlier reports revealed that nitric oxide (NO), calcium (Ca2+)/calmodulin (CaM) and other signaling molecules are required for induced up and down regulation of the expression of plant defense related molecules like PR-protein, phenol, antioxidant enzymes etc. (Sang et al. 2008; Zhang et al. 2009; Ma et al. 2012). Tian et al. (2006) showed that application of CaCl2 as an abiotic elicitor reduces post-harvest damages of pear fruits by induction of the amplified level of defense enzymes activities. Elevation of cytosolic Ca2+ signature has been reported as an important event during host pathogen interaction which induces innate immune responses of plant (Dangl et al. 1996). Previously, we also have shown that foliar application of CaCl2 reduces blister blight incidence of tea plants by activating defense related gene expression, accumulation of different enzymes and phenols along with higher production of NO in leaves (Chandra et al. 2014a). However, the interrelationship between CaCl2 and NO needs further investigation.
Under these circumstances, the main objective of this study were to evaluate the potentiality of calcium chloride to induce resistance against Fusarium wilt disease in tomato plant by activation of defense enzymes, phenols, flavonoids etc. and also to find out the signaling role of NO in this process.
Materials and methods
Plant material
Seeds of tomato (Globe beauty cultivar) were surface sterilized with 0.1% mercuric chloride for 3 min and then washed with sterile distilled water for three times. Sterilized seeds were then planted in the earthen pots (6 × 6 × 10 cm) with a potting mixture (clay/coco peat/sand, 3:2:1, v/v). Plants were maintained at 24 ± 2 °C and 12 h photoperiod. The plants watered on every alternate day and with a balanced nutrient solution once a week according to (Chakraborty et al. 2015b).
Pathogen
Fusarium oxysporum f. sp. lycopersici was maintained in Potato Dextrose Agar medium. Fungal inoculum was prepared with sterile distilled water according to Manzo et al. (2016). Pathogen was grown on PDA for 15 days at 25 °C. Petri dishes containing pathogen were flooded with 10 ml of sterile distilled water. Conidia were scraped out using sterile spatulas and kept in sterile 50 ml tubes. The conidial suspensions were then adjusted to a final concentration of 1 × 103 conidia ml−1 by hemocytometer under light microscope.
Treatment
Forty-five days old tomato plants were sprayed with CaCl2 at a concentration 0.5% (Chakraborty et al. 2015b). The concentration was selected on the basis of the previous study (Chakraborty et al. 2016). To analyze the participation of NO in the regulation of defense response by CaCl2, the sets were also primed as follows: CaCl2 (0.5%) + L-NAME (10 µM); CaCl2 (0.5%) + C-PTIO (100 µM); SNP (100 mM) and CaCl2 (0.5%) + L-NAME (10 µM) + C-PTIO (100 µM). Here, Sodium nitroprusside (SNP) act as a potential NO donor, NG-nitro-l-arginine methyl ester (L-NAME) acts as nitric oxide synthase (NOS) inhibitor and 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (C-PTIO) acts as NO scavenger. NOS inhibitor and NO scavenger were used 8 h prior to the application of elicitor or pathogen in respective sets. Treatment of same-aged plants with distilled water served as control. Each experiment was carried out with three replications and ten plants at time.
Enzyme assays
The leaf tissue was collected from different sets of plants 48 h (except the only pathogen treated set) after treatment and was grounded in mortar under liquid nitrogen. 500 mg of fine powder from each set was suspended in 2 ml of extraction buffer containing 0.1% polyvinylpyrrolidone and 20 ml of 1 mM phenylmethylsulfonyl fluoride: 0.1 M of sodium acetate buffer (pH 5.0) for β-1,3 glucanase and chitinase; 0.1 M sodium borate buffer (pH 8.7) for PAL; and 0.1 M of sodium phosphate buffer (pH 7.0) for PO, PPO, CAT and APX. All the extraction procedures were conducted at 4 °C. The sample was centrifuged at 12,000×g for 20 min at 4 °C. The supernatants were used as the crude enzyme source for the enzymatic assay. Then it was transferred to a 2 ml microcentrifuge tube and stored at −80 °C for further use.
Peroxidase assay (PO)
PO activity was carried out, following the method of Hemeda and Klein (1990). Substrate was prepared by mixing of 5 ml of 1% guaiacol, 5 ml of 0.3% H2O2 and 50 ml of 0.05 M sodium phosphate buffer (pH 6.5). The reaction mixture contained 2.95 ml of prepared substrate and 0.05 ml of enzyme extract and change in absorption was measured at 470 nm for 3 min. PO activity was determined by the increase in the absorbance due to guaiacol oxidation and was expressed as µmol min−1 mg−1 of protein (E = 26.6 mM−1 cm−1).
Polyphenol oxidase assay (PPO)
PPO activity was estimated using the method of Kumar and Khan (1982). The reaction mixture was prepared by 2 ml of 0.1 M sodium phosphate buffer (pH 6.5), 0.5 ml of crude enzyme extract and 1 ml of 0.1 M catechol. The assay mixture was then incubated for 10 min at room temperature. Reaction was stopped by adding 1 ml of 2.5 N H2SO4. The absorption of purpurogallin formed was examined at 495 nm. The blank was prepared by adding 2.5 N H2SO4 at zero time for the same assay mixture. The PPO activity was expressed in U min−1 mg−1 protein (U = change in 0.1 absorbance min−1 mg−1 protein).
Phenylalanine ammonia-lyase assay (PAL)
PAL activity was determined according to the method of Dickerson et al. (1984). 200 μl of crude enzyme extract was incubated with 1.3 ml of 0.1 M borate buffer (pH 8.7) and 0.5 ml of 12 mM l-phenyl alanine for 30 min at 30 °C. The absorbance was measured at 290 nm. Enzyme activity was expressed as production of Transcinnamic acid (in nmol quantities) min−1 g−1 protein.
β-1,3-glucanase assay
β-1,3-glucanase activity was assayed according to Pan et al. (1991). 50 µl of crude enzyme extract was mixed with equal amount of the substrate (1% Laminarin) and was kept for 1 h at room temperature. After incubation the reaction was blocked by adding 300 µl of Dintrosalicylic acid reagent and followed by boiling for 10 min. The resulting colored solution was diluted with the addition of distilled water to make the total volume up to 2 ml and vortexed and the absorption was measured at 520 nm. The blank set was prepared with equal amounts of crude enzyme and laminarin without incubation. The enzyme activity was expressed as μmol of glucose produced min−1 g−1 protein.
Chitinase assay
Chitinase assay was performed according to the method of Bansode and Bajekals (2006) with slight modifications. The reaction mixture was prepared with 2 ml sodium acetate buffer (pH-5.0), 2 ml of substrate (1% colloidal chitin) and crude enzyme extract (0.5 ml). The mixture was then incubated for 1 h at room temperature. The reaction was stopped by adding 2.5 ml of 10% dinitrosalicylic acid reagent and followed by heating in a boiling water bath for 5 min. The mixture was then centrifuged at 10,000×g for 10 min. The absorption of the supernatant was measured at 540 nm. The enzyme activity was expressed as μmol of glucose equivalent released min−1 g−1 protein.
Ascorbate peroxidase assay (APX)
APX activity was determined according to Nakano and Asada (1981). The reaction mixture prepared by adding 50 mM potassium phosphate (pH 7.0), 0.2 mM EDTA, 0.5 mM ascorbic acid, 2% H2O2, and 0.1 ml enzyme extract in a final volume of 3 ml. The decrease in absorbance at 290 nm for 1 min was recorded and the amount of ascorbate oxidized was calculated using extinction coefficient (Є = 2.8 mM−1 APX was defined as 1 mmol ml−1 per min cm−1). One unit of ascorbate oxidized as 1 mmol ml−1 ascorbate oxidized per min.
Catalase assay (CAT)
CAT was measured following the technique of Cakmak and Horst (1991) with slight modifications. The reaction mixture prepared with enzyme extract (100 μl), 50 μl of hydrogen peroxide (0.3%) and final volume was made up to 3 ml by adding up of phosphate buffer (50 mM, pH-7.0). Decrease in absorbance was recorded for 3 min for at 240 nm. The enzyme activity was expressed as nmol min−1 g−1 of protein with help of a molar extinction coefficient Є = 39,400 M−1 cm−1.
Estimation of total protein content
The standard Bradford assay (1976) was employed, using bovine serum albumin as a standard, to test the protein concentration of each extract.
Estimation of Total Phenol
Estimation of total phenol was determined following the method of Zieslin and Ben Zaken (1993). 250 mg of fresh leaf tissue was homogenized in 2 ml of 80% methanol and the material was maintained at 65 °C for 15 min. The sample was then centrifuged at 10,000×g for 10 min at room temperature and the supernatant was collected and used to estimate the phenol content. The reaction mixture was prepared by adding up 1 ml of crude sample extract to the mixture of 5 ml distilled water and 250 µl of 1 N Folin ciocalteu reagent. The reaction mixture was incubated for 30 min at room temperature. Phenolic content was measured at 725 nm using standard curve of gallic acid. The amount of total phenol was expressed as μg gallic acid produced g−1 tissue.
Estimation of total flavonoid content
Total flavonoid content was determined by following the method of Chang et al. (2002). 150 mg of fresh leaf tissue was grounded in 2 ml of 80% ethanol and the material was incubated in dark place for 30 min. It was then centrifuged at 10,000×g for 5 min at room temperature. The reaction mixture was prepared with 1 ml of crude extract (supernatant) mixed with 4.3 ml of 80% aqueous ethanol, 0.1 ml of 10% aluminum nitrate, and 0.1 ml of 1 M aqueous sodium acetate. The reaction mixture was then kept in dark place for 30 min. Absorption was measured at 415 nm. The amount of total flavonoid was expressed as mg g−1 of fresh tissue.
Nitric oxide estimation (NO)
Production of NO was measured by haemoglobin assay according to the method of Delledonne et al. (2001). Leaf tissues of control and treated sets were incubated in a reaction mixture containing 10 mM l-arginine and 10 mM haemoglobin in a total volume of 5 ml of 0.1 M phosphate buffer (pH 7.4). Production of NO was measured spectrophotometrically at 401 nm and NO levels were calculated using an extinction coefficient of 38,600 M−1 cm−1. After 2 h of incubation, NO content in the reaction mixture was measured as nmol of NO produced g−1 tissue h−1.
Real time NO production was observed by using membrane permeable flurochrome 4-5 diaminofluorescein diacetate (DAF-2DA) dye (Bartha et al. 2005). Lower epidermis of leaf was peeled off and placed in a brown bottle containing 1 ml of loading buffer prepared by 10 mM KCl, 10 mM Tris HCl (pH 7.2) with DAF-2DA at a final concentration of 10 mM. It was then incubated for 20 min in dark. Fluorescence was observed with Lieca DMLS microscope at excitation wavelength 480 nm and emission wavelength 500–600 nm. Green fluorescence indicates the generation of NO.
Quantification of phenolic compounds by HPLC
Leaf samples collected from different sets tomato plants after 48 h of incubation. Samples were crushed in HPLC grade methanol (100%) and prepared for phenolic acid estimation with an HPLC system (Agilant, USA) equipped with a DAAD detector and an Agilent Eclipse plus C18 column (100 mm × 4.6 mm, 3.5 μm). Separation was achieved using a flow rate of 0.8 ml/min at 25 °C. The mobile phase consisted of eluent A (acetonitrile) and eluent B (aqueous phosphoric acid solution, 0.1% v/v). A gradient program was used for elution: 0–2 min, 5% A; 2–5 min, 15% A; 5–10 min, 40% A; 10–15 min, 60% A; 15–18 min, 90% A (Khatua et al. 2015). Gallic acid, p-coumaric acid, myricetin, caffeic acid, vanillic acid, ferulic acid, quercetin, cinnamic acid and pyrogallol (M.P. Biomedicals. USA) were used as standards according to Khatua et al. (2015). The DAAD detection was conducted at 278 nm for the quantification (Chandra et al. 2014a). Sample compounds were identified on the basis of absorption spectra and retention times of standard materials. A concentration of each compound was determined by comparing peak areas of reference compounds with those in the samples run under the same elution conditions.
Analysis of defense-related gene expression by semiquantitative RT-PCR
Expression of defense related and antioxidative genes were analyzed by semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR). Total RNA was extracted from different sets of tomato leaves after 48 h of incubation with TRIzol Reagent (Invitrogen, USA). The cDNA was synthesized from the total RNA according to Chandra et al. (2014a). To analyze the expression of a specific gene, 1 µl of the cDNA was taken in a 20 µl PCR mixture containing 1 × DreamTaq PCR buffer, 0.2 mM of each dNTPs, 1 µM of each gene specific primer, and 1 units of Dream Taq DNA polymerase (Fermentas, USA). PCR cycles were carried out under the following conditions: 94 °C for 4 min, then 30 cycles of 94 °C for 30 s, annealing temperature (Tm) for specific primer for 30 s and 72 °C for 60 s with a final extension step of 7 min at 72 °C in a thermal cycler (Applied BioSystem, USA). List of used primers are presented in Table 1. PR-1b (basic PR1), PR-2a (acidic glucanase), PR-2b (basic glucanase), PR-3a (Chitinase 3, acidic), PR-3b (Chitinase 9, basic), PR-5 (Osmotin-like), PR-7 (P69A, subtulisin-like), PAL, Prot In (Proteinase inhibitor), PO, GST (Glutathione-S-transferase), CAM (Calmodulin) and NR (Nitrate reductase) genes were amplified individually (Van Kan et al. 1992; Danhash et al. 1993; Tornero et al. 1996; Rep et al. 2002; Peng et al. 2014; Sanz-Alférez et al. 2008; Jin et al. 2009; Medeiros et al. 2009). The primer pair for the glyceraldehyde phosphate dehydrogenase (GAPDH) gene was used as an internal control (Shih et al. 1992). Linearity between the amount of input RNA and the final RT-PCR products was verified and confirmed. The PCR products were resolved in 1% agarose gel, stained with ethidium bromide, visualized and photographed in Bio-Rad Gel Doc EZ Imager system. Densitometric analysis of the photographed gels was carried out by ImageJ software.
Table 1.
List of primers used
| Gene of interest | Sequences of primers | Tm (°C) | References |
|---|---|---|---|
| PR-1b (basic PR1) | FOR 5′ CCAAGACTATCTTGCGGTTC 3′ | 55 | Van Kan et al. (1992) |
| REV 5′ GAACCTAAGCCACGATACCA3′ | |||
| PR2a (acidic glucanase) | FOR 5′ TATAGCCGTTGGAAACGAAG3′ | 55 | Van Kan et al. (1992) |
| REV 5′ TGATACTTTGGCCTCTGGTC3′ | |||
| PR2b (basic glucanase) | FOR 5′ CAACTTGCCATCACATTCTG 3′ | 52 | Van Kan et al. (1992) |
| REV 5′ CCAAAATGCTTCTCAAGCTC 3′ | |||
| PR 3a (chitinase 3, acidic) | FOR 5′ CAATTCGTTTCCAGGTTTTG 3′ | 52 | Danhash et al. (1993) |
| REV 5′ ACTTTCCGCTGCAGTATTTG3′ | |||
| PR 3b (chitinase 9, basic) | FOR 5′ AATTGTCAGAGCCAGTGTCC 3′ | 59 | Danhash et al. (1993) |
| REV 5′ TCCAAAAGACCTCTGATTGC 3′ | |||
| PR 5 (osmotin-like) | FOR 5′ AATTGCAATTTTAATGGTGC 3′ | 49 | Rep et al. (2002) |
| REV 5′ TAGCAGACCGTTTAAGATGC 3′ | |||
| PR 7 (P69A, subtulisin-like) | FOR 5′ AACTGCAGAACAAGTGAAGG 3′ | 50 | Tornero et al. (1996) |
| REV 5′ AAC GTGATTGTAGCAACAGG 3′ | |||
| PAL | FOR 5′ TTCAAGGCTACTCTGGC 3′ | 52 | Peng et al. (2014) |
| REV 5′ CAAGCCATTGTGGAGAT 3′ | |||
| Prot In (proteinase inhibitor) | FOR 5′ CGGAGAATCTGAATGGGTAAGCGA 3′ | 63 | Medeiros et al. (2009) |
| REV 5′ ACAAGCCGTGGTAAAGGTCCACAA 3′ | |||
| PO (peroxidase) | FOR 5′ ACGGAGCAAGCGACAATTGACAAC 3′ | 65 | Medeiros et al. (2009) |
| REV 5′ CGATTGATTCACCGCAAAGCTCGT 3′ | |||
| GST (glutathione-S-transferase) | FOR 5′ TGTCCCAACCTTCTCGTGCAGTTA 3′ | 65 | Medeiros et al. (2009) |
| REV 5′ TGAGTGATGCCAGTCCAACACAGA 3′ | |||
| Calmodulin | FOR 5′ GCACGGAAGATGAAGGACAC 3′ | 55 | Sanz-Alférez et al. (2008) |
| REV 5′ GCAAGCATCATACGGACAAAC 3′ | |||
| NR | FOR 5′ CAAGCAATCCATCTCCCAT 3′ | 57 | Jin et al. (2009) |
| REV 5′ CATCTCTGTATCGTCTTCAGGA 3′ | |||
| GAPDH (glyceraldehyde phosphate dehydrogenase) | FOR 5′ GAAATGCATCTTGCACTACCAACTGTCTTGC 3′ | 63 | Shih et al. (1992) |
| REV 5′ CTGTGAGTAACCCCATTCATTATCA TACCAAGC 3′ |
Efficacy of CaCl2 against Fusarium wilt
Percent disease incidence was measured according to Ramamoorthy et al. (2002). Thirty-five days old seedlings were transplanted in earthen pots filled with sterilized potting soil containing balanced macro and micro nutrient solutions. Ten days after transplanting elicitor solution (CaCl2-0.5%) was sprayed on the leaves until runoff. Two days after foliar application of elicitor, 60 ml of conidial suspension (103 microconidia ml−1) of F. oxysporum f. sp. lycopersici was poured per pot. After 15 days of elicitor application second spray was done. Plants sprayed with sterile water and inoculated with microconidial suspension was considered as control. Wilt incidence was recorded 30 days after inoculation using following formula:
Percent Disease Incidence (PDI) = (Number of diseased plants/Total number of plants) × 100
Ten pots per replication were maintained and there were three replications. The experiment was repeated thrice.
Staining of lignin
To measure lignin production in the stems of elicitor treated plants, fresh sections were left for 5 min in 2% phloroglucinol in 95% ethanol and mounted in 6 N HCl (Vallet et al. 1996). Appearance of reddish-pink product ensures the presence of lignin in the tissue. Phloroglucinol in acidic condition reacts with mainly the cinnamaldehyde groups present in lignin and give colored product.
Effect of elicitor on physiological parameters of tomato
To study the effect of elicitor on certain physiological parameters tomato seeds were first surface sterilized with 0.1% HgCl2 for 2 min and followed by three washings with the sterile distilled water. Sterilized seed were dipped in the solution of CaCl2 (0.5%) and SNP (100 μM) for 8 h and were placed on a wet blotting paper to study their effect on seed germination and seedling vigor index. The blotting papers were incubated at 24 °C with relative humidity above 85%. Number of germinated seedling was recorded after 14 days and mean seed germination was calculated according to Raut et al. (2014). The root and shoot length (cm) of randomly selected 10 normal seedlings was measured and seedling vigor index was calculated (Raut et al. 2014). Total chlorophyll of elicitor treated and untreated plants was estimated following Arnon’s method (Arnon 1949) with slight modification as described by Chakraborty et al. (2015a, b). Mean trichome density of elicitor treated and untreated plants was measured according to Boughton et al. (2005). Yield of the elicitor treated and untreated control plant was compared according to Nahar and Ullah (2012).
Statistics
All data presented were mean ± standard deviation (S.E.) of three replicates. Statistical analyses were performed by analysis of variance (ANOVA) using SPSS software version 20 (Chakraborty et al. 2015b) and the significance of difference between the treatments was determined using Duncan’s Multiple Range Test (p < 0.05).
Results
Effects of CaCl2 on defense enzyme activity in tomato plant
Foliar application of CaCl2 at a concentration 0.5% showed increased amount of all the defense enzymes production in tomato leaves compared to water treated control. Elicitor treatment showed higher amount of PO, PPO, PAL, β-1,3-glucanase and chitinase production along with elevated level of antioxidative enzymes like CAT and APX. Among all the treatments, tomato plants pre-treated with CaCl2 (0.5%) showed highest inductive ability for all the defense enzymes. The induction of the PPO and chitinase was significantly higher, and about 4 and 3.6 fold increase in enzyme production was observed in tomato plants treated with CaCl2 over the water treated control set (Table 2). Similarly, accumulation of PO, PAL and β-1,3 glucanase activity was noted 2.71, 2.16 and 3.1 fold higher in elicitor treated plants than control, respectively (Table 2). This results also coincide with increased production of antioxidative enzymes. In CaCl2 treated plants 4.83 and 1.61-fold increased amount of APX and CAT enzyme activity was recorded when compared to water treated control plant (Table 2). Compare to other sets plants treated with both the NO modulator (C-PTIO and L-NAME) and elicitor showed significant reduction for all the enzymes studied compared to control plant.
Table 2.
Effect of foliar application of CaCl2 (0.5%), SNP (100 μM), C-PTIO (100 μM), L-NAME (10 μM) and pathogen inoculation alone or in different combinations on the production of defense enzymes, Phenol and flavonoid in leaves of tomato
| Enzymes | Control | CaCl2 | CaCl2 + L-NAME | CaCl2 + C-PTIO | SNP | CaCl2 + L-NAME + C-PTIO |
|---|---|---|---|---|---|---|
| Peroxidase (PO) [µmol min−1 mg−1 protein] | 2.53 ± 0.2c | 6.11 ± 0.54b | 2.01 ± 0.16cd | 1.56 ± 0.28de | 6.1 ± 0.43b | 1.3 ± 0.2de |
| Polyphenol oxidase (PPO) [U min−1 mg−1 protein] | 33.96 ± 4.1c | 124.1 ± 16ab | 22 ± 6.69c | 26.18 ± 5.5c | 104.96 ± 13.62b | 19.14 ± 3.51c |
| Phenylalanine ammonia-lyase (PAL) [nmol of transcinnamic acid min−1 g−1 protein] | 114.35 ± 9.9c | 224.79 ± 16.43a | 90.22 ± 10.29d | 74.69 ± 6.31de | 192.28 ± 12.23b | 48.09 ± 9.07f |
| β-1,3-glucanase [µmol glucose produced min−1 g−1 protein] | 28.8 ± 5.33c | 88.51 ± 11.88a | 26.21 ± 6.67c | 20.71 ± 4.29c | 66.37 ± 11.83b | 17.52 ± 2.09c |
| Chitinase [µmol glucose equivalent released min−1 g−1 protein] | 0.85 ± 0.05d | 2.37 ± 0.24b | 0.62 ± 0.08de | 0.53 ± 0.14e | 2.08 ± 0.09c | 0.38 ± 0.06e |
| Ascorbate peroxidase (APX) [µmol min−1 g−1 protein] |
0.18 ± 0.01c | 0.69 ± 0.03b | 0.15 ± 0.03cd | 0.12 ± 0.02cd | 0.85 ± 0.05a | 0.11 ± 0.03d |
| Catalase (CAT) [nmol min−1 g−1 protein] |
6.66 ± 0.25c | 10.34 ± 1.1b | 4.86 ± 0.53d | 3.97 ± 0.64d | 12.27 ± 0.68a | 3.35 ± 0.35d |
| Total phenol [μg gallic acid produced g−1 tissue] |
283.43 ± 35.19b | 564.11 ± 41.98a | 210.15 ± 13.24bc | 157.36 ± 15.71c | 550.74 ± 43.14a | 225.38 ± 61.81bc |
| Total flavonoid[mg g−1 of the tissue] | 740.73 ± 87.2c | 1254.01 ± 12.79b | 474.42 ± 85.53d | 425.96 ± 59.73de | 1381.17 ± 128.25b | 327.66 ± 30.88e |
Values represent mean ± SE of three separate experiments, each in triplicate
CaCl 2 Calcium chloride, SNP sodium nitroprusside, C-PTIO 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, L-NAME NG-nitro-l-arginine methyl ester
Different letters within the row indicate significant difference (p < 0.05) from the control set using Duncan’s multiple range test. Same letter within the row denotes no significant difference between the groups
Effects of CaCl2 on defense-related gene expression in tomato plant
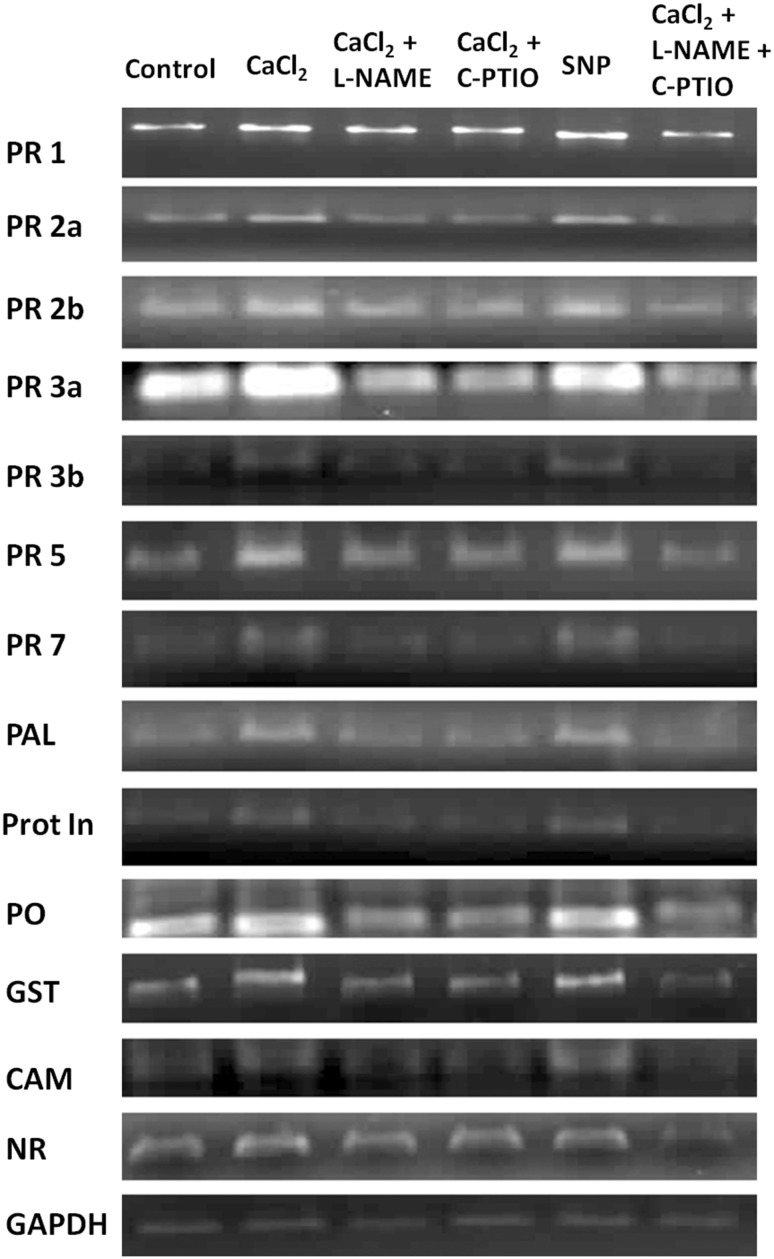
To justify spectrophotometric results and also to elucidate effect of elicitor treatment on defense related genes, expression in the transcript level was determined by semi-quantitative RT-PCR analysis. Differential alteration of defense-related genes like PR-1, PR-2a, PR-2b, PR-3a, PR-3b, PR-5, PR-7, PAL, Prot In, PO, GST, CAM and NR expression in different treated tomato plants (Fig. 1). Elevated defense enzyme productions were also reflected in the gene expression analysis in different treated tomato plants. From the Fig. 1 it is clearly observed that for all the genes examined, marked change observed in CaCl2 sprayed tomato plants. Higher amount of GST gene expression in elicitor treated set implies greater protection of plants against oxidative stress. Furthermore, the expression analysis of NR gene revealed that CaCl2 also have inductive role in overexpression of nitrite reductase gene for the higher accumulation of NO which might act as a signaling compound in downstream gene expression system. Among all the sets NO scavenger and NOS inhibitor treated plants showed significant decreased expression of mRNA for all the tested genes.
Fig. 1.
Semi-quantitative RT-PCR analyses of different sets treated with CaCl2, Pathogen and NO modulators either alone or in combinations. Expression of different defense related PR genes, antioxidant enzyme-coding genes (PO, GST), calcium sensor gene (CAM) and gene of nitrate reductase (NR) are represented in the subsequent order of treatment. GAPDH band represents internal control
Effects of CaCl2 on production of total phenol and flavonoid in tomato plant
Studies on induction of phenolic compounds revealed that higher accumulation total phenol and flavonoid levels were observed in CaCl2 treated tomato plants challenge inoculated by F. oxysporum f. sp. lycopersici as well as in the plants solely sprayed with CaCl2. The maximum accumulation of total phenol and flavonoid was recorded as 2.1 and 2-fold higher in elicitor treated sets than control plants, respectively (Table 2). However, NO modulator treated plants showed minimum amount of phenolic compound production. Furthermore, through HPLC analysis specific phenolic acids were quantified from the total phenol extracts of different sets of treated tomato plants. Phenolic compounds present in the samples were identified by comparing both retention times and UV–Vis spectra with those of pure standards (Table 3). Production of gallic acid, p-coumaric acid and vanillic acid were not found in control plants but those compounds were observed in different sets of treated plants (Table 3). Among all the sets significant increase of all the phenolic compounds were found to be higher in the plants sprayed with CaCl2. Production of caffeic acid, ferulic acid, myricetin, quercetin, cinnamic acid and pyrogallol were noticed 26.33, 55.56, 8.46, 26.11, 27.69 and 2.17 fold higher than untreated control in CaCl2 treated set.
Table 3.
Quantitative analysis of phenolic acid content in tomato leaves due to foliar application of CaCl2 (0.5%), SNP (100 μM), C-PTIO (100 μM), L-NAME (10 μM) and pathogen inoculation alone or in different combinations
| Standard compounds | Quantity (µg/g of fresh weight) | |||||
|---|---|---|---|---|---|---|
| Control | Cacl2 | CaCl2 + L-NAME | CaCl2 + C-PTIO | SNP | CaCl2 + L-NAME + C-PTIO | |
| Gallic acid | NF | 25.84 ± 1.36b | NF | NF | 20.89 ± 0.59c | 4.62 ± 0.70d |
| Caffeic acid | 2.58 ± 0.30f | 67.95 ± 0.87c | 6.64 ± 0.79d | 3.77 ± 0.18ef | 80.03 ± 1.07a | 5.02 ± 0.42e |
| Vanillic acid | NF | 36.12 ± 0.86a | 12.36 ± 0.68c | 10.69 ± 0.72d | 27.22 ± 0.34b | NF |
| P-Coumaric acid | NF | 79.57 ± 2.48b | 26.03 ± 0.40f | 22.05 ± 0.36g | 59.86 ± 0.79c | 4.53 ± 0.17h |
| Ferulic acid | 2.67 ± 0.30h | 148.36 ± 3.95b | 55.91 ± 0.58d | 45.21 ± 0.60e | 111.28 ± 1.33c | 5.47 ± 1.07g |
| Myricetin | 6.74 ± 0.71d | 57.07 ± 1.81a | NF | NF | 14.81 ± 0.64c | NF |
| Quercetin | 4.92 ± 0.28f | 128.47 ± 2.0b | 15.02 ± 0.40d | 11.44 ± 0.87e | 130.47 ± 0.98a | 4.4 ± 0.32f |
| Cinnamic acid | 2.57 ± 0.28e | 71.17 ± 4.11b | 8.01 ± 0.68d | 4.79 ± 0.45e | 56.70 ± 0.86c | NF |
| Pyrogallol | 3.62 ± 0.31d | 7.88 ± 1.84b | 12.93 ± 0.71a | 9.06 ± 0.44b | 6.28 ± 0.36c | 4.88 ± 0.23d |
Values represent mean ± SE of three separate experiments, each in triplicate
CaCl 2 Calcium chloride, SNP sodium nitroprusside, C-PTIO 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, L-NAME NG-nitro-l-arginine methyl ester, NF not found
Different letters within the row indicate significant difference (p < 0.05) from the control set using Duncan’s multiple range test. Same letter within the row denotes no significant difference between the groups
Effects of CaCl2 on production of NO in tomato plant
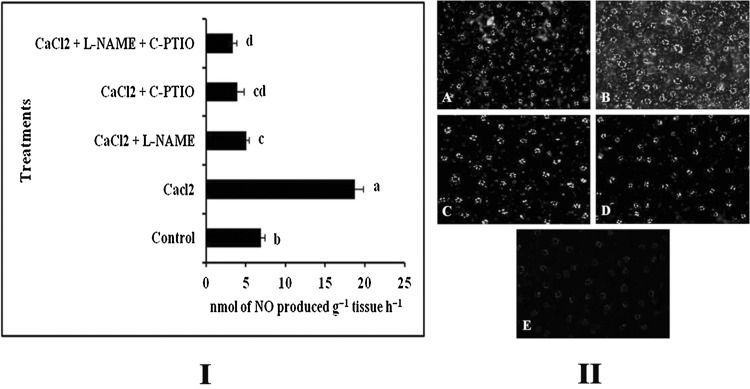
It has already been well-known that NO plays a key role to modulate the immune responses in plants during elicitor treatment (Chakraborty et al. 2015b; Chandra et al. 2014a, 2015). To investigate whether increase of defense in our model plant by CaCl2 is NO mediated, we checked the NO production in both CaCl2 and various combinations of NO modulator treated tomato leaves and compared them with the water treated control. In CaCl2 treated plants, an increased level of NO production was observed when compared to the control. However, significant reduction of NO production was observed in the plants treated with C-PTIO and L-NAME (Fig. 2I). Almost around 2.7 fold increase in NO production was observed in elicitor treated set compared to control.
Fig. 2.
Effect of foliar application of CaCl2 (0.5%) along with different NO modulators on the production of NO. I Spectrophotometric analysis of NO production in the leaf tissue. II Real-time detection of NO. NO production in leaf epidermal cells was stained by DAF-2DA. (A) Control; (B) CaCl2 (0.5%); (C) CaCl2 (0.5%) + L-NAME (10 µM); (D) CaCl2 (0.5%) + C-PTIO (100 µM); (E) CaCl2 (0.5%) + L-NAME (10 µM) + C-PTIO (100 µM). Results are mean ± SE of three separate experiments done in triplicate. Different letters in the bar graph specify significant difference (p < 0.05) from the control set using Duncan’s multiple range test whereas same letter denotes no significant difference between the groups
NO production was further justified by using a NO specific fluorophore DAF-2DA on leaf peals, which converts fluorescent triazol derivative upon reaction with NO. Similar kind of increase in NO production was observed in elicitor treated sets as monitored by spectrophotometry (Fig. 2II).
Effect of NO donor (SNP) and NO modulators (L-NAME and C-PTIO) on defense response
Induction of defense response and production of signaling molecule NO in the CaCl2 treated tomato plants are clear from the above results. To further substantiate the involvement of NO in the observed CaCl2-mediated defense stimulation, we treated tomato plants with NO donor (SNP) as well as NO scavenger (C-PTIO) and NOS inhibitor (L-NAME) either singly or in combination with elicitor. In SNP treated sets, a significant increase of defense enzymes namely, PO (2.41-fold), PPO (3.9-fold), PAL (1.68-fold), β-1,3-glucanase (2.3-fold) and chitinase (2.4-fold) along with the induced activity of antioxidant enzymes CAT (1.84-fold) and APX (4.72-fold) was observed (Table 2). Simultaneous higher accumulation of total phenolics (1.94-fold) and total flavonoids (1.86-fold) were detected (Table 2). Interestingly, increased amount of different phenolic acids were also increased compared to water treated control plant (Table 3). Similarly, expression of defense related genes and antioxidant enzyme-coding gene as well as the gene of CAM and NR were also increased (Fig. 1). It was evident that CaCl2 treatment showed higher accumulation of NO in tomato plants. However, tomato plants co-treated with elicitor as well as NOS inhibitor, NG-nitro-l-arginine methyl ester (L-NAME) or NO scavenger 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (C-PTIO) in various combinations, restricted the NO production noticeably, as detected by spectrophotometrically or microscopically (Fig. 2). Consequently, accumulation of defense enzymes, antioxidant enzymes, total phenolic and flavonoid contents (Table 2) and different phenolic acids (Table 3) along with the expression profiles of different defense related genes, including genes of antioxidant enzyme and CAM and NR gene were found to be observed reduced or at basal level like control plants (Fig. 1).
Effects of CaCl2 treatment on incidence of Fusarium wilt disease
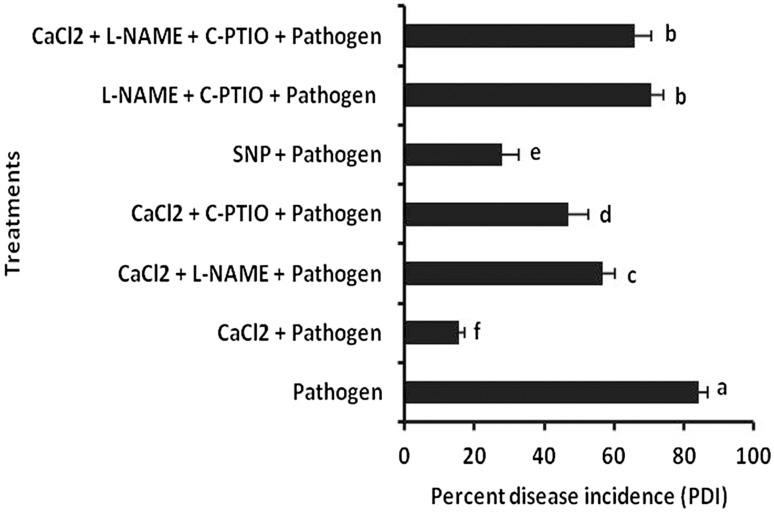
The treatments of tomato seedlings with CaCl2 significantly reduced wilt development by F. oxysporum f. sp. lycopersici (Fig. 3). The amount of reduction from F. oxysporum f. sp. lycopersici treated plant was recorded 84.24%. However, SNP treated set showed 66.80% reduction than pathogen treated control. Furthermore, co-treatment of NO scavenger (C-PTIO) and elicitor as well as NO synthase inhibitor (L-NAME) and elicitor or in a combination of both scavenger and inhibitor with elicitor showed significant increase in disease incidence compared to plants treated with CaCl2 alone. In those sets, the amount of reduction of disease incidence was observed as 44.26, 32.81 and 21.73% than pathogen treated control plant respectively.
Fig. 3.
Effect of exogenous application of CaCl2 (0.5%) and pathogen inoculation along with different NO modulators on percent disease incidence on tomato plants. Results are mean ± SE of three separate experiments done in triplicate. Different letters in the bar graph specify significant difference (p < 0.05) from the control set using Duncan’s multiple range test whereas same letter denotes no significant difference between the groups
Effects of CaCl2 on production of lignin in tomato plant
Histochemical observations demonstrate that CaCl2 also have inductive role for the production of lignin which starts to deposit in primary cell walls prior to the secondary walls on secondary xylem and other tissues. From Fig. 4, it was clear that elicitor treated plants showed higher amount of lignifications in the tissue 10 days after elicitation compared to water treated control plants. However, plants inoculated with pathogen also showed greater lignifications in the stem tissue after 10 days of incubation. Interestingly, highest lignifications occurred in the plants treated with CaCl2 (0.5%) and challenge inoculated with pathogen.
Fig. 4.
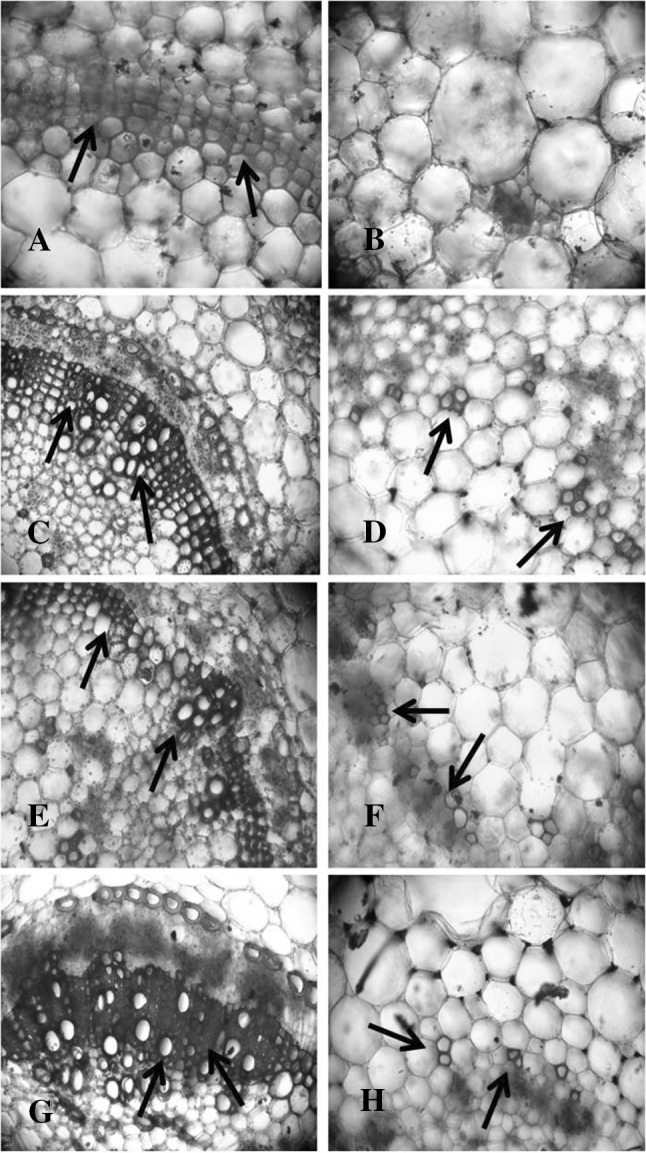
Effect of exogenous application of CaCl2 (0.5%) on lignin production in the stem of tomato plants. A and B Control (water treated); C and D CaCl2-0.5% (observed after 10 days of elicitor application); E and F Pathogen inoculated (observed after 10 days); G and H CaCl2 (0.5%) + pathogen inoculated plant (observed after 10 days). Arrows indicate the sites of lignin synthesis
Effects of CaCl2 on seed germination, shoot length, root length, seedling vigor, chlorophyll content, mean trichome density and yield in tomato plant
The increase in seed germination by CaCl2 and SNP was recorded 9.39 and 7.66% respectively (Table 4). Shoot length and root length was also significantly increased in elicitor treated sets compared to water treated control. In comparison to SNP, seeds treated with CaCl2 showed greater shoot and root length. Amount of increase in shoot and root length for both CaCl2 and SNP treated seeds showed 1.46, 1.27, 1.36 and 1.18 fold compare to control respectively (Table 4). The extent of seedling vigor was observed highest in CaCl2 treated seeds. Value was recorded 1.56 and 1.33-fold increase in CaCl2 and SNP treated seeds respectively compared to control (Table 4). Furthermore, chlorophyll content in CaCl2 treated plants was 1.58-fold increased. However, in SNP treated plants it remains same like control (Table 4). Mean trichome density was significantly higher in both CaCl2 and SNP sprayed plants and recorded 1.75 and 1.41-fold increase compared to water treated control plants (Table 4). Yield of tomato plants were significantly altered with the application of CaCl2 and SNP compared to control plant. 1.51 and 1.44-fold increase in total yield was recorded in CaCl2 and SNP treated plants compared to control respectively.
Table 4.
Effect of CaCl2 (0.5%) and SNP (100 μM) as seed and foliar treatment on seed germination, shoot length, root length, seedling vigor index, total chlorophyll content, trichome density and yield in tomato plants
| Sets | % Seed germination | Shoot length (cm) | Root length (cm) | Seedling vigor index | Total Chlorophyll [mg of total chlorophyll g fresh tissue−1] | Mean trichome density (no./microscopic field) | Yield (g/pot) |
|---|---|---|---|---|---|---|---|
| Control | 80.33 ± 1.52b | 6.53 ± 0.35c | 5.2 ± 0.5c | 941.93 ± 46.41c | 0.70 ± 0.08b | 9.66 ± 1.52c | 115.66 ± 4.04b |
| Cacl2 | 88.66 ± 1.15a | 9.56 ± 0.85a | 7.1 ± 0.26a | 1478.4 ± 110.26a | 1.11 ± 0.15a | 17 ± 1.73a | 175 ± 7.21a |
| SNP | 87 ± 1a | 8.33 ± 0.51b | 6.16 ± 0.35b | 1261.7 ± 43.15b | 0.73 ± 0.08b | 13.66 ± 1.52b | 167 ± 6.24a |
Values represent mean ± SE of three separate experiments, each in triplicate
CaCl 2 Calcium chloride, SNP sodium nitroprusside
Different letters within the row indicate significant difference (p < 0.05) from the control set using Duncan’s multiple range test. Same letter within the row denotes no significant difference between the groups
Discussion
Plants acquire an array of mechanisms to shield themselves against regular assault of potential pathogens and other stresses (Chakraborty and Acharya 2017). During course of time they have advanced their defense strategies that include both constitutive as well as pathogen-induced molecules (Vanitha et al. 2009). In fact, natural defense response of plants against various pathogens depends upon early recognition of intra or extracellular components of pathogen (Chandra et al. 2015). Plants treated with different abiotic or biotic elicitor molecules have been shown to encourage plant’s innate immune system to over express different defense related enzymes and genes, increased accumulation of phenolic compounds, cell wall material synthesis, over production of different signaling molecule etc. (Nürnberger et al. 2004; Acharya et al. 2011a; Chandra et al. 2014b).
In this study, we examined the efficacy of CaCl2, as an abiotic elicitor, on tomato plants against Fusarium oxysporum f. sp. lycopersici. Biochemical, molecular and enzymatic studies revealed that all the parameters examined were significantly altered due to CaCl2 treatment. Notable elevation of defense response was observed in tomato plants by foliar application of CaCl2. There has been quantitative increase in the levels of biochemicals such as phenols and flavonoids and an increase in defense enzymes like PO, PPO, PAL, β-1,3-glucanase and chitinase activities in CaCl2 treated plants was recorded after 48 h of incubation. Furthermore, transcript analysis of CaCl2 treated plants showed higher expression of PR 1, PR 2a, PR 2b, PR 3a, PR 3b, PR 5, PR 7, PAL, Prot In and PO gene compared to control. In which PR 2a and PR 2b represents β-1,3-glucanase; PR 3a and PR 3b represents chitinase; PR 5 represents osmotin like proteins. Among these defense molecules, PO and PPO are recognized to be involved in the strengthening of the plant’s cell wall by accumulation of lignin which leads to protection against different invading pathogens (Lamb and Dixon 1997; Bruce and West 1989; Acharya et al. 2011b; Chandra et al. 2015). These observations were reflected in our study as the higher accumulation of PO and PPO ultimately leads to the greater production of lignin in the CaCl2 treated plants. On the other hand, induced expression of β-1,3-glucanase beside its well known antifungal activities would also enhance varied physiological roles (Balasubramanian et al. 2012). Thus over expression of β-1,3-glucanase by foliar application of CaCl2 might become valuable at the time of pathogen foray as this enzyme is directly implicated in hydrolyzing the components of fungal cell wall. Another PR protein, chitinases (PR 3) also has the potential to hydrolyze major components of fungal cell wall like chitin (Leah et al. 1991). Application of CaCl2 have been shown to induce better expression of PO, PPO, PAL, β-1,3-glucanase, chitinase and other defense molecules in different host plants (Chandra et al. 2014a; Tian et al. 2006; Chakraborty et al. 2015a). Thus induction of these enzymes in the host plant during pathogenesis possesses a great importance as shown in our result.
Furthermore, PAL is the entry-point enzyme in the phenylpropanoid biosynthesis pathway and plays an important role in phenolic compound synthesis (Parmar and Subramanian 2012). The vital role of phenolic compounds is to induce disease resistance. Their accumulation due to application of different elicitors have already been reported (Nicholson and Hammerschmidt 1992; Sánchez-Estrada et al. 2009; Dong et al. 2010; Chandra et al. 2015). Phenolic and flavonoid compounds are also known to have free radical scavenging, antimicrobial activity, inhibition of hydrolytic and oxidative enzymes and anti-inflammatory action (Mandal et al. 2009; Srivastava et al. 2013). In this study, higher accumulation of phenolic compounds has been recorded in elicitor treated plants as a consequence of increased amount of PAL activity. Previously, it was reported that higher accumulation of ferulic acid in tomato roots may become esterified and cross linked to form lignin-like polymers which in turn provide defense against impending pathogens (Mandal et al. 2009). In addition to this, 4-coumaric acid and ferulic acid has been found to be effective to reduce the growth of Fusarium species in vitro at all concentrations tested (McKeehen et al. 1999). However, reduced level of ferulic acid, 4-hydroxybenzoic acid and 4-coumaric acid along with lower level of PAL and PO enzyme activity, increase the susceptibility of tomato plant against wilt pathogen (Mandal et al. 2009). Our results agree with this view as HPLC analysis showed that in elicitor treated sets production of phenolic compounds like gallic acid, caffeic acid, p-coumaric acid, ferulic acid, quercetin, cinnamic acid and pyrogallol was significantly increased. These compounds are helpful to plants to surmount adverse environmental conditions as well as to give resistance against pathogens (Gould and Lister 2005; Chandra et al. 2015; Ruiz-García and Gómez-Plaza 2013). According to Ruiz et al. (2003) foliar application of CaCl2 (10 µM) in Citrus increases PAL activity which leads to the accumulation of phenolic compounds and provide resistance against Alternaria alternata. In our work, increased amount of phenol accumulation by foliar application of CaCl2 significantly reduce Fusarium wilt disease incidence in tomato plants.
Generation of reactive oxygen species (ROS) is thought to be one of the earlier event towards the recognition of a pathogen by the plants (Baker and Orlandi 1995; Chakraborty et al. 2015b). To cope up with this, plants have developed different scavenging system including production of antioxidant enzymes (Grob et al. 2013). APX and CAT are two main antioxidant enzymes produced by the plants in response to ROS. Increasing production of these two enzymes were already reported in tomato plants by foliar application of sub-lethal dose of CuCl2 (Chakraborty et al. 2015b). In this study, amount of APX and CAT was increased in elicitor treated plants. Not only that the mRNA expression of GST, another antioxidant enzyme, was also elevated moderately due to elicitor application. These results signify that CaCl2 might also provide the requisite guard to the plants from the oxidative stress coupled with pathogenic attack.
Downstream signal transduction pathways after perception of elicitor are an important subject of investigation (Baenas et al. 2014). Beside many other roles, Ca2+ is a well documented important intracellular messenger in plant defense signaling, which is relayed by the calcium sensor like CaM that quickly converts the signal to second messengers like NO and cyclic nucleotides (Chandra et al. 2014a; Peng et al. 2014). Over the last few years, NO has been popularized as an important signaling molecule behind several patho-physiological events and also involved in the activation of plant defense during pathogen attack (Klessig et al. 2000; Wendehenne et al. 2001; Lecourieux et al. 2006; Acharya and Acharya 2007; Romero-Puertas and Delledonne 2003). Ca2+ dependent NO production was recorded in few plant system in response to different elicitors like cryptogein, CaCl2 etc. (Foissner et al. 2000; Lamotte et al. 2004; Chandra et al. 2014a). Calcium induced NO production and induction of antioxidative enzymes in response to different stresses was hampered by application of EGTA (an extra cellular calcium chelator) (Qiao et al. 2015; Rahman et al. 2016). Moreover, characterization of a plant nitric oxide synthase (NOS) from Arabidopsis thaliana showed that the enzyme contains CaM-binding motifs and that signifies complete activation of NOS needs both Ca2+ and CaM (Guo et al. 2003; Lecourieux et al. 2006). We have shown elevation in NO level in several plants like Raphanus sativus, Camellia sinensis and in Capsicum annuum by foliar application of different biotic and abiotic elicitors (Chandra et al. 2014a, b, 2017; Chakraborty et al. 2015b, 2016; Chakraborty and Acharya 2016), showing its involvement in the signal transduction process leading to induced defense responses. In this study, CaCl2-treated tomato plants showed greater NO production than the control set. Nitrate reductase (NR) has been shown to be one of the major source NO in plants including tomato (Meyer et al. 2005; Jin et al. 2009) and NO was found to be one of the major positive regulator of NR (Du et al. 2008). This fact was reflected in our study where elicitor and SNP both differentially modulate the expression of NR gene in treated tomato plants. Furthermore, to establish the connection between NO and CaCl2 induced resistance, separate tomato plant was treated with NO donor, SNP, a strong NOS inhibitor, L-NAME and a potent NO scavenger C-PTIO alone or in combinations.
Production of the defense related enzymes (i.e., PO, PPO, PAL, β-1,3-glucanase and chitinase) including the antioxidant enzymes (CAT and APX) and the expression level of different defense genes (PR 1, PR 2a, PR 2b, PR 3a, PR 3b, PR 5, PR 7, PAL, Prot In and PO) and that of the antioxidant enzyme (GST), NR and CAM as well as total phenol and flavonoid contents were comparatively lower in CaCl2 + L-NAME, CaCl2 + C-PTIO and CaCl2 + L-NAME + C-PTIO treated sets compared to the plants treated with CaCl2 and SNP. Production of NO was also validated by using DAF-2DA stain which showed significant reduction of fluorescence in L-NAME and C-PTIO treated plants than the plants treated with CaCl2. Our results coincide with the results of some previous studies where co-treatment with L-NAME or C-PTIO and elicitor have showed reduced production of NO and as a consequence, reduction in defense induction was observed in pearl millet, Rauvolfia serpentina, tea etc. (Manjunatha et al. 2009; Gupta et al. 2013; Chandra et al. 2015). However, sole treatment of SNP induces defense responses in several plants (Hu et al. 2003; Hasanuzzaman and Fujita 2013; Chandra et al. 2014b). In present work application of SNP showed significant induction for all the defense molecules including defense enzymes, defense gene expressions, antioxidant enzymes production and activation of phenolic compounds which signifies the role of NO in this process.
Furthermore, according to earlier findings some of resistance inducing chemicals like salicylic acid, β-aminobutyric acid, chitosan and 2, 6 dichloroisonicotinic acid are also known to enhance the seed germination and other physiological aspects including, root and shoot length, yield, chlorophyll content etc. (Zhou et al. 2002; Rajaei and Mohamad 2013; Jayalakshmi et al. 2010; Raut et al. 2014). In present study both CaCl2 and SNP showed significantly higher amount of seed germination, root and shoot length, seedling vigor, chlorophyll content and yield compared to control which implies, both elicitor and NO have inductive effects on those parameters also. Trichomes, are one of the defensive structure, which occur on the surfaces of many plants and provide protection to the plants against herbivores (Simmons and Gurr 2004; Boughton et al. 2005). Foliar applications of abiotic elicitors such as methyl jasmonate (MJ) or Benzothiadiazole (BTH) induces increased densities of defense-related glandular trichomes on new leaves of tomato plants (Boughton et al. 2005). In our case also we have also found mean trichome density becoming much higher in CaCl2 and SNP treated sets than untreated control.
From these results, it could be concluded that foliar application of CaCl2 could provide promising integrated alternatives in suppression of Fusarium wilt disease of tomato. In this connection, Ca2+ ion acts as an external signal which induces to generate an internal signature signal like NO that ultimately leads to the over expressions of different defensive responses to the tomato plant. Furthermore, CaCl2 also have the potential to improve plant growth. So, this work presents an alternative, safer and user friendly approach for the management of Fusarium wilt disease of tomato. Further studies regarding the use of CaCl2 alone or in combination should be examined under different combinations of host and pathogen for better understanding and to establish it as a multiuser compound for sustainable agriculture in a broader range.
Acknowledgments
Author’s contribution
KA designed whole research. NC and SC conducted experiments and analyzed data. NC wrote the manuscript. All authors read and approved the manuscript.
Funding
There was no funding for this study.
Abbreviations
- CaCl2
Calcium chloride
- HPLC
High performance liquid chromatography
- RT-PCR
Semi-quantitative reverse transcription-polymerase chain reaction
- NO
Nitric oxide
- NR
Nitrate reductase
- CAM
Calmodulin
- PGPR
Plant growth promoting rhizobacteria
- PO
Peroxidase
- GST
Glutathione S-transferases
- SNP
Sodium nitroprusside
- L-NAME
NG-nitro-l-arginine methyl ester
- NOS
Nitric oxide synthase
- C-PTIO
2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide
- PPO
Polyphenol oxidase
- PAL
Phenylalanine ammonia-lyase
- CAT
Catalase
- APX
Ascorbate peroxidase
- DAF-2DA
4,5-Diaminofluorescein diacetate
- Prot In
Proteinase inhibitor
- GAPDH
Glyceraldehyde phosphate dehydrogenase
- ROS
Reactive oxygen species
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abdel-Kader MM, El-Mougy NS, Aly MDE, Embaby EI. Occurrence of Sclerotinia foliage blight disease of cucumber and pepper plants under protected cultivation system in Egypt II. Bio-control measures against Sclerotinia spp. in vitro. Adv Life Sci. 2012;1:59–70. [Google Scholar]
- Acharya R, Acharya K. Evaluation of nitric oxide synthase status during disease progression in resistant and susceptible varieties of Sesamum indicum against Macrophomina phaseolina. Asian Australas J Plant Sci Biotechnol. 2007;1:61–63. [Google Scholar]
- Acharya K, Chakraborty N, Dutta AK, et al. Signaling role of nitric oxide in the induction of plant defense by exogenous application of abiotic inducers. Arch Phytopathol Plant Prot. 2011;44:1501–1511. doi: 10.1080/03235408.2010.507943. [DOI] [Google Scholar]
- Acharya K, Chandra S, Chakraborty N, Acharya R. Nitric oxide functions as a signal in induced systemic resistance. Arch Phytopathol Plant Prot. 2011;44:1335–1342. doi: 10.1080/03235408.2010.496552. [DOI] [Google Scholar]
- Agrios GN. Plant pathology. 5. San Diego: Academic; 2005. [Google Scholar]
- Amini J. Induced resistance in tomato plants against Fusarium wilt ivoked by nonpathogenic Fusarium, Chitosan and Bion. Plant Pathol J. 2009;25:256–262. doi: 10.5423/PPJ.2009.25.3.256. [DOI] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenas N, García-Viguera C, Moreno DA. Elicitation: a Tool for enriching the bioactive composition of foods. Molecules. 2014;19:13541–13563. doi: 10.3390/molecules190913541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Phytopathology. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Balasubramanian V, Vashisht D, Cletus J, Sakthivel N. Plant β -1,3-glucanases: their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol Lett. 2012;34:1983–1990. doi: 10.1007/s10529-012-1012-6. [DOI] [PubMed] [Google Scholar]
- Bansode Y, Bajekals S. Characterization of chitinase from microorganisms isolated from Lonar Lake. Indian J Biotechnol. 2006;5:357–363. [Google Scholar]
- Bartha B, Kolbert Z, Erdei L. Nitric oxide production induced by heavy metals in Brassica juncea L. Czern and Pisum sativum L. Acta Biol Szeged. 2005;49:9–12. [Google Scholar]
- Biswas SK, Pandey NK, Rajik M. Inductions of defense response in tomato against Fusarium wilt through inorganic chemicals as inducers. J Plant Pathol Microbiol. 2012;3:1–7. [Google Scholar]
- Boughton AJ, Hoover K, Felton GW. Methyl jasmonate application induces increased densities of glandular trichomes on tomato, Lycopersicon esculentum. J Chem Ecol. 2005;31:2211–2216. doi: 10.1007/s10886-005-6228-7. [DOI] [PubMed] [Google Scholar]
- Bruce RJ, West CA. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989;91:889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Horst J. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. doi: 10.1111/j.1399-3054.1991.tb00121.x. [DOI] [Google Scholar]
- Chakraborty N, Acharya K. Ex vivo analyses of formulated bio-elicitors from a phytopathogen in the improvement of innate immunity in host. Arch Phytopathol Plant Prot. 2016;49:485–505. doi: 10.1080/03235408.2016.1242196. [DOI] [Google Scholar]
- Chakraborty N, Acharya K. “NO way”! Says the plant to abiotic stress. Plant Gene. 2017 [Google Scholar]
- Chakraborty N, Chandra S, Acharya K. Sublethal heavy metal stress stimulates innate immunity in tomato. Sci World J. 2015;2015:1–7. doi: 10.1155/2015/208649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Chandra S, Acharya K. Boosting of innate immunity in chilli. Res J Pharm Technol. 2015;8:885–892. doi: 10.5958/0974-360X.2015.00144.4. [DOI] [Google Scholar]
- Chakraborty N, Ghosh S, Chandra S, et al. Abiotic elicitors mediated elicitation of innate immunity in tomato: an ex vivo comparison. Physiol Mol Biol Plants. 2016;22:307–320. doi: 10.1007/s12298-016-0373-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Chakraborty N, Chakraborty A, et al. Abiotic elicitor-mediated improvement of innate immunity in Camellia sinensis. J Plant Growth Regul. 2014;33:849–859. doi: 10.1007/s00344-014-9436-y. [DOI] [Google Scholar]
- Chandra S, Chakraborty N, Chakraborty A, et al. Induction of defence response against blister blight by calcium chloride in tea. Arch Phytopathol Plant Prot. 2014;47:2400–2409. doi: 10.1080/03235408.2014.880555. [DOI] [Google Scholar]
- Chandra S, Chakraborty N, Dasgupta A, et al. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci Rep. 2015;5:15195. doi: 10.1038/srep15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra S, Chakraborty N, Panda K, Acharya K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol Biochem. 2017;115:298–307. doi: 10.1016/j.plaphy.2017.04.008. [DOI] [PubMed] [Google Scholar]
- Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10:178–182. [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don’t have no mercy: cell death programs in plant–microbe interactions. Plant Cell. 1996;8:1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danhash N, Wagemakers AM, van Kan JAL, de Wit PJGM. Molecular characterization of four chitinase cDNAs obtained from Cladosporium fulvum-infected tomato. Plant Mol Biol. 1993;22:1017–1022. doi: 10.1007/BF00028974. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Zeier J, Marocco A, Lamb C. Signal interactions between nitric oxide and reactive oxygen intermediates in the plant hypersensitive disease resistance response. Proc Natl Acad Sci USA. 2001;98:13454–13459. doi: 10.1073/pnas.231178298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson DP, Pascholati SF, Hagerman AE, et al. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol Plant Pathol. 1984;25:111–123. doi: 10.1016/0048-4059(84)90050-X. [DOI] [Google Scholar]
- Dong J, Wan G, Liang Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol. 2010;148:99–104. doi: 10.1016/j.jbiotec.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Du ST, Zhang YS, Lin XY, et al. Regulation of nitrate reductase by its partial product nitric oxide in Chinese cabbage pakchoi (Brassica chinensis L.) Plant Cell Environ. 2008;31:195–204. doi: 10.1111/j.1365-3040.2007.01750.x. [DOI] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Longteraals P, Durner J. Technical advance: in vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J. 2000;23:817–824. doi: 10.1046/j.1365-313X.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- Gould KS, Lister C. Flavonoid functions in plants. In: Andersen OM, Markham KR, editors. Flavonoids chemistry, biochemistry and applications. Boca Raton: Taylor & Fransis/CRC Press; 2005. pp. 397–441. [Google Scholar]
- Grob F, Durner J, Gaupels F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front Plant Sci. 2013;4:419. doi: 10.3389/fpls.2013.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover A, Gowthaman R. Strategies for development of fungus-resistant transgenic plants. Curr Sci. 2003;84:330–340. [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM. Identification of a plant nitric oxide synthase gene involved in hormonal signalling. Science. 2003;302:100–103. doi: 10.1126/science.1086770. [DOI] [PubMed] [Google Scholar]
- Gupta NS, Banerjee M, Basu SK, Acharya K. Involvement of nitric oxide signal in Alternaria alternata toxin induced defense response in Rauvolfia serpentina benth. ex Kurz calli. Plant Omics. 2013;6:157–164. [Google Scholar]
- Hasanuzzaman M, Fujita M. Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology. 2013;22:584–596. doi: 10.1007/s10646-013-1050-4. [DOI] [PubMed] [Google Scholar]
- Hemeda HM, Klein BP. Effects of naturally occurring antioxidants on peroxidase activity of vegetable extracts. J Food Sci. 1990;55:184–185. doi: 10.1111/j.1365-2621.1990.tb06048.x. [DOI] [Google Scholar]
- Hu X, Neill SJ, Cai W, Tang Z. NO-mediated hypersensitive responses of rice suspension cultures induced by incompatible elicitor. Chin Sci Bull. 2003;48:358–363. doi: 10.1007/BF03183230. [DOI] [Google Scholar]
- Jayalakshmi P, Suvarnalatha Devi P, Prasanna ND, Revathi G, Shaheen SK. Morphological and physiological changes of groundnut plants by foliar application with salicylic acid. Bioscan. 2010;5:193–195. [Google Scholar]
- Jetiyanon K, Kloepper JW. Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control. 2002;24:285–291. doi: 10.1016/S1049-9644(02)00022-1. [DOI] [Google Scholar]
- Jin CW, Du ST, Zhang YS, et al. Differential regulatory role of nitric oxide in mediating nitrate reductase activity in roots of tomato (Solanum lycocarpum) Ann Bot. 2009;104:9–17. doi: 10.1093/aob/mcp087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatua S, Dutta AK, Acharya K. Prospecting Russula senecis: a delicacy among the tribes of West Bengal. PeerJ. 2015;3:e810. doi: 10.7717/peerj.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klessig DF, Durner J, Noad R, et al. Nitric oxide and salicylic acid signalling in plant defences. Proc Natl Acad Sci USA. 2000;97:8849–8855. doi: 10.1073/pnas.97.16.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K, Khan P. Peroxidase and polyphenol oxidase in excised ragi (Eleusine corocana cv PR 202) leaves during senescence. Ind J Exp Biol. 1982;20:412–416. [PubMed] [Google Scholar]
- Lamb C, Dixon RA. The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- Lamotte O, Gould K, Lecourieux D, et al. Analysis of nitric oxide signalling functions in tobacco cells challenged by the elicitor cryptogein. Plant Physiol. 2004;135:516–529. doi: 10.1104/pp.104.038968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leah R, Tommerup H, Svendsen I, Mundy J. Biochemical and molecular characterization of three barley seed proteins with antifungal properties. J Biol Chem. 1991;266:1564–1573. [PubMed] [Google Scholar]
- Lecourieux D, Ranjeva R, Pugin A. Calcium in plant defence-signalling pathways. New Phytol. 2006;171:249–269. doi: 10.1111/j.1469-8137.2006.01777.x. [DOI] [PubMed] [Google Scholar]
- Ma F, Lu R, Liu H, et al. Nitric oxide-activated calcium/calmodulin-dependent protein kinase regulates the abscisic acid-induced antioxidant defence in maize. J Exp Bot. 2012;63:4835–4847. doi: 10.1093/jxb/ers161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal S, Mallick N, Mitra A. Salicylic acid-induced resistance to Fusarium oxysporum f. sp. lycopersici in tomato. Plant Physiol Biochem. 2009;47:642–649. doi: 10.1016/j.plaphy.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Manjunatha G, Niranjan-Raj S, Prashanth GN, et al. Nitric oxide is involved in chitosan-induced systemic resistance in pearl millet against downy mildew disease. Pest Manag Sci. 2009;65:737–743. doi: 10.1002/ps.1710. [DOI] [PubMed] [Google Scholar]
- Manzo D, Ferriello F, Puopolo G, et al. Fusarium oxysporum f. sp. radicis-lycopersici induces distinct transcriptome reprogramming in resistant and susceptible isogenic tomato lines. BMC Plant Biol. 2016;16:53. doi: 10.1186/s12870-016-0740-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeehen JD, Busch RH, Fulcher RG. Evaluation of wheat (Triticum aestivum L.) phenolic acids during grain development and their contribution to Fusarium resistance. J Agric Food Chem. 1999;47:1476–1482. doi: 10.1021/jf980896f. [DOI] [PubMed] [Google Scholar]
- Medeiros FCL, Resende MLV, Medeiros FHV, et al. Defense gene expression induced by a coffee-leaf extract formulation in tomato. Physiol Mol Plant Pathol. 2009;74:175–183. doi: 10.1016/j.pmpp.2009.11.004. [DOI] [Google Scholar]
- Meyer C, Lea US, Provan F, et al. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res. 2005;83:181–189. doi: 10.1007/s11120-004-3548-3. [DOI] [PubMed] [Google Scholar]
- Nahar K, Ullah SM. Morphological and physiological characters of tomato (Lycopersicon esculentum Mill) cultivars under water stress. Bangladesh J Agric Res. 2012;37:355–360. doi: 10.3329/bjar.v37i2.11240. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbato specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Nicholson RL, Hammerschmidt R. Phenolic compounds and their role in disease resistance. Annu Rev Phytopathol. 1992;30:369–389. doi: 10.1146/annurev.py.30.090192.002101. [DOI] [Google Scholar]
- Nürnberger T, Brunner F, Kemmerling B, Piater L. Innate immunity in plants and animals: striking similarities and obvious differences. Immunol Rev. 2004;198:249–266. doi: 10.1111/j.0105-2896.2004.0119.x. [DOI] [PubMed] [Google Scholar]
- Pan SQ, Ye XS, Kuć J. Association of β-1,3-glucanase activity and isoform pattern with systemic resistance to blue mould in tobacco induced by stem injection with Peronospora tabacina or leaf inoculation with tobacco mosaic virus. Physiol Mol Plant Pathol. 1991;39:25–39. doi: 10.1016/0885-5765(91)90029-H. [DOI] [Google Scholar]
- Parmar P, Subramanian R. Biochemical alteration induced in tomato (Lycopersicum esculentum) in Response to Fusarium oxysporum f. sp. lycopersici. Afr J Basic Appl Sci. 2012;4:186–191. [Google Scholar]
- Peng H, Yang T, Jurick WM. Calmodulin gene expression in response to mechanical wounding and Botrytis cinerea infection in tomato fruit. Plants. 2014;3:427–441. doi: 10.3390/plants3030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punja Z. Transgenic carrots expressing a thaumatin-like protein display enhanced resistance to several fungal pathogens. Can J Plant Pathol. 2005;27:291–296. doi: 10.1080/07060660509507227. [DOI] [Google Scholar]
- Qiao M, Sun J, Liu N, et al. Changes of nitric oxide and its relationship with H2O2 and Ca2+ in defense interactions between wheat and Pucciniat triticina. PLoS ONE. 2015;10:1–19. doi: 10.1371/journal.pone.0132265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Nahar K, Hasanuzzaman M, Fujita M. Calcium supplementation improves Na+/K+ ratio, antioxidant defense and glyoxalase systems in salt-stressed rice seedlings. Front Plant Sci. 2016;7:1–16. doi: 10.3389/fpls.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaei P, Mohamad N. Effect of beta aminobutyric acid (BABA), ABA and ethylene synthesis inhibitor (CoCl2) on seed germination and seedling growth of Brassica napus L. Eur J Exp Biol. 2013;3:437–440. [Google Scholar]
- Ramamoorthy V, Raguchander T, Samiyappan R. Induction of defense-related proteins in tomato roots treated with Pseudomonas fluorescens Pf1 and Fusarium oxysporum f. sp. lycopersici. Plant Soil. 2002;239:55–68. doi: 10.1023/A:1014904815352. [DOI] [Google Scholar]
- Raupach GS, Kloepper JW. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology. 1998;88:1158–1164. doi: 10.1094/PHYTO.1998.88.11.1158. [DOI] [PubMed] [Google Scholar]
- Raut SA, Borkar SG, Nagrale DT. Effect of disease (Alternaria leaf blight) resistance elicitors on growth parameters of tomato plant. The Bioscan. 2014;9:1157–1159. [Google Scholar]
- Rep M, Dekker HL, Vossen JH, et al. Mass spectrometric identification of isoforms of PR proteins in xylem sap of fungus-infected tomato. Plant Physiol. 2002;130:904–917. doi: 10.1104/pp.007427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Puertas M, Delledonne M. Nitric oxide signaling in plant–pathogen interactions. IUBMB Life. 2003;55:579–583. doi: 10.1080/15216540310001639274. [DOI] [PubMed] [Google Scholar]
- Ruiz JM, Rivero RM, Lopez-Cantarero I, Romero L. Role of Ca in the metabolism of phenolic compounds in tobacco leaves (Nicotiana tabacum L.) Plant Growth Regul. 2003;41:173–177. doi: 10.1023/A:1027358423187. [DOI] [Google Scholar]
- Ruiz-García Y, Gómez-Plaza E. Elicitors: a tool for improving fruit phenolic content. Agriculture. 2013;3:33–52. doi: 10.3390/agriculture3010033. [DOI] [Google Scholar]
- Sánchez-Estrada A, Tiznado-Hernández ME, Ojeda-Contreras AJ, et al. Induction of enzymes and phenolic compounds related to the natural defence response of netted melon fruit by a bio-elicitor. J Phytopathol. 2009;157:24–32. doi: 10.1111/j.1439-0434.2008.01440.x. [DOI] [Google Scholar]
- Sang JR, Zhang AY, Lin F, et al. Cross-talk between calcium–calmodulin and nitric oxide in abscisic acid signaling in leaves of maize plants. Cell Res. 2008;18:577–588. doi: 10.1038/cr.2008.39. [DOI] [PubMed] [Google Scholar]
- Sanz-Alférez S, Mateos B, Alvarado R, Sánchez M. SAR induction in tomato plants is not effective against root-knot nematode infection. Eur J Plant Pathol. 2008;120:417–425. doi: 10.1007/s10658-007-9225-6. [DOI] [Google Scholar]
- Shanmugam V, Kanoujia N. Biological management of vascular wilt of tomato caused by Fusarium oxysporum f. sp. lycospersici by plant growth-promoting rhizobacterial mixture. Biol Control. 2011;57:85–93. doi: 10.1016/j.biocontrol.2011.02.001. [DOI] [Google Scholar]
- Shih MC, Heinrich R, Goodman HM. Cloning and chromosomal mapping of nuclear genes encoding chloroplast and cytosolic glyceraldehyde-3-phosphate-dehydrogenase from Arabidopsis thaliana. Gene. 1992;119:317–319. doi: 10.1016/0378-1119(92)90290-6. [DOI] [PubMed] [Google Scholar]
- Simmons AT, Gurr GM. Trichome-based host plant resistance of Lycopersicon species and the biocontrol agent Mallada signata: are they compatible? Entomol Exp Appl. 2004;113:95–101. doi: 10.1111/j.0013-8703.2004.00210.x. [DOI] [Google Scholar]
- Srivastava R, Khalid A, Singh US, Sharma AK. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato wilt. Biol Control. 2010;53:24–31. doi: 10.1016/j.biocontrol.2009.11.012. [DOI] [Google Scholar]
- Srivastava MP, Tiwari R, Sharma N. Assessment of phenol and flavonoid content in the plant materials. J New Biol Rep. 2013;2:163–166. [Google Scholar]
- Tian SP, Qin GZ, Xu Y. Induction of defense responses against alternaria rot by different elicitors in harvested pear fruit. Appl Microbiol Biotechnol. 2006;70:729–734. doi: 10.1007/s00253-005-0125-4. [DOI] [PubMed] [Google Scholar]
- Tornero P, Conejero V, Vera P. Primary structure and expression of a pathogen-induced protease (PR-P69) in tomato plants: similarity of functional domains to subtilisin-like endoproteases. Proc Natl Acad Sci USA. 1996;93:6332–6337. doi: 10.1073/pnas.93.13.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallet C, Chabbert B, Czaninski Y, Monties B. Histochemistry of lignin deposition during sclerenchyma differentiation in alfalfa stems. Ann Bot. 1996;78:625–632. doi: 10.1006/anbo.1996.0170. [DOI] [Google Scholar]
- Van Kan JAL, Joosten MHAJ, Wagemakers CAM, et al. Differential accumulation of messenger-RNAs encoding extracellular and intracellular PR proteins in tomato induced by virulent and avirullent races of Cladosporium fulvum. Plant Mol Biol. 1992;20:513–527. doi: 10.1007/BF00040610. [DOI] [PubMed] [Google Scholar]
- Vanitha SC, Niranjana SR, Umesha S. Role of phenylalanine ammonia lyase and polyphenol oxidase in host resistance to bacterial wilt of tomato. J Phytopathol. 2009;157:552–557. doi: 10.1111/j.1439-0434.2008.01526.x. [DOI] [Google Scholar]
- Wang X, Liu W, Chen X, et al. Differential gene expression in incompatible interaction between wheat and stripe rust fungus revealed by cDNA-AFLP and comparison to compatible interaction. BMC Plant Biol. 2010;10:9. doi: 10.1186/1471-2229-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendehenne D, Pugin A, Klessig DF, Durner J. Nitric oxide: comparative synthesis and signalling in animal and plant cells. Trends Plant Sci. 2001;6:177–183. doi: 10.1016/S1360-1385(01)01893-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tan J, Guo Z, et al. Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant Cell Environ. 2009;32:509–519. doi: 10.1111/j.1365-3040.2009.01945.x. [DOI] [PubMed] [Google Scholar]
- Zhou YG, Yang YD, Qi YG, et al. Effects of chitosan on some physiological activity in germinating seed of peanut. J Peanut Sci. 2002;31:22–25. [Google Scholar]
- Zieslin N, Ben Zaken R. Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem. 1993;31:333–339. [Google Scholar]
- Zvirin T, Herman R, Brotman Y, et al. Differential colonization and defence responses of resistant and susceptible melon lines infected by Fusarium oxysporum race 1.2. Plant Pathol. 2010;59:576–585. doi: 10.1111/j.1365-3059.2009.02225.x. [DOI] [Google Scholar]