Abstract
Background
The study evaluated the change in the prevalence of airflow obstruction in the U.S. population 40–79 years of age from years 1988–1994 to 2007–2010.
Methods
Spirometry data from two representative samples of the U.S. population, the National Health and Nutrition Examination Surveys (NHANES) conducted in 1988–1994 and 2007–2010, were used. The American Thoracic Society/European Respiratory Society (ATS/ERS) criteria were used to define airflow obstruction.
Results
Based on ATS/ERS criteria, the overall age-adjusted prevalence of airflow obstruction among adults aged 40–79 years decreased from 16.6% to 14.5% (p < 0.05). Significant decreases were observed for the older age category 60–69 years (20.2% vs. 15.4%; p < 0.01), for males (19.0% vs. 15.4%; p < 0.01), and for Mexican American adults (12.7% vs. 8.4%; p < 0.001). The prevalence of moderate and more severe airflow obstruction decreased also (6.4% vs. 4.4%; p < 0.01). Based on ATS/ERS criteria, during 2007–2010, an estimated 18.3 million U.S. adults 40–79 years had airflow obstruction, 5.6 million had moderate or severe airflow obstruction and 1.4 million had severe airflow obstruction.
Conclusions
The overall age-adjusted prevalence of airflow obstruction among U.S. adults aged 40–79 years decreased from 1988–1994 to 2007–2010, especially among older adults, Mexican Americans, and males.
Keywords: airflow obstruction, chronic obstructive pulmonary disease, NHANES, spirometry
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality worldwide (1). In the United States, an estimated 15 million people had doctor-diagnosed COPD in 2010 and 12 million had undiagnosed COPD. COPD ranks third in causes of mortality resulting in about 100,000 deaths annually (2–4). COPD is a costly disease; direct and indirect healthcare costs are higher among COPD patients when compared to other patients. In a case-control study, COPD patients used 50–60% more medical services (inpatient, emergency department and office visits) than controls (5). The National Heart, Lung, and Blood Institute estimated the total annual cost of COPD for 2010 to be $49.9 billion (6). COPD associated disability also reduces the probability of being employed by 8.6% (3). Despite the decreased prevalence of smoking over the last several decades (7, 8), a recent study reported that there was little change in the overall prevalence of COPD among 20–79 year olds between the periods 1988–1994 and 2007–2010 based on the NHANES pre-bronchodilator (pre-BD) spirometry data and using mainly the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria (9) to define COPD (10).
Spirometry measurements of the forced expiratory volume in the first second (FEV1) and the FEV1 to forced vital capacity (FVC) ratio are recommended for the diagnosis of COPD and for the study of COPD prevalence (11). The interpretation of the ratio differs, however, depending on what professional recommendations are used. The American Thoracic Society/European Respiratory Society (ATS/ERS) recommends that a FEV1/FVC ratio below the lower 5th percentile (the lower limit of normal, or LLN) as determined from a representative sample of healthy non-smokers, be used to define airflow obstruction (12). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) (9, 13) recommends that post-bronchodilator (post-BD) spirometry with a ratio of FEV1/FVC below 0.7 be used to identify COPD (14). Several studies have shown that the GOLD criteria can potentially over-diagnose COPD and over-estimate the COPD burden in older adults, especially if pre-BD spirometry is used for the determination (15–18). Since COPD is a costly disease, it is important to ensure that the estimates of the COPD prevalence and burden in the current U.S. population are consistent and reliable to assure sufficient planning and funding for prevention, treatment, and research.
The aims of our study were as follows: 1) to evaluate changes in the prevalence of airflow obstruction among older adults (40–79 years) for two periods of the NHANES study 1988–1994 and 2007–2010 focusing on the ATS/ERS criteria to define airflow obstruction (in contrast to the Ford et al. study (10)); 2) to evaluate the change in the COPD GOLD Stage 1 status when using pre-BD and post-BD spirometry in the NHANES participants who had both tests; and 3) to test how well the NHANES III (1988–1994) reference equations (19) for spirometry fit the 2007–2010 data for healthy nonsmokers in order to assess reliability of the estimates (NHANES III equations were applied to both samples to determine the presence of airflow obstruction).
Methods
The National Center for Health Statistics of the Centers for Disease Control and Prevention conducts the NHANES, which is a cross-sectional survey of the civilian, non-institutionalized U.S. population (20). Household interviews and standardized physical examinations [in mobile exam centers (MEC)] are used to collect data. The NHANES survey samples are selected through a complex, multistage, probability design. The NHANES 2007–2010 survey cycles oversampled major U.S. demographic subgroups and the study procedures are detailed in the references (21, 22). Informed consent was obtained from all participants and the National Center for Health Statistics Research Ethics Review Board approved the protocol.
This study analyzed publicly available NHANES 1988–1994 and 2007–2010 spirometry data. During 1988–1994, 8495 persons aged 40–79 years were examined (79% unweighted response rate). Of these, 7667 (90%) had valid spirometry [the spirometry test met reliability/reproducibility quality control check (spprelia=1) and there were two or more successful maneuvers (sppmaneu>1)] and height data, and were used in our study. During 2007–2010, 7104 persons aged 40–79 years attended the MEC exam (71% unweighted response rate) and were eligible for spirometry. 1281 participants were excluded from spirometry for various reasons [safety reasons (649), limited time available in the MEC (337), subject refusals (113), or other reasons (182)], and 346 had invalid spirometry tests or missing height information (1). Of those examined, 5476 (77%) had valid spirometry data and were included in our study.
Spirometry
NHANES 2007–2010 spirometry testing was performed in accordance with recommendations of the ATS (23) using Ohio 822/827 dry-rolling seal volume spirometers with biological filters (A-M Systems PFT Filter Kit B) to minimize infection risks. These were the same spirometers that were used in the NHANES III (1988–1994) study. The spirometry methods were similar for both surveys except for the following: 1) The spirometry software displayed both the volume-time and flow-volume curves in 2007–2010; only the flow-volume curve was displayed in 1988–1994, 2) NHANES III did not use in-line filters, 3) the minimum number of maneuvers performed per test session was three for NHANES 2007–2010 and five for NHANES 1988–1994, and 4) the 2007–2010 survey included annual refresher training and bi-weekly, as opposed to monthly, quality control reports by the National Institute for Occupational Safety and Health (NIOSH). In both studies, height was measured with a stadiometer.
In the 2007–2010 survey, participants whose test results indicated airflow obstruction (FEV1/FVC <LLN or FEV1/FVC <0.7) were selected for post-BD spirometry. Of the 1137 (20.8%) selected 40–79-year old adults, because of time constraints or safety reasons, only 577 (50.7%) had the post-BD test done. Since a previous study used the GOLD criteria to define COPD and pre-BD spirometry as opposed to the recommended post-BD spirometry (10), we evaluated the potential bias due to using pre-BD tests in the estimation of COPD prevalence. For this purpose, we used the 577 participants with both the pre-BD and post-BD spirometry in NHANES 2007–2010.
Airflow obstruction definitions
Using the ATS/ERS criteria, mild or more severe airflow obstruction (Mild+) was defined as the ratio of FEV1 to FVC below the lower 5th percentile (i.e., LLN) (12) and moderate or more severe airflow obstruction (Moderate+) was defined as FEV1/FVC <LLN plus FEV1 <70% predicted. We used modified GOLD Stage 1 criteria, where mild or or more severe (Mild+) airflow obstruction was defined as pre-BD FEV1/FVC <0.7 (13) and moderate or more severe (Moderate+) COPD was defined as pre-BD FEV1/FVC <0.7 plus FEV1 <80% predicted. Severe airflow obstruction was defined as for moderate, but using FEV1 <50% predicted, for both ATS/ERS and GOLD. U.S. population reference equations developed from NHANES III data were used to derive the predicted and LLN values for both periods (19). Reference values are available for non-Hispanic white, non-Hispanic black, and Mexican Americans. In this study, for the “other” race category, we applied a correction factor of 0.88 to the reference values for FEV1 and FVC for non-Hispanic whites (24). For the “other Hispanic” group, we applied the reference values for Mexican Americans.
Demographic variables used in the analysis for both time periods included gender, self-reported age, self-reported race/ethnicity including non-Hispanic (NH) white, NH black, and Mexican American, and self-reported education. Potential risk factors included self-reported smoking status (never, former, and current smokers), pack-years, and body mass index (kg/m2).
To evaluate how well the NHANES III reference equations fit the NHANES 2007–2010 spirometry data, we selected all “healthy” nonsmokers aged 20–79 years from the NHANES 2007–2010 data and evaluated the the difference between the observed and predicted values derived from the NHANES III equations. We defined healthy nonsmokers as adults who were never smokers who reported no respiratory symptoms of chronic bronchitis, wheezing, or health care provider diagnosed respiratory disease (e.g., emphysema or chronic bronchitis) (19).
Data analysis
Prevalence estimates were computed using SAS, version 9.2 software procedure Proc SurveyReg (SAS Institute Inc. Cary, NC) (25). This procedure allowed us to account for the complex survey design (20) and to apply the NHANES examination weights assigned to each individual. To make estimates between the two time periods comparable with respect to the population age distribution, estimates were age adjusted by the direct method using the year 2000 Census Bureau projections for the U.S. civilian, non-institutionalized population with the following age groups: 40–49, 50–59, 60–69 and 70–79 (21, 26). The weighted frequencies and means were derived using SAS procedures Proc SurveyFreq and Proc SurveyMeans; both procedures accounted for the complex survey design and examination weights. To compare the prevalence estimates from NHANES 1988–1994 and 2007–2010, we used the t-test and the Bonferroni adjustment of the p values to account for multiple comparisons within each categorical variable. To estimate the burden of COPD for the U.S. population, the average U.S. population size for years 2007–2010 was estimated using the national Current Population Survey (27) population size tables.
To investigate the suitability of the NHANES III based reference equations for the 2007–2010 data, we fit the NHANES III reference equations to a group of “healthy-nonsmokers” 20–79 as defined above. For each person we calculated the predicted values for FEV1, FVC, and the FEV1/FVC ratio based on the person’s ethnicity, age, height, and gender using the NHANES III reference equations. Next, we calculated the standardized differences between the observed and predicted values [i.e., z-score=(observed-predicted)/residual standard deviation]. The mean z-score and the lower 95% confidence limit were then plotted against categorized age, for adults 20 to 79 years of age and a t-test was used to test whether the mean z-score values differed significantly from zero.
To evaluate the differences in the percentage of those with COPD defined by GOLD Stage 1 criteria using pre-BD spirometry and post-BD spirometry, we used the data for those participants who had post-BD spirometry and calculated the unweighted percentage with COPD for pre-BD spirometry and post-BD spirometry.
We also evaluated the differences between the prevalences of airflow obstruction defined by the GOLD Stage 1 and 2 and the ATS/ERS criteria, for the 2007–2010 time period.
Results
Table 1 provides the descriptive statistics for the NHANES 1988–1994 and 2007–2010 participants aged 40–79 with valid spirometry and height, n =7667 and n =5476, respectively. The two study samples were similar with respect to mean age (55.4 vs. 54.9 years; p >0.05) (data not shown) and gender distribution (p >0.05). The 2007–2010 sample population had, however, a higher level of education, where 47.6% had at least some college education or higher vs. 27.2% in 1988–1994 (p <0.001). Also, the NHANES 2007–2010 sample had a higher proportion of never-smokers (49.6% vs. 42.6%; p <0.001) and the combined categories of former smokers and current smokers showed a decrease in mean pack-years of smoking (23.3 vs. 29.1; p <0.001). The prevalence of obesity (body mass index (BMI) of 35 kg/m2 or greater) increased in 2007–2010 (16.9% vs. 10.0%; p <0.001).
Table 1.
Descriptive characteristics of adults age 40–79 with valid spirometry data, NHANES 1988–1994 and NHANES 2007–2010 data
| NHANES | Demographic data
|
|||||
|---|---|---|---|---|---|---|
| NHANES 1988–1994
|
NHANES 2007–2010
|
|||||
| Sample n | Population N (thousand)* | Frequency Percent† | Sample‡ n | Population N (thousand)* | Frequency Percent† | |
| Overall | 7,667 | 89,151 | 5,476 | 126,199 | ||
| Age | ||||||
| 40–49 | 2,408 | 33,187 | 31.4 | 1,654 | 43,667 | 30.2 |
| 50–59 | 1,719 | 21,999 | 22.4 | 1,472 | 39,984 | 26.9 |
| 60–69 | 2,042 | 20,100 | 26.6 | 1,428 | 26,573 | 26.1 |
| 70–79 | 1,498 | 13,864 | 19.5 | 922 | 15,975 | 16.8 |
| Gender | ||||||
| Males | 3,675 | 41,913 | 47.9 | 2,724 | 62,708 | 49.7 |
| Females | 3,992 | 47,237 | 52.1 | 2,752 | 65,634 | 50.3 |
| Race | ||||||
| NH white | 3,601 | 71,943 | 47.0 | 2,704 | 92,301 | 49.4 |
| NH black | 1,972 | 8,608 | 25.7 | 1,046 | 13,399 | 19.1 |
| Mexican American | 1,800 | 3,162 | 23.5 | 923 | 7,813 | 16.9 |
| Other Hispanic | — | — | 591 | 10.8 | ||
| Other | 294 | 3.8 | 212 | 3.9 | ||
| Education | ||||||
| <High school grad | 3,333 | 43.7 | 1,569 | 28.7 | ||
| High school | 2,218 | 29.1 | 1,293 | 23.6 | ||
| Some college | 1,035 | 13.6 | 1,435 | 26.2 | ||
| College graduate | 1,036 | 13.6 | 1,173 | 21.4 | ||
| Body mass index | ||||||
| <30 | 5,332 | 69.4 | 3,262 | 59.8 | ||
| ≥30 – <35 | 1,551 | 20.1 | 1,269 | 23.3 | ||
| ≥35 | 774 | 10.0 | 923 | 16.9 | ||
| Smoking status | ||||||
| Never smoker | 3,268 | 42.6 | 2,714 | 49.6 | ||
| Former smoker | 2,511 | 32.8 | 1,643 | 30.0 | ||
| Current smoker | 1,888 | 24.6 | 1,117 | 20.4 | ||
| Pack-years (n, mean, (SE)) | ||||||
| Former smoker + current smoker | 4,280 | 29.1 (0.6) | 2,659 | 23.3 (1.1) | ||
Current Population Survey.
Age-specific and age-standardized weighted prevalence.
Sample excludes those with missing values for height.
Table 2 shows the age-standardized prevalence of Mild+airflow obstruction by ATS criteria (FEV1/FVC <LLN) for the 40–79 year olds. The overall prevalence decreased from 16.6% (SE 0.8) during 1988–1994 to 14.5% (SE 0.7) during 2007–2010. The decrease in prevalence between 1988–1994 and 2007–2010 also reached statistical significance for 60–69 years old, males, and Mexican-American adults.
Table 2.
Age-specific and age-standardized prevalence of ATS/ERS defined Mild+ (FEV1/FVC<LLN); Moderate+ (FEV1/FVC<LLN and FEV1<70%); and severe airflow obstruction (FEV1/FVC<LLN and FEV1<50%) among U.S. adults aged 40–79 years, by periods 1988–1994 and 2007–2010
| NHANES | Mild + airflow obstruction
|
Moderate + airflow obstruction
|
Severe airflow obstruction
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| 1988–94 | 2007–10 | t-test of Difference | 1988–94 | 2007–10 | t-test of Difference | 1988–94 | 2007–10 | t-test of Difference | |
| P† (SE) | P† (SE) | p-value | P† (SE) | P† (SE) | p-value | P† (SE) | P† (SE) | p-value | |
| Overall | 16.6 (0.8) | 14.5 (0.7) | * | 6.4 (0.5) | 4.4 (0.4) | ** | 2.2 (0.2) | 1.1 (0.2) | *** |
| Age | |||||||||
| 40–49 | 12.8 (1.0) | 12.7 (0.9) | 3.0 (0.6) | 2.3 (0.6) | 1.0 (0.3) | 0.3 (0.2) | |||
| 50–59 | 16.6 (1.2) | 15.2 (1.7) | 6.2 (0.7) | 4.5 (0.6) | 2.0 (0.3) | 1.1 (0.3) | |||
| 60–69 | 20.2 (1.2) | 15.4 (1.3) | ** | 10.0 (1.0) | 5.8 (0.7) | ** | 4.1 (0.6) | 1.7 (0.6) | ** |
| 70–79 | 22.1 (1.6) | 17.2 (1.5) | 11.0 (1.2) | 7.8 (1.0) | 3.6 (0.8) | 2.3 (0.5) | |||
| Gender | |||||||||
| Males | 19.0 (1.1) | 15.4 (0.9) | ** | 6.8 (0.5) | 4.5 (0.5) | ** | 2.4 (0.3) | 1.1 (0.3) | ** |
| Females | 14.6 (0.9) | 13.8 (1.0) | 6.1 (0.6) | 4.2 (0.4) | ** | 2.2 (0.3) | 1.0 (0.2) | ** | |
| Race | |||||||||
| NH White | 17.3 (0.8) | 15.9 (0.8) | 6.7 (0.5) | 4.7 (0.5) | ** | 2.5 (0.3) | 1.0 (0.2) | *** | |
| NH Black | 15.9 (1.0) | 13.6 (1.2) | 5.8(0.7) | 4.7 (0.7) | 1.5 (0.3) | 1.8 (0.5) | |||
| Mexican American | 12.7 (0.8) | 8.4 (0.8) | *** | 3.7 (0.6) | 1.7 (0.3) | * | 0.8 (0.2) | 0.6 (0.3) | |
| Education | |||||||||
| <High school grad | 18.3 (1.4) | 15.3 (1.3) | 7.7 (0.8) | 6.6 (1.1) | 2.9 (0.5) | 2.0 (0.5) | |||
| High school grad | 17.2 (1.1) | 17.6 (1.6) | 7.7 (0.8) | 5.9 (0.6) | 2.2 (0.4) | 1.6 (0.4) | |||
| Some college | 16.6 (1.3) | 15.0 (1.1) | 6.3 (0.8) | 3.7 (0.6) | ** | 3.1 (0.7) | 0.9 (0.3) | ** | |
| College graduate | 12.9 (1.5) | 10.9 (1.3) | 2.7 (0.6) | 2.3 (0.5) | 0.6 (0.3) | 0.1 (0.1) | |||
| Smoking status | |||||||||
| Never smoker | 8.0 (0.8) | 8.2 (0.8) | 1.9 (0.4) | 1.1 (0.2) | 0.7 (0.2) | 0.3 (0.1) | |||
| Former smoker | 16.2 (1.0) | 14.5 (1.3) | 6.0 (0.7) | 4.5 (0.7) | 1.8 (0.3) | 1.1 (0.3) | |||
| Current smoker | 31.8 (1.3) | 34.4 (1.8) | 14.9 (1.0) | 14.9 (1.3) | 5.8 (0.7) | 3.4 (0.7) | * | ||
Mild+ is Mild or more severe airflow obstruction.
Moderate+ is Moderate or more severe airflow obstruction.
Age-specific and age-standardized weighted prevalence.
p < 0.05;
p < 0.01;
p < 0.001; after Bonferroni adjustment.
The age-standardized prevalence of Moderate+airflow obstruction defined as FEV1/FVC <LLN and FEV1 <70% predicted is also seen in Table 2. The overall prevalence dropped from 6.4% (SE 0.5) during 1988–1994 to 4.4% (SE 0.4) during 2007–2010. Again, the 2007–2010 prevalence was lower, reaching statistical significance for the 60–69 year age group, both genders, NH white and Mexican American persons, and those with some college education.
Further, Table 2 includes the the age-standardized prevalence for severe airflow obstruction based on the ATS/ERS criteria, for 40–79 year olds. The overall prevalence was 2.2% (SE 0.2) for years 1988–1994 and 1.1% (SE 0.2) for 2007–2010. There was significant decline in the prevalence of severe airflow obstruction for adults 60–69 years, both genders, NH white adults, and those with some college education. There was also a decline among current smokers (p <0.05). Often, the prevalence for the years 2007–2010 was about half of the prevalence for 1988–1994 for the demographic factors.
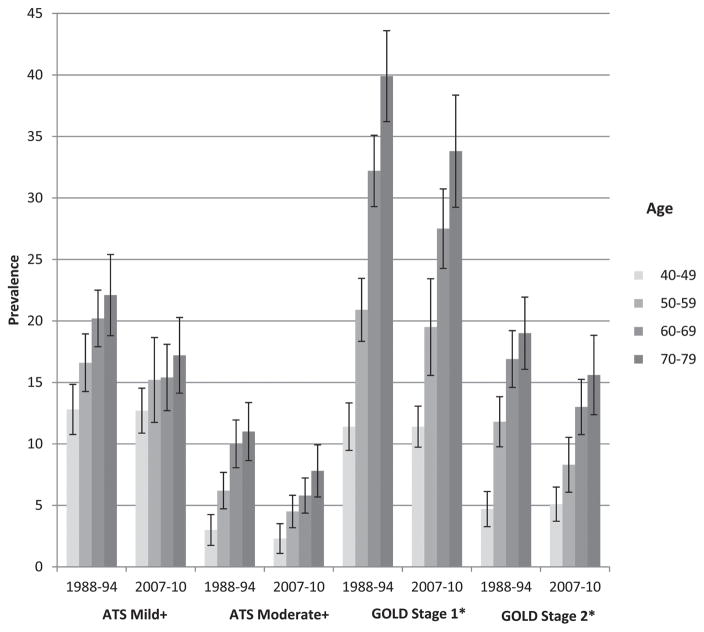
Figure 1 shows the prevalence of airflow obstruction (ATS/ERS and modified GOLD criteria) by age categories and time period. Table 3 shows the statistical differences between the estimates of prevalence of airflow obstruction based on ATS/ERS and GOLD Stage 1 and 2 criteria, for NHANES 2007–2010. The prevalence of ATS/ERS Mild+airflow obstruction overall, and age, gender, race, education and smoking status-specific prevalence were all significantly lower, with the exception of the 40–49 years olds. For that 40–49 age category, the prevalence of airflow obstruction was less for GOLD than for ATS/ERS which is consistent with the GOLD underestimation in the younger age categories. For Moderate+airflow obstruction, ATS/ERS was consistently significantly less than GOLD for all categories.
Figure 1.
Prevalence of airflow obstruction by severity, by age category, by time period. ATS/ERS Mild+: Mild or more severe airflow obstruction. ATS/ERS Moderate+: Moderate or more severe airflow obstruction. *Modified GOLD criteria. GOLD Stage 1: Mild or more severe airflow obstruction. GOLD Stage 2: Moderate or more severe airflow obstruction.
Table 3.
Age-specific and age-standardized prevalence of GOLD and ATS/ERS defined Mild+ and Moderate+ airflow obstruction among U.S. adults aged 40–79 years, 2007–2010
| NHANES | Mild + airflow obstruction
|
Moderate + airflow obstruction
|
||||
|---|---|---|---|---|---|---|
| GOLD Stage 1 | ATS/ERS | Matched t-test of Difference | GOLD Stage 2 | ATS/ERS | Matched t-test of Difference | |
| P† (SE) | P† (SE) | p-value | P† (SE) | P† (SE) | p-value | |
| Overall | 20.0 (1.0) | 14.5 (0.7) | *** | 9.0 (0.6) | 4.4 (0.4) | *** |
| Age | ||||||
| 40–49 | 11.4 (0.8) | 12.7 (0.9) | * | 5.1 (0.7) | 2.3 (0.6) | *** |
| 50–59 | 19.5 (1.9) | 15.2 (1.7) | *** | 8.3 (1.1) | 4.5 (0.6) | *** |
| 60–69 | 27.5 (1.6) | 15.4 (1.3) | *** | 13.0 (1.1) | 5.8 (0.7) | *** |
| 70–79 | 33.8 (2.2) | 17.2 (1.5) | *** | 15.6 (1.6) | 7.8 (1.0) | *** |
| Gender | ||||||
| Males | 24.0 (1.1) | 15.4 (0.9) | *** | 9.4 (0.8) | 4.5 (0.5) | *** |
| Females | 16.3 (1.2) | 13.8 (1.0) | *** | 8.6 (0.7) | 4.2 (0.4) | *** |
| Race | ||||||
| NH White | 21.9 (1.1) | 15.9 (0.8) | *** | 9.8 (0.7) | 4.7 (0.5) | *** |
| NH Black | 16.3 (1.2) | 13.6 (1.2) | *** | 8.1 (0.8) | 4.7 (0.7) | *** |
| Mexican American | 10.3 (0.8) | 8.4 (0.8) | * | 4.0 (0.5) | 1.7 (0.3) | *** |
| Education | ||||||
| <High school grad | 20.6 (1.7) | 15.3 (1.3) | *** | 11.9 (1.4) | 6.6 (1.1) | *** |
| High school grad | 21.8 (1.8) | 17.6 (1.6) | *** | 9.8 (0.8) | 5.9 (0.6) | *** |
| Some college | 20.6 (1.5) | 15.0 (1.1) | *** | 9.3 (0.8) | 3.7 (0.6) | *** |
| College graduate | 17.2 (1.4) | 10.9 (1.3) | *** | 6.0 (0.8) | 2.3 (0.5) | *** |
| Smoking status | ||||||
| Never smoker | 11.8 (1.1) | 8.2 (0.8) | *** | 3.9 (0.5) | 1.1 (0.2) | *** |
| Former smoker | 22.5 (1.2) | 14.5 (1.3) | *** | 9.4 (1.0) | 4.5 (0.7) | *** |
| Current smoker | 40.4 (1.9) | 34.4 (1.8) | *** | 24.0 (1.4) | 14.9 (1.3) | *** |
Mild+ is Mild or more severe airflow obstruction GOLD (FEV1/FVC<70%); ATS/ERS (FEV1/FVC<LLN); Moderate+ is Moderate or more severe airflow obstruction GOLD (FEV1/FVC<70% and FEV1<80%); ATS/ERS (FEV1/FVC<LLN and FEV1<70%).
Age-specific and age-standardized weighted prevalence.
p < 0.05;
p < 0.01;
p < 0.001; after Bonferroni adjustment.
Using the average U.S. population size for 40–79 year olds for years 2007–2010 of 126,199,000 and prevalence of ATS/ERS defined airflow obstruction of 14.5% for Mild+, 4.4% for Moderate+, and 1.1% for severe degree, we estimated the number of individuals in the United States. with COPD. The burden of COPD is approximately 18.3 million with Mild+(95% CI 16.4–20.3), 5.6 million with Moderate+(95% CI 4.4–6.6) and 1.4 million with severe (95% CI 0.9–1.8).
Post-bronchodilator spirometry is required for the GOLD definition of COPD to determine reversibility of airflow obstruction (9). In the NHANES 2007–2010 study of the 5476 adults 40–79 years of age with valid spirometry, 1137 (20.8%) were selected for BD follow-up because their spirometry results indicated the presence of airflow obstruction defined by the ATS/ERS or GOLD Stage 1 criteria (i.e., FEV1/FVC <LLN or FEV1/FVC <0.7). Of the 1137 cases, only 577 (50.7%) had the bronchodilator test done. Of those with a post-BD test and pre-BD GOLD Stage 1 (549), only 354 (64.4%) remained in the GOLD Stage 1 category after post-BD testing and 35.5% became “normal.” However, of the 560 who didn’t have the post-BD test, 315 were not tested because of safety reasons (such as uncontrolled blood pressure, irregular pulse on examination, taking medication for major arrhythmia or certain other medications, implanted defibrillator, or history of congenital heart disease) and 245 were not tested for other reasons (refusal, insufficient time, and others). Exclusion of those for safety reasons may have potentially decreased the reversibility rate. However, based on nonparametric testing, there were no significant differences between the group that had post-BD test and the two groups that did not have the post-BD test for safety reasons or for other reasons for the percent predicted FEV1/FVC ratios on pre-BD spirometry.
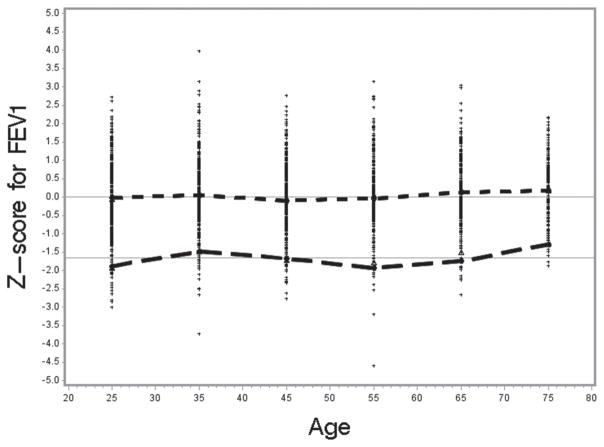
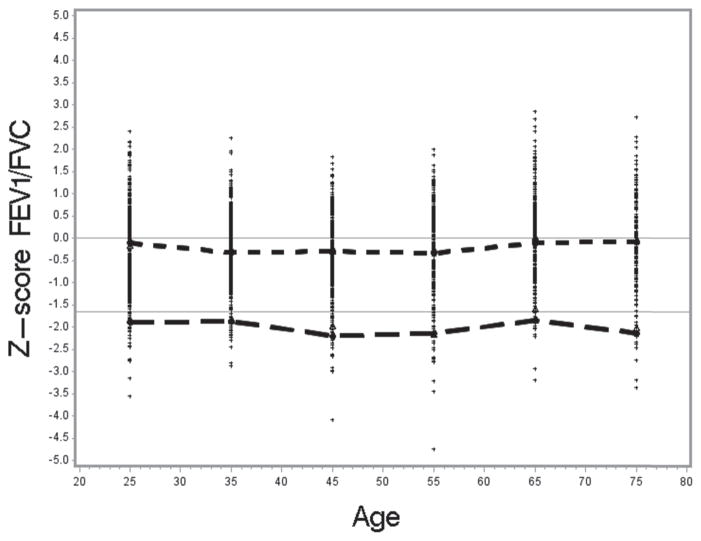
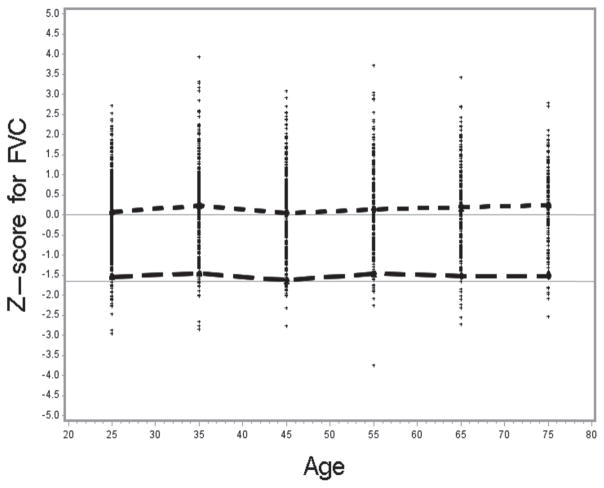
To test how well the NHANES III reference values fit the NHANES 2007–2010 sample population, we selected all “healthy” non-smokers 20–79 years of age from the NHANES 2007–2010 data (n =1980). The mean standardized differences between the observed and predicted values (z-score) were as follows: for FEV1 0.047 (SE 0.02; t =2.1; p <0.05), for FVC 0.151 (SE 0.02; t =6.4; p <0.001), and for the FEV1/FVC ratio −0.188 (SE 0.02; t = −8.5; p <0.001). The t-test values indicate that the observed mean z-scores were significantly different from zero especially for FVC and the ratio. Figures 2–4 show the distribution of the z-scores, the mean z-scores and their lower 95th percentile, by age categories.
Figure 2.
Z-score for FEV1 by age; −1.645 line is the lower limit of normal – 5th percentile; Short dashed line connects the mean z-score for each age category in relation to zero; Long dashed line connects the mean LLN for each age category.
Figure 4.
Z-score for ratio by age; −1.645 line is the lower limit of normal – 5th percentile; Short dashed line connects the mean z-score for each age category in relation to zero; Long dashed line connects the mean LLN for each age category.
The mean z-scores were close to zero for FEV1 for most age categories, but consistently above zero for FVC. The higher z-score values for FVC reflect higher observed FVC values for the NHANES 2007–2010 sample than was predicted based on the NHANES III-based reference equations. The higher observed FVC values then result in the lower mean FEV1/FVC values for NHANES 2007–2010, in comparison to the predicted values derived from the NHANES III reference equations and thus a negative z-score. The mean z-scores [(Observed-Predicted)/Relative Standard Deviation] are negative for most of the age categories since predicted FEV1/FVC values based on the NHANES III data are higher than the observed mean FEV1/FVC values. Consequently, because the distribution of the observed FEV1/FVC values is shifted towards zero with respect to the predicted and LLN values, the LLN values derived from the NHANES III equations identified more than 5% as being abnormal. Of the healthy non-smokers from the NHANES 2007–2010 sample, on average 7.4% were identified as having FEV1/FVC<LLN.
Discussion
We analyzed NHANES spirometry data from time periods 1988–1994 and 2007–2010 to evaluate changes in the prevalence of airflow obstruction, a main feature of chronic obstructive pulmonary disease, among older adults (age 40–79). Our results show that the ATS/ERS-defined prevalence of airflow obstruction declined significantly from 16.6 to 14.5% (p <0.05) for Mild+airflow obstruction and from 6.4 to 4.4% (p <0.01) for Moderate+airflow obstruction. There was a statistically significant decline in the prevalence of airflow obstruction in the age category 60–69 from 20.2% to 15.4% (p <0.01). In addition, severe airflow obstruction declined significantly in current smokers. Reasons for the decline may include decrease in the prevalence of smoking in the U.S. population, and reductions in occupational exposures and air pollution since 1988–1994.
Although our study shows decline in the prevalence of airflow obstruction, COPD remains one of the leading causes of death in the U.S. (28). The high mortality rate from COPD may be explained by higher life expectancy and decreased early mortality from cardiovascular disease (28–31). Results from the National Health Interview Survey (NHIS) 2007–2009 showed that the prevalence of COPD (self-reported doctor-diagnosed chronic bronchitis in the past year or ever emphysema) increased systematically with age from 5.7% for the 45–54 year olds to 7.6% among those 55–64, and 9.5% for the 65–74 year old U.S. adults (32). This lower doctor-diagnosed NHIS estimates reflects different diagnostic methods other than spirometry results, i.e. symptoms of chronic bronchitis or shortness of breath in the doctor-diagnosed COPD, medical diagnosis of COPD without pulmonary function testing (33), or differences in the survey methods. The lower prevalence of doctor-diagnosed disease may also indicate underdiagnosis of COPD in the U.S. population. Nevertheless, the observed declining trend in the prevalence of airflow obstruction based on spirometry measurements, as observed in our study, is likely to be reflected in declining morbidity and mortality from COPD in the future if the trend in the decreasing exposure to particulates continues (34, 35).
A previous study by Ford et al. (10) reported no significant differences between NHANES III and NHANES 2007–2010 sample populations in the prevalence of COPD (overall prevalence 14.6% vs. 13.5%, p >0.05) for ages 20–79 years using GOLD Stage 1 and 2 criteria and pre-BD spirometry. Ford et al. estimated the prevalence of airflow obstruction at 29.4% in the 60–79-year age group. Ford et al. did not apply any reliability/reproducibility exclusion criteria to the NHANES III data while our NHANES III analysis was limited to those with reliable spirometry exams and at least 2 maneuvers, which may explain some of the differences with our study. Our results also show that the differences in the prevalence of GOLD Stage 1 and Stage 2 for the two time periods are not statistically significant, for the age categories (Figure 1). One of the reasons for the different findings by the two studies is that the GOLD Stage 1 and GOLD Stage 2 defined on pre-bronchodilator spirometry over-estimate the disease prevalence. In this study, the GOLD defined prevalence of COPD was significantly higher than the prevalence of ATS/ERS defined airflow obstruction, for Mild+and Moderate+categories, but not for the severe category (Table 3).
The burden of COPD was likely also overestimated by Ford et al. using the GOLD criteria based on the fixed ratio and using pre-BD spirometry. Ford et al. estimates that in the larger U.S. population 20–79 age group 28.9 million have mild or more severe airflow obstruction based on GOLD Stage 1, 12.9 million have moderate and more severe obstruction based on GOLD Stage 2, and 1.5 million have severe airflow obstruction based on GOLD Stage 3. For the U.S. population aged 40–79, we estimated that the burden of COPD based on the prevalence of ATS-defined airflow obstruction, the main feature of COPD, for the U.S. population 40–79 age group was approximately 18.3 (95% CI 16.4–20.3) million for Mild+airflow obstruction, 5.6 (95% CI 4.4–6.6) million for Moderate+airflow obstruction, and 1.4 (95% CI 0.9–1.8) million for severe airflow obstruction.
Our estimate for the 40–79 age group based on pre-BD criteria was approximately 25.2 (95% CI 22.3–27.8) million for GOLD Stage 1 (Mild+), 11.4 (95% CI 9.7–12.9) million for GOLD Stage 2 (Moderate+), and 1.4 (95% CI 0.9–1.9) million for GOLD stage 3 (severe). Thus there are large differences in the estimated burden of COPD when using the GOLD and ATS-based estimates for Mild+obstruction (25.2 vs. 18.3 million ≈6.9 million) and for Moderate+obstruction (11.4 vs. 5.6 million ≈5.8 million) for the U.S. population aged 40–79.
Many publications have reported that the usage of the fixed FEV1/FVC ratio criterion of 0.7, especially when using pre-BD spirometry, leads to over estimation of the COPD prevalence in older adults (15–18, 36, 37). The PLATINO study indicated that applying GOLD 1 criteria to pre-BD spirometry, rather than post-BD spirometry as required by GOLD recommendations, may overestimate the COPD prevalence by approximately 35% (38). However, Tashkin et al. (39) reported substantial acute bronchodilator reversibility in patients with early COPD who had no other features of asthma suggesting that BD reversibility may not be a completely reliable way of distinguishing asthma and COPD.
We have determined using the z-score analysis that usage of the NHANES III equations may potentially lead to a slight overestimation of the prevalence of airflow obstruction in the 2007–2010 data. Although the reference equations fit well to the FEV1 data, FEV1 z-scores were around zero, z-scores for FVC (i.e., for both the predicted and LLN values were systematically above zero and above −1.645, respectively, for most age categories) and the mean z-score was significantly higher than zero. As expected, the resulting z-score values for the ratio of FEV1/FVC were lower than zero for the predicted value and below −1.645 for the LLN values, and the mean z-score was significantly lower than zero. This may be due to better spirometry quality in the 2007–2010 testing period.
Consequently, more “healthy never smokers” from 2007–2010 population sample were classified with airflow obstruction using the NHANES III LLN criteria for the FEV1/FVC ratio (7.4%) rather than the expected 5%, potentially leading to overestimation of the prevalence of airflow obstruction (Figure 4). This, in turn, likely underestimates the true difference between the NHANES III and current NHANES estimates. This result indicates that a new set of reference equations may be needed for the current U.S. population. Applicability of the Global Lung Initiative 2012 reference equations (40) for the current U.S. population should be evaluated.
The results show reduction in the prevalence of airflow obstruction mainly in the 60+ages (Figure 1), and the decline was significant in the 60–69 age category. There are limitations, however, that need to be considered when interpreting these results. First, the exclusion criteria, the participation rate in the spirometry testing and the valid spirometry rate were different in the NHANES 2007–2010 study than in the NHANES III, resulting in lower overall rate in those with valid spirometry (90% vs. 77%). However, NHANES is a demographically based survey with a complex multistage design incorporating probability, stratified and cluster sampling at different stages.
The statistical software uses design variables (primary sampling units, strata) and non-response adjusted sample weights to produce adjusted prevalence estimates. Secondly, the definition of airflow obstruction was based on NHANES III reference equations (19) and we have shown in this article that their usage may potentially lead to overestimation of the prevalence of airflow obstruction in NHANES 2007–2010. Generally, because of stringent spirometry review of the flow-volume curves, it is unlikely that participants with airflow obstruction would be excluded from these two studies due to invalid spirometry.
Conclusion
In conclusion, the prevalence of ATS/ERS-defined airflow obstruction decreased significantly from 1988–1994 (16.6%) to 2007–2010 (14.5%), p <0.05. We estimated, based on the prevalence of ATS/ERS-defined airflow obstruction, a main determinant of COPD, that approximately 18.3 million adults aged 40–79 have Mild+airflow obstruction, 5.6 million have Moderate+airflow obstruction, and 1.4 million have severe airflow obstruction in the current U.S. population.
Figure 3.
Z-score for FVC by age; −1.645 line is the lower limit of normal – 5th percentile; Short dashed line connects the mean z-score for each age category in relation to zero; Long dashed line connects the mean LLN for each age category.
Acknowledgments
The authors thank Diana Freeland (NIOSH) for providing technician training and quality review oversight and the NHANES technicians for their dedication to the subjects.
Footnotes
Declaration of Interest Statement
The authors have no financial, consulting, and personal relationships that could influence this work product. The National Institute for Occupational Safety and Health, National Center for Health Statistics, or National Heart, Lung, and Blood Institute supported the salaries of the authors. This work was performed by U.S. Federal Government employees as part of their work; no nongovernmental funding supported this work. The authors alone are responsible for the content and writing of the paper. The findings and conclusions of this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the National Heart, Lung, and Blood Institute.
References
- 1.World Health Organization (WHO) Chronic obstructive pulmonary disease (COPD) Fact sheet [Online] 2012 Nov; Available from: http://www.who.int/mediacentre/factsheets/fs315/en/index.html.
- 2.National Heart, Lung, and Blood Institute. Morbidity and mortality 2012 chartbook on cardiovascular, lung, and blood diseases. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service. National Institute of Health; Available from: http://www.nhlbi.nih.gov/research/reports/2012-mortality-chart-book.htm. [Google Scholar]
- 3.Snider JT, Romley JA, Wong KS, Zhang J, Eber M, Goldman DP. The disability burden of COPD. COPD. 2012;9:513–521. doi: 10.3109/15412555.2012.696159. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Chronic obstructive disease among adults—United States, 2011. MMWR Surveill Summ. 2012;61:938–943. [PubMed] [Google Scholar]
- 5.Akazawa M, Halpern R, Riedel AA, Stanford RH, Dalal A, Blanchette CM. Economic burden prior to COPD diagnosis: a matched case-control study in the United States. Respir Med. 2008;102:1744–1752. doi: 10.1016/j.rmed.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 6.National Heart, Lung, and Blood Institute. Morbidity and mortality 2009 chartbook on cardiovascular, lung, and blood diseases. Bethesda, MD: U.S. Department of Health and Human Services, Public Health Service. National Institute of Health; 2009. Available from: http://www.docin.com/p-419824684.html. [Google Scholar]
- 7.Agaku I, King B, Dube SR. Current cigarette smoking among adults—United States, 2011. MMWR. 2012;61(44):889–894. [PubMed] [Google Scholar]
- 8.Syamlal G, Mazurek JM, Malarcher AM. Current cigarette smoking prevalence among working adults—United States, 2004—2010. MMWR. 2011;60(38):1305–1309. [PubMed] [Google Scholar]
- 9.Global Initiative for Chronic Obstructive Lung Disease, Inc. [accessed February 26, 2013];Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2013. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- 10.Ford ES, Mannino DM, Wheaton AG, Giles WH, Presley-Cantrell L, Croft JB. Trends in the prevalence of obstructive and restrictive lung function among adults in the United States: findings from the National Health and Nutrition Examination Survey from 1988–1994 to 2007–2010. Chest. 2013;143(5):1395–1406. doi: 10.1378/chest.12-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes TA, Fromer L. Spirometry use: detection of chronic obstructive pulmonary disease in the primary care setting. Clin Interven Aging. 2011;6:47–52. doi: 10.2147/CIA.S15164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 13.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinkski J Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 14.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370:765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 15.Hnizdo E, Glindmeyer HW, Petsonk EL, Enright P, Buist AS. Case definitions for chronic obstructive pulmonary disease. COPD. 2006;3:95–100. doi: 10.1080/15412550600651552. [DOI] [PubMed] [Google Scholar]
- 16.Medbo A, Melbye H. Lung function testing in the elderly—can we still use FEV1/FVC <70% as a criterion of COPD? Respir Med. 2007;101:1097–1105. doi: 10.1016/j.rmed.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Swanney MP, Ruppel G, Enright PL, Pedersen OF, Crapo RO, et Miller MR, Jensen RL, Falaschetti E, Schouten JP, Hankinson JL, Stocks J, Quanjer PH. Using the lower limit of normal for the FEV1/FVC ratio reduces the misclassification of airway obstruction. Thorax. 2008;63:1046–1051. doi: 10.1136/thx.2008.098483. [DOI] [PubMed] [Google Scholar]
- 18.Miller MR, Quanjer PH, Swanney MP, Ruppel G, Enright PL. Interpreting lung function data using 80% predicted and fixed threshold misclassifies more than 20% of patients. Chest. 2011;139:52–59. doi: 10.1378/chest.10-0189. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz, Fedan KB. Spirometric reference valuses from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) [accessed March 25, 2013];The national health and nutrition examination survey: sample design, 1999–2006. 2012 Available from: http://www.cdc.gov/nchs/data/series/sr_02/sr02_155.pdf.
- 21.Centers for Disease Control and Prevention (CDC) [accessed April 12, 2013];Continuous NHANES web tutorial, age standardization and population counts. 2013 Available from: http://www.cdc.gov/nchs/tutorials/NHANES/NHANESAnalyses/agestandardization/age_standardization_intro.htm.
- 22.Centers for Disease Control and Prevention (CDC) [accessed April 12, 2013];NHANES Spirometry Exam Manual. 2008 Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_07_08/spirometry.pdf.
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Hankinson JL, Kawut SM, Shahar E, mith LJ, Stukovsky KH, Barr RG. Performance of American Thoracic Society-recommended spirometry reference values in a multiethnic sample of adults the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Chest. 2010;137:138–145. doi: 10.1378/chest.09-0919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.SAS Institute Inc. Base SAS 9.3 Procedures Guide: Statistical Procedures. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- 26.Klein RJ, Schoenborn CA. Health People Statistical Notes. 20. Hyattsville, Maryland: National Center for Health Statistics; 2001. Age adjustment using the 2000 projected U.S. population. [PubMed] [Google Scholar]
- 27. [accessed August 1, 2012];CPS Current Population Survey. 2011 Available from: http://www.census.gov/cps.
- 28.Kochanek KE, Xu J, Murphy SL, Minino AM, Kung H. Deaths: final data for 2009. Natl Vital Stat Rep. 2011;60(3):1–116. [PubMed] [Google Scholar]
- 29.Huertas A, Lalange P. COPD a multifactorial systemic disease. Therapeutic Adv Respir Dis. 2011;5(3):217–224. doi: 10.1177/1753465811400490. [DOI] [PubMed] [Google Scholar]
- 30.Huertas A, Lalange P. circulating endothelial progenitor cells and chronic pulmonary diseases. Eur Respir J. 2011;37:426–431. doi: 10.1183/09031936.00034810. [DOI] [PubMed] [Google Scholar]
- 31.Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- 32.CDC/NCHS. [accessed April 15, 2013];Health Data Interactive and National Health Interview Survey, 2007–2009. [Online] Available from: http://205.207.175.93/HDI/ReportFolders/reportFolders.aspx.
- 33.Koefoed MM, Christensen RD, Sondergaard J, Jarbol DE. Lack of spirometry use in Danish patients initiating medication targeting obstructive lung disease. Respir Med. 2012;106:1743–1748. doi: 10.1016/j.rmed.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality extended follow-up of the Harvard Six Cities Study. Am J Respir Crit Care Med. 2006;173:667–672. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pope CA, III, Dockery DW. Health effect of fine particulate air pollution: lines that connect. J Air Waste Mgmt Assoc. 2006;56(6):709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 36.Sterk PJ. Let’s not forget: the GOLD criteria for COPD are based on post-bronchodilator FEV1. Eur Respir J. 2004;23:497–498. doi: 10.1183/09031936.04.00017104. [DOI] [PubMed] [Google Scholar]
- 37.Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax. 2005;60:842–847. doi: 10.1136/thx.2005.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez-Padilla R, Hallal PD, Vazquez-Garcia JC, Márquez MN, Jardim JR, Pertuzé J, Lisboa C, Muiño A, López MV, Tálamo C, de Oca MM, Valdivia G, Menezes AM Latin American COPD Prevalence Study (PLATINO) Team. Impact of broncholdilator use on the prevalence of COPD in population-based samples. COPD. 2007;4:113–120. doi: 10.1080/15412550701341012. [DOI] [PubMed] [Google Scholar]
- 39.Tashkin DP, Ceilli B, Decramer M, Liu D, Burkhart D, Cassino C, Kesten S. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008;31:742–750. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 40.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Halll GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, Stocks J ERS Global Lung Function Initiative. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]